Kadok Leaves
Piper sarmentosum Roxb.
Piperaceae
DEFINITION
Kadok leaves consist of the whole mature leaves of a vine of P. sarmentosum Roxb. (Piperaceae)
SYNONYM
Chavica sarmentosa (Roxb. ex Hunter) Miq.
VERNACULAR NAMES
Wild betel leaf (English) [ 1 ], chabai, kadok batu (Malay), jia ju, qing ju, xi ye qing wei teng (Chinese) [ 2 ].
CHARACTER
IDENTIFICATION
Plant Morphology
Erect or ascending terrestrial, often stoloniferous, dioecious herb or shrublet and glabrous with long creeping procumbent fruit-bearing branches measuring 0.3-1.5 m tall with large, stout, sweet fruits on maturity [ 3 ]; most parts very finely powdery pubescent when young. Stems has thick cuticle, with scattered 1-2-celled non-glandular trichomes; swollen at nodes; short aerial. Leaves dark green, variable in shape and size, simple, alternate, heart shape, tender and cordate with 2-8 cm long petiole; lower leaves ovate-cordate, 7-15 cm x 5-14 cm, base cordate, apex cuminate, 5-7-veined; upper leaves oblong, ovate-oblong or obliquely oblong, 7-11 cm x 3-5 cm, ovate to obliquely or rounded at base, shortly acuminate at apex, 3-veined; primary veins 7, palmate, pair of veins usually arise 0.5-2 cm above from the base, prominently raised; cuticle thin, smooth; stomata rarely observed; glandular trichomes present mainly in the laminar region; non-glandular trichomes found on the veins; midrib prominently raised abaxially and slightly raised adaxially; trichomes mostly confined to the abaxial surface. Inflorescence an erect, axillary cylindrical spike, 1-2 cm long; spikeoblongoid, 2-5 cm long and 0.8-1 cm thick; peduncle as long as spike [ 5 , 6 ]. Flower unisexual ovary with either male or female flowers; 0.7 cm long; bracts circular, white, about 1 cm in diameter; stamens short; stigmas 3-4. Fruit an obovoid berry, 1.5 cm x 1 cm, connate to each other and adnate to bract but with free apex and sweet taste. Aerial roots are short, slender, cylindrical, brownish outgrowth from the lower nodes of stem.
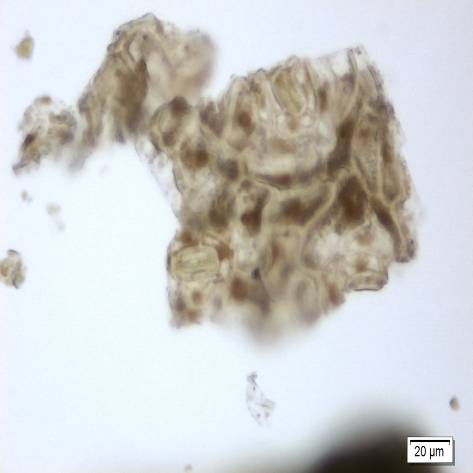
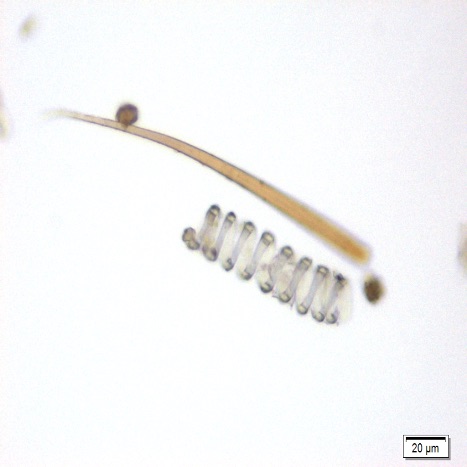
Microscopy
Microscopic characters of P. sarmentosum leaves consist of fragments of epidermis cells and abaxial epidermis shows numerous anisocytic, anomocytic, actinocytic, tetracytic and cyclocytic stomata, with 3-7 subsidiary cells arranged in one or more whorls, measuring 24-30 µm long and 20-27 µm wide; adaxial epidermis consists of an outer epidermal layer having square or tangentially elongated rectangular shaped cells, measuring 14-40 µm high, 12-53 µm long, in surface view, stomata rarely observed; secretory cells 20-28 µm in diameter which are present in the mesophyll cells; fragments of spirally thickened vessel and simple unicellular trichome [ 7 ].
Colour Tests
Observed colour of solution after treatment with various reagents:
| HCl (conc.) | Green |
| 5% FeCl3 | Green |
Thin Layer Chromatography (TLC)

Figure 3 : TLC profiles of beta-sitosterol (S), methanol extracts of P. sarmentosum dried leaves powder collected from different locations (L1-L3) observed under (a) UV at 254 nm and (b) UV at 366 nm before spraying and (c) visible light after spraying with anisaldehyde -sulphuric acid reagent.
| Test Solutions | Weigh about 1.0 g of P. sarmentosum dried leaves powder in a 50 mL screw-capped conical flask and add 25 mL methanol. Sonicate for 60 min at temperature 25°C and filter. Use the filtrate as test solution. |
| Standard solution | Dissolve 5.0 mg of beta-sitosterol standard [CAS no.: 83-46-5] in 5 mL methanol to produce 1000 µg/mL stock solution. Dilute this stock solution in methanol to produce 500 µg/mL. |
| Stationary Phase | HPTLC Silica gel 60 F254, 10 x 10 cm |
| Mobile phase | Toluene : ethyl acetate; (5:1) (v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
High Performance Liquid Chromatography (HPLC)
| Test solution | Extract about 5.0 g of the P. sarmentosum dried leaves powder with 10 mL of methanol. Sonicate the mixture for 60 min at temperature 25°C. Filter the mixture solution through a 0.45 µm syringe filter and inject the filtrate into the HPLC column. | |||||||||||||||
| Standard solution | Dissolve 5.0 mg of beta-sitosterol standard [CAS no.: 83-46-5] in 5 mL methanol to produce 1000 µg/mL stock solution. Dilute this stock solution in methanol to produce 500 µg/mL. | |||||||||||||||
| Chromatographic system |
Detector: UV 210 nm |
|||||||||||||||
| Mobile Phase (gradient mode) |
|
|||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the standard solutions (500 µg/mL). The requirements of the system suitability parameters are as follow:
|
|||||||||||||||
| Acceptance criteria |
|
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 15% |
| Acid-insoluble ash | Not more than 3% |
| Loss on Drying |
| Not more than 12% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 22% |
| Cold method | Not less than 15% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 13% |
| Cold method | Not less than 8% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Essential oil of the leaves has been found to contain monoterpenes (e.g. α-thujene, α-pinene, β-pinene, myrcene, limonene, trans-β-ocimene, linalool, 4-terpineol, α-terpineol, α-copaene, methyleugenol, β-elemene, α-phellandrene, eugenol and b-elemene), sesquiterpenes (e.g. α-cadinene, β-cadinene, cis-caryophyllene, trans-caryophyllene, germacrene-D, (-)-alloaromadendrene, α-humulene, germacrene B, δ-cadinene, caryophyllene oxide, myristicine, spathulenol, β-caryophyllene, α-farnesene, γ-cadinene, E,Z-farnesol and E,E-farnesol), a sesquiterpenoid (e.g. β-eudesmol) and others (e.g. methyl 3-phenylpropionate, safrole, tridecane, bicycloelemene, β-borbonene, elemicine, eusarone, nerol, cis-asarone, cedarene, cadinol, trans-asarone, n-heptadecane, isobutyl phthalate, butyl phthalate, phytol, bis(2-ethylhexyl) phthalate, piperitone, cinnamyl alcohol, α-ionone, β-guaiene, ethyl laurate, bicylogermacrene, cadinadiene, guaiol, dehydrocarveol, t-muurolol, β-bisabolol, δ-cadinol and α-cadinol) [ 8 , 9 ].
Methanol extract of the leaves has been found to contain phenylpropanoyl amides (e.g. chaplupyrrolidone A, chaplupyrrolidone B and deacetylsarmentamide) [ 10 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally, a decoction of P. sarmentosum leaves is used as an embrocation (application by rubbing) to treat bone pain. The leaves are also used as poultice on the children’s heads for headache and as an embrocation for tinea [ 11 ].
Biological and pharmacological activities supported by experimental data
Antiplasmodial activity
Methanol (0.8 mg/mL) and chloroform (0.05 mg/mL) extracts of P. sarmentosum leaves caused 100% inhibition of Plasmodium falciparum after 48 hours of incubation [ 4 ].
Methanol extract of P. sarmentosum leaves (5 mg/kg/day) was administered intraperitoneally to Plasmodium berghei infected adult female ddY mice for four days. There is a lower parasitaemia (0.08-20.31%) in the treated group compared to control (0.16-31.17%) [ 4 ].
Antibacterial activity
Methanol extract of P. sarmentosum leaves inhibited the growth of Staphylococcus aureus (MIC 2000 µg/disc), Methicillin Resistant S. aureus (MRSA) (MIC 1000 µg/disc) and Pseudomonas aeruginosa (MIC 2000 µg/disc) using disk diffusion method [ 12 ].
Methanol extract of P. sarmentosum leaves (100 mg/disc) inhibited the growth of MRSA (inhibition zone = 10 mm) compared to distilled water (6 mm) using disk diffusion assay. The extract showed MIC value of 50 mg/mL and minimum bactericidal concentration (MBC) value of 100 mg/mL. The MIC index (MBC/MIC) was 2 for MRSA [ 13 ].
Antifungal activity
Ethanol extract of P. sarmentosum leaves (0.9 mg/disc) inhibited the growth of oral Candida albicans (inhibition zone = 10.81 ± 0.30 mm) using disk diffusion method. MBC value of the extract was 1.25 mg/mL for C. albicans and 2.5 mg/mL for Aggregatibacter actinomycetemcomitans using broth microdilution method [ 14 ].
Methanol extract of P. sarmentosum leaves (2.5% w/v) inhibited 100% of Colletotrichum gloesporioides mycelial growth while the chloroform extract (2.5% w/v) inhibited only 77.75% growth. Both extracts (2.5% w/v) also inhibited 100% of C. gloeosporioides spore germination [ 15 ].
Antiviral activity
Ethanol extract of P. sarmentosum leaves showed antiviral activity on vesicular stomatitis virus (VSV) (MIC value 0.02 mg/mL) using simplified plaque reduction assay [ 16 ].
Antituberculosis activity
Methanol extract and its ethyl acetate and chloroform fractions of P. sarmentosum leaves inhibited the growth of Mycobacterium tuberculosis with MIC value of 12.5 µg/mL (methanol extract), 3.12 µg/mL (for both ethyl acetate and chloroform fractions). The combination of isoniazid and ethyl acetate fraction of P. sarmentosum leaves (100 µg/mL) at a ratio of 1:3 in serial dilution gave fractional inhibitory concentration index (FICI) of 0.58 and MIC value of 0.5 µg/mL towards M. tuberculosis [ 18 ].
Both ethanol and aqueous extracts of P. sarmentosum leaves inhibited the growth of M. tuberculosis with MIC value of 12.5 µg/mL and MBC value of 12.5 µg/mL comparable to isoniazid (0.50 µg/mL) [ 19 ].
Anti-oxidant activity
Ethanol extract of P. sarmentosum leaves (0.1 mg/mL) also showed anti-oxidant activities with DPPH radical scavenging activity (21.82 ± 2.13%) compared to butylated hydroxyanisole (BHA) (34.93 ± 2.41%), quercetin (28.13 ± 1.95%) and vitamin E (10.98 ± 1.68%). The extract (0.1 mg/mL) also showed anti-oxidant activity (17.59 ± 3.2%) comparable to BHA (21.49 ± 2.41%) using β-carotene linoleate assay [ 19 ].
Methanol extract of P. sarmentosum leaves was also reported to have high β-carotene bleaching activity with anti-oxidant index of 13.0 ± 0.84 [ 17 ]. The extract (250 µg/mL) also showed xanthine/xanthine oxidase superoxide scavenging activity of 87.6% while naringenin isolated from the methanol extract showed 75.7% activity which is comparable to superoxide dismutase standard (100%) [ 20 ].
Rutin and vitexin (10-400 µm, each) isolated from the aqueous extract of P. sarmentosum leaves showed cytoprotective effect against oxidative stress caused by hydrogen peroxide (H2O2) in human umbilical vein endothelial (HUVEC) cells[ 21 ].
Methanol, aqueous and hexane extracts of P. sarmentosum leaves (150-300 µg/mL) significantly (p < 0.05) decreased malondialdehyde level, superoxide dismutase, catalase and glutathione peroxidase in HUVEC cells treated H2O2 compared to HUVEC cells treated with H2O2 only [ 22 ].
Methanol extract of P. sarmentosum leaves showed antioxidant activity with 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity (IC50 = 129.65 ± 1.04 µg/mL) compared to ascorbic acid (IC50 = 4.15 ± 0.50 µg/mL). Total phenolic content of the extract was found to be 50.01 mg GAE/g dry weight (DW) [ 23 ].
Methanol extract of P. sarmentosum leaves (125 mg/kg/day) was administered orally to male Wistar rats for 28 days before the induction of oxidative stress using carbon tetrachloride (CCl4). A significant (p < 0.05) decrease in lung thiobarbituric acid reactive substances (TBARS) level and lung glutathione peroxidase (GPx) activity was observed in treated rats compared to CCl4 induced control group [ 24 ].
Ethanol extract of P. sarmentosum leaves (250 and 500 mg/kg/day) was administered orally to male Sprague Dawley rats for 14 days before the induction of oxidative stress using carbon tetrachloride (CCl4). A significant decrease in TBARS level (p < 0.05) as well as increase in superoxide dismutase level (p < 0.05), catalase level (p < 0.05) and total plasma antioxidant activity (p < 0.05) were observed in treated rats compared to CCl4 induced control group [ 25 ].
Aqueous extract of P. sarmentosum leaves showed anti-oxidant activities with DPPH radical scavenging activity of 46.84% and β-carotene antioxidant protection factor of 1.11.Total phenolic content of the extract was found to be 10.98 mg GAE/g DW [ 26 ].
Aqueous extract showed antioxidant activities with DPPH radical scavenging activity (15.44%) compared to vitamin C (≈90%), ferric reducing antioxidant potential (FRAP) activity (377.41 mmol) compared to vitamin C (≈1700 mmol) and β-Carotene bleaching activity (17.6%) compared to vitamin C (≈11%) [ 27 ].
The boiled aqueous extract showed antioxidant activity with DPPH radical scavenging activity (38-40%) compared to butylated hydroxytoluene (BHT) (~85%). The FRAP activity (98.76 mmol) was lower compared to BHT (1000 mmol). Total phenolic content of the extract was found to be 10.92 mg/g DW [ 27 ].
Aqueous extract of P. sarmentosum leaves showed antioxidant activity with DPPH radical scavenging activity (IC50 = 27.12 mg/mL) compared to vitamin C [ 28 ].
Anti-inflammatory activity
Methanol extract of P. sarmentosum leaves (50-100 µg/mL) significantly decreased nitric oxide (NO) production at 100 µg/mL (p < 0.001) and at 50 µg/mL (p < 0.05) in murine monocytic macrophages (RAW 264.7) cells treated with recombinant mouse interferon/ lipopolysaccharide (IFN-g/LPS) compared to IFN-g/LPS treated group only. The extract also showed moderate NO inhibitory activity (IC50 = 60.24 ± 2.39 µg/mL) compared to L-NG-nitroarginine methyl ester (L-NAME) (0.008 ± 0.1 µg/mL) in RAW 264.7 cells treated with IFN-g/LPS [ 23 ].
Methanol extract of P. sarmentosum leaves (50-200 mg/kg) was administered orally as a single dose to male Wistar rats before the induction of paw edema using carrageenan. After three hours, a significant inhibition of paw edema (p < 0.05 at 50 mg/kg, p < 0.01 at 100 mg/kg and p < 0.01 at 200 mg/kg) was observed in treated rats compared to distilled water treated group [ 29 ].
Methanol extract of P. sarmentosum leaves (300 mg/kg) was administered orally as a single dose to Wistar albino rats one hour before the induction of paw edema using carrageenan. A significant (p ≤ 0.01) inhibition of paw edema was observed in treated rats compared to control group [ 31 ].
Methanol extract of P. sarmentosum leaves (300 mg/kg) was administered orally as a single dose to Wistar albino rats before the induction of paw edema using dextran. After three hours, a significant (p ≤ 0.01) inhibition of paw edema was observed in treated rats compared to control group [ 31 ].
Aqueous extract of P. sarmentosum leaves (30-300 mg/kg) was administered subcutaneously as a single dose to Sprague Dawley rats 30 minutes before the induction of paw edema using carrageenan. A significant (p < 0.05) decrease in paw edema volume was observed in treated rats compared to dimethylsulfoxide treated group [ 30 ].
Antinociceptive activity
Methanol extract of P. sarmentosum leaves (200 mg/kg) was administered intraperitoneally as a single dose to male Balb/c mice for 30 minutes before the induction of abdominal constriction using acetic acid. A significant (p < 0.05) decrease in the number of abdominal constriction was observed in treated mice compared to 5% Tween-20 treated group
[ 23 ].
Aqueous extract of P. sarmentosum leaves (30-300 mg/kg) was administered subcutaneously as a single dose to male Balb/c mice for 30 minutes before the induction of abdominal constriction using acetic acid. A significant (p < 0.05) dose dependent decrease in writhing response was observed in treated mice compared to saline treated group
[ 30 ].
Aqueous extract of P. sarmentosum leaves (30-300 mg/kg) was administered subcutaneously as a single dose to male Balb/c mice 30 minutes before the hot plate test. A significant (p < 0.05) dose dependent increase in the mean of latency time to discomfort reaction was observed in treated mice compared to saline treated group [ 30 ].
Anti-atherosclerosis activity
Standard diet enriched with 1% cholesterol and aqueous extract of P. sarmentosum leaves (500 mg/kg/day) administered orally to male New Zealand white rabbits for a duration of 10 weeks significantly (p < 0.05) decreased inflammation markers (vascular cell adhesion molecule (VCAM-1), intercellular adhesion molecule (ICAM-1) and C-reactive protein (CRP) compared to cholesterol diet group [ 28 ].
Standard diet enriched with 1% cholesterol and aqueous extract of P. sarmentosum leaves (500 mg/kg/day) administered orally to male New Zealand white rabbits for a duration of 10 weeks significantly (p < 0.05) decreased atherosclerotic lesion area and reduced the thickening of tunica intima layer in rabbits aorta compared to cholesterol diet group [ 32 ].
Standard diet enriched with 1% cholesterol and methanol extract of P. sarmentosum leaves (62.5-250 mg/kg/day) administered orally to male New Zealand white rabbits for duration of 10 weeks significantly decreased the area of fatty streak (p ≤ 0.01) and reduced thickening of the intima and foam cell (p ≤ 0.01) in rabbit abdominal aorta compared to cholesterol diet group [ 33 ].
Concomitant treatment of aqueous extract of P. sarmentosum leaves (150 µg/mL) and H2O2 significantly decreased mRNA expression of cellular adhesion molecule (ICAM-1) (p < 0.01) and NADPH oxidase 4 (Nox4) (p < 0.05). The treatment also significantly increased the antioxidant enzymes; SOD1 (p < 0.05), catalase (p < 0.01) and GPx (p < 0.05) in HUVEC cells compared to control [ 34 ].
Aqueous extracts of P. sarmentosum leaves (150 µg/mL) significantly (p < 0.05) increased nitric oxide, endothelial nitric oxide synthase (eNOS) protein level, eNOS mRNA expression and eNOS enzyme activity in HUVEC cells compared to control [ 35 ].
Antidiabetic activity
Chaplupyrrolidones B isolated from the methanol extract of P. sarmentosum leaves inhibited α-glucosidase activity (IC50 430 ± 1.2 µm) compared to acarbose (404 ± 0.4 µm) [ 10 ].
Aqueous extract of P. sarmentosum leaves showed approximately 70% inhibition of α-glucosidase activity [ 26 ].
Aqueous extract of P. sarmentosum leaves (0.125 g/kg/day) was administered orally for 28 days to streptozotocin-induced diabetic male Sprague Dawley rats. A significant decrease in fasting blood glucose (p < 0.05) and increase in body weight (p < 0.05) was observed in treated rats compared to diabetic group. Less ultrastructural degenerative changes in cardiac tissue and proximal aorta were also observed in treated rats compared to diabetic group [ 36 ].
Anti-osteoporosis activity
Aqueous extract of P. sarmentosum leaves (125 mg/kg/day) was administered orally for six weeks to ovariectomized female Sprague Dawley rats after closed fracture of the rats’ right femora. The median fracture healing score was significantly (p < 0.05) higher in treated rats compared to ovariectomized control rats. Histologically, the formation of woven bone and the remodeling of woven bone to lamellar bone were observed in treated rats [ 37 ].
A radiological study showed a significant decrease in total callus axial volume (p < 0.05) and callus score (p < 0.05) and increase in fracture healing score (p < 0.05) in treated rats compared to ovariectomized control rats. The soft calluses had been replaced by hard callus (woven bone), which was being remodelled into lamellar bone in treated rats [ 38 ].
Aqueous extract of P. sarmentosum leaves (125 mg/kg/day) was administered orally together with intramuscular dexamethasone for two months to adrenalectomized male Sprague Dawley rats (aged 43 months old). A significant increase in 11 β-HSD1 dehydrogenase activity (p < 0.05) that deactivated cortisol was observed. Subsequently this lead to the decreased expression of 11 β-HSD1 (p < 0.05) in bones of treated rats compared to dexamethasone-treated adrenalectomized rat [ 39 ].
Cytotoxicity activity
The ethanol extract of P. sarmentosum leaves showed cytotoxicity effect on human cervical carcinoma cells (HeLa) (cytotoxic dose at 50% (CD50) = 0.1 mg/mL) using microtitration cytotoxicity assay [ 16 ].
Methanol, aqueous and hexane extracts of P. sarmentosum leaves (0-1000 µg/mL) showed no cytotoxicity effect on HUVEC cells after 72 hours of incubation using MTT assay. The extracts also protected HUVEC cells against the cytotoxicity effect of H2O2 (Effective concentration at 50% (EC50) = 150 µg/mL) after 24 hours of incubation using MTT assay [ 22 ].
Ethanol extract of P. sarmentosum leaves showed cytotoxicity effect (IC50 = 12.5 µg/mL) on human hepatocellular carcinoma (HepG2) compared to tamoxifen (IC50 = 3 µg/mL) after 72 hours of incubation using MTT assay. There was no cytotoxicity effect on Chang cell (normal liver cell) compared to tamoxifen (IC50 = 18.6 µg/mL). The extract (1.5-200 µg/mL) significantly (p < 0.05) decreased the viable HepG2 cells compared to untreated cells after 72 hours of incubation using trypan blue staining assay [ 40 ].
Anti-angiogenic activity
Pellitorine (50 µg/mL) and sarmentine (50 µg/mL) isolated from chloroform extract of P. sarmentosum leaves showed low anti-angiogenic activity (30%) compared to suramin sodium (100%) in rat aorta ring model. The antiangiogenic activity of chloroform extract (45 µg/mL) was 50% [ 41 ].
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute toxicity
Methanol extract of P. sarmentosum leaves (5000 mg/kg) was administered orally as a single dose to male and female mice. The toxicity effect was observed for seven days and showed no toxic effect (LD50 > 5000 mg/kg) [ 29 ].
Ethanol extract of P. sarmentosum leaves (2000 mg/kg) was administered orally as a single dose to female Sprague Dawley rats (aged 8-12 weeks old). The toxicity effect was observed for 14 days and showed no toxic effect (LD50 > 2000 mg/kg) [ 35 ].
Oral single dose acute toxicity study using aqueous mixture of dried powder of P. sarmentosum leaves on female Sprague Dawley rats (aged between 8 and 12 weeks old) showed no toxic effect on the parameters observed which includes behaviors, body weight, food and water intakes. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. Approximate lethal dose (LD50) is more than 2,000 mg/kg body weight [ 42 ].
Clinical Studies
Information and data have not been established.
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- Global information hub on integrated medicine. [Internet Piper sarmentosum; [cited on 8th September 2014]. Available from: http://www.globinmed.com/index.php?option=com_content&view=article&id=79219;piper-sarmentosum-roxb-ex-hunt-piperaceae&catid=718;p
- Multilingual multiscript plant name database. [Internet] Australia: The University of Melbourne;2007 [cited on 8th September 2014]. Available from: http://www.plantnames.unimelb.edu.au/Sorting/Piper.html
- Rukachaisirikul T, Siriwattanakit P, Sukcharoenphol K, Wongvein C, Ruttanweang P, Wongwattanavuch P, Suksamrarn A. Chemical constituents and bioactivity of Piper sarmentosum. Journal of Ethnopharmacology. 2004;93:173-176.
- Rahman NNNA, Furuta T, Kojima S, Takane K, Mohd MA. Antimalarial activity of extracts of Malaysian medicinal plants. Journal of Ethnopharmacology, 1999;64:249-254.
- Jansen PCM. Piper sarmentosum Roxb. ex Hunter, In: C. C. de Guzman and J. S. Siemonsma, Eds., Plant resources of South-East Asia no. 13: spices. Backhuys Publisher, Leiden. 1999.
- Tseng YC, Xia N, Gilbert MG. Piperaceae. in: W. Zhengyi and P. H. Raven, Eds., Flora of China-Cy- cadaceae through Fagaceae, Vol. 4, Science Press, Bei- jing and Missouri Botanical Garden Press, St. Louis. 1999.
- Raman V, Galal AM, Khan IA. An investigation of the vegetative anatomy of Piper sarmentosum, and a comparison with the anatomy of Piper betle (Piperaceae). American Journal of Plant Science. 2012;3:1135-1144.
- Qin W, Huang S, Li C, Chen S, Peng Z. Biological activity of the essential oil from the leaves of Piper sarmentosum Roxb. (Piperaceae) and its chemical constituents on Brontispa longissima (Gestro) (Coleoptera: Hispidae). Pesticide, Biochemistry and Physiology. 2010;96:132-139.
- Chieng TC, Assim ZB, Fasihuddin BA. Toxicity and antitermite activities of the essential oils from Piper sarmentosum. Malaysian Journal of Animal Science. 2008;12(1):234-239.
- Damsud T, Adisakwattana S, Phuwapraisirisan P. Three new phenylpropanoyl amides from the leaves of Piper sarmentosum and their α-glucosidase inhibitory activities. Phytochemistry Letters. 2013;6(3):350-354.
- Burkill I. A dictionary of the economic products of the malay peninsula. Vol. 2. Kuala Lumpur: Ministry of Agriculture Malaysia. 1935;p.1752-1753.
- Zaidan MRS, Noor Rain A, Badrul AR, Adlin A, Norazah A, Zakiah I. In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Tropical Biomedicine. 2005;22(2):165–170.
- Fernandez L, Daruliza K, Sudhakaran S, Jegathambigai R. Antimicrobial activity of the crude extract of Piper sarmentosum against methicilin-resistant Staphylococcus aureus (MRSA), Escherichia coli,Vibrio cholera and Streptococcus pneumoniae. European Review for Medical and Pharmacological Sciences. 2012;16:105-111.
- Taweechaisupapong S, Singhara S, Lertsatitthanakorn P, Khunkitti W. Antimicrobial effects of Boesenbergia pandurata and Piper sarmentosum leaf extracts on planktonic cells and biofilm of oral pathogens. Pakistan Journal of Pharmaceutical Sciences. 2010;23(2):224-231.
- Bussaman P, Namsena P, Rattanasena P, Chandrapatya A. Effect of crude leaf extracts on Colletotrichum gloeosporioides (Penz.) Sacc. Journal of Entomology. 2012;2012:1-6.
- Ali AM, Mackeen MM, Salehh.Ei-Sharkawy, A.Hamid J, Ismail NH, Ahmad F, Lajis MN. Antiviral and cytotoxic activities of some plants used in malaysian indigenous medicine. Pertanika Journal of Tropical Agricultural Science. 1996;19:129-136.
- Chanwitheesuk A, Teerawutgulrag A, Rakariyatham N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chemistry. 2005;92(3):491-497.
- Khalid H, Zhari I, Amirin S, Pazilah I. Analysis of proteins, polysaccharides, glycosaponins contents of Piper sarmentosum Roxb. and anti-TB evaluation for bio-enhancing/interaction effects of leaf extracts with isoniazid (INH). Natural Product Radiance. 2008;7(5):402-408.
- Hussain K, Ismail Z, Sadikun A, Ibrahim P. Antioxidant, anti-TB activities, phenolic and amide contents of standardised extracts of Piper sarmentosum Roxb. Natural Product Research. 2009;23(3):238–249.
- Subramaniam V, Adenan MI, Ahmad AR, Sahdan R. Natural antioxidants: Piper sarmentosum (kadok) and Morinda elliptica (mengkudu). Malaysian Journal of Nutrition. 2003;9(1):41-51.
- Ugusman A, Zakaria Z, Hui CK, Nordin NAMM, Mahdy ZA. Flavonoids of Piper sarmentosum and its cyto-protective effects against oxidative stress. EXCLI Journal. 2012;11:705-714.
- Hafizah AH, Zaiton Z, Zulkhairi A, Mohd Ilham A, Nor Anita MM, Zaleha AM. Piper sarmentosum as an antioxidant on oxidative stress in human umbilical vein endothelial cells induced by hydrogen peroxide. Journal of Zhejiang University Science B. 2010;11(5):357-365.
- Lee KH, Padzil AM, Syahida A, Abdullah N, Zuhainis SW, Maziah M, Sulaiman MR, Israf DA, Shaari K, Lajis NH. Evaluation of anti-inflammatory, antioxidant and anti-nociceptive activities of six Malaysian medicinal plants. Journal of Medicinal Plants Research. 2011;5(23):5555-5563.
- Mohd Fahami Nur A, Kamisah Y, Rahman RF, Faizah O. Piper sarmentosum Roxb protects lungs against oxidative stress induced by carbon tetrachloride in rats. Journal of Medicinal Plants Research. 2011;5(26):6128-6135.
- Hussain K, Ismail Z, Sadikun A, Ibrahim P. Standardization and in vivo antioxidant activity of ethanol extracts of fruit and leaf of Piper sarmentosum. Planta Medica. 2010;76:418–425.
- Wongsa P, Chaiwarit J, Zamaludien A. In vitro screening of phenolic compounds, potential inhibition against a-amylase and a-glucosidase of culinary herbs in Thailand. Food Chemistry. 2012;131:964-971.
- Sumazian Y, Syahida A, Hakiman M, Maziah M. Antioxidant activities, flavonoids, ascorbic acid and phenolic contents of Malaysian vegetables. Journal of Medicinal Plants Research. 2010;4(10):881-890.
- Amran AA, Zakaria Z, Othman F, Das S, Al-Mekhlafi HM, Nordin NA. Changes in the vascular cell adhesion molecule-1, intercellular adhesion molecule-1 and c-reactive protein following administration of aqueous extract of Piper sarmentosum on experimental rabbits fed with cholesterol diet. Lipids in Health and Disease. 2011;10:2.
- Ridtitid W, Ruangsang P, Wongnawa M, Reanmongkol W. Studies of the anti-inflammatory and antipyretic activities of the methanolic extract of Piper sarmentosum Roxb. leaves in rats. Songklanakarin Journal of Science and Technology. 2007;29(6):1519-1526.
- Zakaria ZA, Patahuddin H, Mohamad AS, Israf DA, Sulaiman MR. In vivo anti-nociceptive and anti-inflammatory activities of the aqueous extract of the leaves of Piper sarmentosum. Journal of Ethnopharmacology. 2010;128(1):42-48.
- Vaghasiya Y, Nair R, Chanda S. Investigation of some Piper species for anti-bacterial and anti-inflammatory property. International Journal of Pharmacology. 2007;3(5):400-405.
- Amran AA, Zakaria Z, Othman F, Das S, Raj S, Nordin NA. Aqueous extract of Piper sarmentosum decreases atherosclerotic lesions in high cholesterolemic experimental rabbits. Lipids in Health and Disease. 2010;9:44.
- Amran AA, Zakaria Z, Othman F, Das S, Al-Mekhlafi HM, Raj S, Nordin N. Effect of methanolic extract of Piper sarmentosum leaves on neointimal foam cell infiltration in rabbits fed with high cholesterol diet. EXCLI Journal. 2012;11:274-283.
- Ugusman A, Zakaria Z, Hui CK, Nordin NA. Piper sarmentosum inhibits ICAM-1 and Nox4 gene expression in oxidative stress-induced human umbilical vein endothelial cells. BMC Complementary and Alternative Medicine. 2011;11:31.
- Ugusman A, Zakaria Z, Hui CK, Nordin NA. Piper sarmentosum increases nitric oxide production in oxidative stress: a study on human umbilical vein endothelial cells. Clinics. 2010;65(7):709-714.
- Thent ZC, Lin TS, Das S, Zakaria Z. Effect of Piper sarmentosum extract on the cardiovascular system of diabetic Sprague-Dawley rats: electron microscopic study. Evidence-Based Complementary and Alternative Medicine. 2012;2012:628750.
- Estai MA, Soelaiman IN, Shuid AN, Das S, Ali AM, Suhaimi FH. Histological changes in the fracture callus following the administration of water extract of Piper sarmentosum (daun kadok) in estrogen-deficient rats. Iran Journal of Medical Science. 2011;36(4):281-288.
- Estai MA, Suhaimi FH, Das S, Fadzilah FM, Alhabshi SMI, Shuid AN, Soelaiman IN. Piper sarmentosum enhances fracture healing in ovariectomized osteoporotic rats: a radiological study. Clinics. 2011;66(5):865-872.
- Ramli ESM, Soelaiman IN, Othman F, Ahmad F, Shuib AN, Mohamed N, Muhammad N, Hj Suhaimi F. The effects of Piper sarmentosum water extract on the expression and activity of 11β-hydroxysteroid dehydrogenase type 1 in the bones with excessive glucocorticoids. Iran Journal of Medical Science. 2012;37(1):39-46.
- Omar WHHW, Ariffin SHZ, Ariffin ZZ, Safian MF, Senafi S, Wahab RMA. Penyaringan antikanser ekstrak etanol daripada famili piperaceae terpilih dan penentuannya melalui pewarnaan tripan biru. Sains Malaysiana. 2010;39(6):941-949.
- Hussain K, Ismail Z, Sadikun A, Ibrahim P. Cytotoxicity evaluation and characterization of chloroform extract of leaf of Piper sarmentosum possessing antiangiogenic activity. Pharmacologyonline. 2009;2:379-391.
- Teh BP, Hamzah NF, Norazila Z, Norliyana MY, Wan Abdul Hakim WL. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2015. Report no.: HMRC 11-045/01/PS/L/A.