Lada Hitam Fruit
Piper Nigrum L
Piperaceae



Figure 1 : Piper nigrum (a) Tree; (b) leaves and fruits; (c) green fresh fruits, dried white peppercorns, dried black peppercorns (from left to right).
DEFINITION
Lada hitam fruit consists of dried fruit powder of Piper nigrum L. (Piperaceae) [ 1 ]
SYNONYM
None
VERNACULAR NAMES
Black pepper (English); lada hitam (Malay); hei hu jiao (Chinese); milagoo, milaagu, yavanappiriyam (Tamil) [ 1 , 2 ].
CHARACTER
IDENTIFICATION
Plant Morphology
P. nigrum is a woody shrub and a climber, may reach 5 m long. Stem bark dark green with roots arising from the nodes, the stem branches by dichotomy and nodes are slightly swollen. Leaves opposite, ovate to lanceolate, cordate, upper surface of leaf dark green while underneath lighter green, 8-20 cm long, 5-15 cm wide. Inflorescence spike, 25 cm long,opposite leaf, the flower stalk 1-3.5 cm long, with a lanceolate sheath, 4-5 x 0.1 cm. Fruits green, unripe fruit is 3.5-6.0 mm diameter, almost globular. Black pepper is the dried fruit, very wrinkled surface and black in colour [ 1 ].
Microscopy
Powdered material consists of abundant large cells containing brown pigment, found in small groups; mostly are oblong; others are round to oval or irregular. Brachysclerids are very few, very large, almost as large as the brown pigmented cells. The powder has thick reticulated walls and pitted lumen, rectangular to oval. The scarce epidermal layer consists of polygonal to rectangular shaped cells with thick cell walls. Rosette calcium oxalate crystals are also observed [ 1 ].












Figure 2 : Microscopic characters of P. nigrum dried fruitpowder. (a) Spiral vessel (magnification 400x); (b) annular vessel (magnification 400x); (c-d) rosette cluster calcium oxalate crystals of tetragonal system (magnification 400x); (e-f) thin-walled pentagonal parenchyma cells (magnification 400x); (g-h) lignified thick-walled parenchyma cells (magnification 400x); (i-j) brachysclereids (magnification 400x); (k-l) starches (magnification 400x). [Scale bars: a-l = 20 µm]
Colour Tests
Observed colour of solution after treatment with various reagents:
| KOH (5%) | Brown |
Thin Layer Chromatography (TLC)

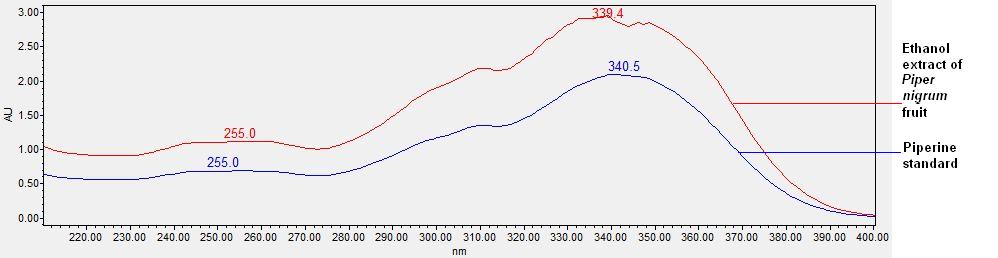
Figure 3 : TLC profiles of a piperine standard (S) and ethanol extract of P. nigrum dried fruit powder(L) observed under (a) UV 254 nm and (b) UV 366 nm.
| Test Solutions | Weigh about 1 g of P. nigrum dried fruit powder in a 250 mL round bottom flask and add 20 mL of ethanol. Sonicate the mixture for 10 min at 60°C. Filter the solution and evaporate to dryness on water bath. Reconstitute with 1 mL methanol and use as test solution. |
| Standard solution | Dissolve piperine standard [CAS no.: 94-62-2] in methanol to produce 0.5 mg/mL solution. |
| Stationary Phase | HPTLC Silica gel 60 F254, 10 x 10 cm |
| Mobile phase | Hexane : ethyl acetate : dichloromethane; 5 : 3 : 2 (v/v/v) |
| Application |
|
| Development distance | 8 cm, automatic developing chamber 2 (ADC2) |
| Drying | Air drying |
| Detection |
|
High Performance Liquid Chromatography (HPLC)
| Test solution | Weigh about 1 g of P. nigrum dried fruit powder with 20 mL ethanol in a flask. Sonicate the mixture for 10 min at 60°C. Filter the solution and evaporate to dryness on water bath. Reconstitute with 1 mL methanol. Further dilute 0.1 mL of the sample and top up with 0.9 mL methanol. Filter the mixture solution through a 0.45 µm syringe filter and inject the filtrate into the HPLC column. |
| Standard solution | Dissolve piperine standard [CAS no.: 94-62-2] in methanol to produce 0.5 mg/mL solution. |
| Chromatographic system |
Detector: UV 254 nm Column: C18 (5 µm, 4.6 mm I.D x 250 mm) Column oven temperature: 27ºC Flow rate: 1.0 mL/min Injection volume: 5 µL |
| Mobile Phase (Isocratic mode) |
Isocratic elution using the mobile phase described below:
|
| System suitability requirement |
Perform at least five replicate injections of piperine standard solutions (0.5 mg/mL). The requirements of the system suitability parameters are as follow:
|
| Acceptance criteria |
The ultraviolet (UV) spectrum of piperine in the test solution is similar to the UV spectrum of the piperine in the standard solution (optional supportive data). |
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 7% |
| Acid-insoluble ash | Not more than 1% |
| Loss on Drying |
| Not more than 17% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 14% |
| Cold method | Not less than 6% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 6% |
| Cold method | Not less than 4% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
FRUIT
Methanol extract of P. nigrum fruits (black pepper) was found to contain alkamides (e.g. retrofractamide A, pipercide, piperchabamide D, pellitorin, dehydroretrofractamide C and dehydropipernonaline) [ 3 ].
Ethanol extracts were found to contain amide alkaloids (e.g. pipericine, piperine, [(2E,4E)-octadienoyl]-N-isobutylamide, [(2E)-hexadecanoyl]pyrrolidine, [(2E,4E)-octadecadienoyl]-N-isobutylamide, (2E,4E)-eicosadienoyl-N-isobutylamide, [(2E,4E)-octadecadienoyl]piperidine, pipercallosine, tricholein, trichostachine, 1-piperettylpyrrolidine, ∆α,β-dihydrowisanidine, retrofractamide A, retrofractamide D, piperettine, pipwaqarine, piperamide-C5:1(2E), piperamide-C7:1(6E), piperamide-C7:2(2E,6E), piperamide-C9(8E), piperamide-C9:2(2E,8E), piperamide-C9:3(2E,4E,8E), 1-[(2E,4E)-2,4-decadienoyl]pyrrolidine, 1-[(2E,4E)-2,4-dodecadienoyl]pyrrolidine, piperylin, pipercide, guineensine and piperolein B and others (e.g. 30,40-methylenedioxycinnamaldehyde, β-caryophyllene, β-elemene and octadecanoic acid) [ 4 , 5 , 6 ].
Acetone extract was found to contain amides (e.g. (E,E)-N-(2-methylpropyl)- 2,4-decadienamide, (E,E,E)-13(1,3-benzodioxol-5-yl)-N(2-methylpropyl)-2,4,12-tridecatrienamide and (E,E,E)-11-(1,3-benzodioxol-5-yl)-N-(2-methylpropyl)-2,4,10-undecatrienamide) [ 7 ].
Chloroform extracts were found to contain alkaloids (e.g. pipercyclobutanamides A and B) and sesquiterpene (e.g. β-caryophyllene) [ 8 , 9 ].
Petroleum ether (60-80°) extract was found to contain alkamide (e.g. isopiperolein B) [ 10 ].
Petroleum ether extracts were found to contain amides(e.g.2E,4E,8Z–N-isobutyleicosatrienamide, pellitorine, trachyone, pergumidiene, isopiperoleine B, pipnoohine, pipyahyine, [(2E,4E)-octadienoyl]-N-isobutylamide, sarmentine, kalecide, [(2E,4E)-dodecadienoyl]pyrrolidine, pellitorine, hexadecanoylpyrrolidine, [(2E)-octadecanoyl]pyrrolidine, 1-[(2E,4E,12Z)-octadecatrienoyl]-N-isobutylamide, piptaline and 1-[7-(3,4-methylenedioxyphenyl)-(2E,4E)-heptadienoyl]-N-isobutylamide) and others (e.g. stigmastanol, β-sitosterol, stigmasterol, stigmastanol 3-O–β–D-glucopyranoside, β-sitosterol 3-O–β–D-glucopyranoside, hexadecanoic ethyl ester, octadecanoic acid and 1-(3,4-methylenedioxyphenyl)-(1E)-tetradecene)
[ 11 , 12 ].
Hexane extract was found to containamides (e.g. piperine, piperolein B, pediculicide and (2E,4E)-N-isobutyl-2,4-decadienamide) [ 13 ].
Essential oil of P. nigrum fruits (black pepper) has been found to contain monoterpenoids (e.g. α-pinene, camphene, β-pinene, myrcene, 3-carene, δ-carene, β-carene, α-phellandrene, β-phellandrene, α-pinene oxide, limonene oxide, safrole, limonen-6-ol, limonene, γ-terpinene, ρ-cymene, terpinolene, α-thujene, citronellol, α-terpinene, δ-limonene, ρ-phellandrene, ocimene, 3-methylbutanal and methylpropanal, 1,8-cineol, linalool, α-terpineol, 1-terpinen-4-ol, 1-terpinen-5-ol, ρ-cymene-8-ol, ρ-cymen-8-ol-methyl ether, trans-carveol, cis-carveol, trans-pinocarveol, dihydrocarveol, borneol, dihyrocarvone, nerol, carvacrol, (E)-β-ocimene, camphor, sabinene, cis– and trans-sabinene hydrate, m-mentha-3(8),6-diene (isosylveterpinolene) and carvone), sesquiterpenoids (e.g. β-farnesene, trans-β-farnesene, (E)(E)-β-farnesene, α-humulene, nerolidol, (E)-nerolidol, δ-gurjunene, α-gurjunene, β-gurjunene, α-cububene, calarene, thujopsene, γ-muurolene, α-muurolol, β-cubebene, γ-cadinene, δ-cadinene, cuparene, α-copaene, copaene, δ-guaiol, calamenene, cadinol, elemol, β-eudesmol and farnesol (Z,E)), bisabolane-type sesquiterpenoids (e.g. β-bisabolene, curcumene, ar-curcumene and α-curcumene), guaiane-type sesquiterpenoids (e.g. δ-guaiene and α-guaiene), elemane-type sesquiterpenoids (e.g. β-elemene, γ-elemene and δ-elemene), germacrane type (e.g. germacrene D, germacrene B and bicyclogermacrene) santalene-type sesquiterpenoid (e.g. α-santalene), bergamotene-type sesquiterpenoids (e.g. α-cis-bergamotene and α-trans-bergamotene), caryophyllane-type sesquiterpenoids (e.g. β-caryophyllene alcohol, isocaryophyllene, caryophyllene oxide, β-caryophyllene, epoxydihydrocaryophyllene, and trans-caryophyllene), sesquisabinane-type sesquiterpenoid (e.g. sesquisarbene), selinene-type sesquiterpenoids (e.g. α-selinene and β-selinene), cedrane-type sesquitepenoid (e.g. cedrene) and others (e.g. cadina-1,4-diene, cis-piperitol, cyclosativene, tricyclene, calamine, phenylacetic acid, cis-ρ.menthen-1-ol, cis-ρ.-2,8-methandien-1-ol, 2-undecanone, myrtenal, carvetonacetone, myrtenol, 1(7),2-p-methadien-6-ol, 4,10,10-trimethyl-7-methylene bicycle [6.2.0]-decane-4-carboxaldehyde, caryophylla-3(12),7(15)-dien-4β-ol, caryophylla-2,7(15)-dien-4β-ol, caryophylla-2,7(15)-dien-4α-ol, cis-ρ-2- methen-1-ol, cis-ρ-2,8-menthadien-1-ol, 2-isopropyl-3-methoxypyrazine, 2,3-diethyl-5-methylpyrazine, 1-formylpiperidine, 1-acetylpiperidine, 2,3,5,6-tetramethylpyrazine, pyrazine, pyridine, cyclohexane, 1-methyl-4-(1-methylethylidene), 3-cyclohexen-1-ol, 4-methyl-1-(1-methylethyl), pivalate, (E)-3(10)-caren-4-ol, bicyclo[3.1.0]hexane, 4-methylene-1-(1-methylethyl), bufa-20,22-dienolide,14-hydroxy-3-oxo-(5á), 2-cyclohexen-1-ol, 1-methyl-4-(1-methylethyl)-, eudesma-4(14),11-diene, trans-9-octadecenoic acid, trimethylsilyl ester, 1,2-dihydropyridine, 1-(1-oxobutyl),1-chloroeicosane, β-pinone, 1,1,4-trimethylcyclohepta-2,4-dien-6-one, 3,8(9)-ρ-menthadien-1-ol, N-formyl piperidine, 1(7),2-p-methadien-4-ol, 5,10(15)-cadiene-4-ol, cryptone, methyl eugenol and myristicin) [ 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 ].
SEEDS
Aqueous extract of P. nigrum seeds (black pepper) was found to contain choline and acetylcholine [ 34 ].
Ethanol extract was found to contain alkamides (e.g. guineensine, retrofracamide C, (2E,4Z,8E)-N-[9-(3,4-methylenedioxyphenyl)-2,4,8-nonatrienoyl]piperidine, pipernonaline, piperrolein B, piperchabamide D, pellitorin and pipkirine), piperine, piperonal and pipercide) and others (e.g. stigmastanol, stigmasterol, stigmastanol glucoside, stigmasterol glucoside, cinnamylideneacetone, methylenedioxyphenylpropiophenone and 2-hydroxy-4,5-methylenedioxypropiophenone) [ 35 , 36 ].
Acetone extract was found to contain amide alkaloids (e.g. piperylin, piperine, N–trans-feruloyltyramine, retrofractamide A, piperamide, piperolein, piperettine and guineensine), lignin (e.g. hinokinin) and sesquiterpenes (e.g. torreyol, α-copaene, β-caryophyllene, β-bisabolene, δ-cadinene, spathulenol and caryophyllene oxide) [ 14 ].
Petroleum ether extract was found to contain amides (e.g. piptigrine, piperine, wisanine, pipsaeedine, pipbinine, (E,E)-N-(2-methylpropyl)dodeca-2,4-dienamide, (E,E)-N-(2-methylpropyl)hexadeca-2,4-dienamide, piptaline, piperanine, ∆,α,β-dihydrowisanidine, ∆,α,β-dihydrowisanine, (E,E,E)-1-{[9-[3,4-(methylenedioxy)phenyl]nona-2,4,8-trienoyl}pyrrolidine, (E,E,E)-1-{11-[3,4-(methylenedioxy) phenyl]undeca-2,4,10-trienoyl}piperidine, 1-piperettylpyrrolidine and piperettine) and fatty acids (e.g. capric, lauric, myristic, palmitic, stearic, oleic, linoleic, malvalic, sterculic and vernolic) [ 37 , 38 , 39 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
P. nigrum has been used in India for centuries as traditional remedies in Ayurvedic, Unani and Siddha medicines. It has been used for illnesses such as constipation, diarrhoea, earache, gangrene, heart disease, hernia, indigestion, insect bites, insomnia, joint pain, lung diseases, liver problems, tooth decay, toothache and treatment of eye problems by applying pepper salves or poultices directly onto the eyes [ 2 ].
P. nigrum has also been used as one of the ingredients of jamu tonic, indigestion mixture with ginger, electuary or paste mixed with honey and preparation for after childbirth. It has been used externally as a counter-irritant, as poultices for colic, rheumatism and headache, and by smearing on the body for after childbirth. P. nigrum oil could be used for cholera. High dose of P. nigrum with wild bamboo shoots may be used for abortion. Malay women have used P. nigrum pills with honey and ginger as an abortifacient [ 40 ].
In China, P. nigrum has also been used to dispel cold from stomach and to eliminate phlegm [ 41 ].
Biological and pharmacological activities supported by experimental data
Antibacterial activity
Amide alkaloids isolated from the petroleum ether extract of P. nigrum fruits exhibited antibacterial activity. 2E, 4E, 8Z–N-isobutyleicosatrienamide, trachyone and pergumidiene inhibited the Gram positive bacteria, i.e. Bacillus sphaericus (17, 30 and 29 μM, respectively), Bacillus subtilis (34, 30 and 58 μM, respectively) and Staphylococcus (34, 30 and 29 μM, respectively), as well as Gram negative bacteria, i.e. Klebsiella aerogenes and Chromobacterium violaceum, with MIC values of 70, 60, 58 μM with respect to the three compounds [ 45 ].
Antifungal activity
Acetone extract and essential oil from P. nigrum fruits (black pepper) were tested for their antifungal activity against various pathogenic fungi by inverted petriplate and food-poisoning techniques. In the food-poisoning technique, the essential oil (6 µL dose) possessed more than 80% activity in controlling the mycelial growth of Fusarium graminearum, Penicillium viridicatum and Curvularia lunata, and the extract (6 µL dose) inhibited 100% mycelial growth of Penicillium viridicatum and Aspergillus ochraceus. In inverted petriplate technique, only the essential oil (6 µL dose) was found to be 100% effective in controlling the mycelial growth of Fusarium graminearum and more than 70% effective against Aspergillus niger, Aspergillus oryzae and Penicillium madriti [ 46 ].
Antioxidant activity
Water and ethanol extracts of P. nigrum (75 mg/mL) significantly (p < 0.01) decreased DPPH free radical scavenging capacity at 55% and 48%, respectively, in comparison with positive controls; butylated hydroxyl anisole (79%), butylated hydroxytoluene (76%) and α-tocopherol (78%) [ 42 ].
Ethanol extract of P. nigrum fruit (250 μg/mL) exhibited a 74.61 ± 0.02% DPPH free radical scavenging capacity with (inhibitory concentration at 50% (IC50))value of 14.51 μg/mg
[ 43 ].
Two fractions (R2 and R3) of petroleum ether extract of P. nigrum fruits showed significant (p < 0.05) antioxidant activities via different mechanisms as compared to the respective positive controls including inhibition of lipid peroxidation in a linoleic acid emulsion (500 μg/mL; R2 = 58.79%, R3 = 60.48%, α-tocopherol = 76.47%), DPPH free radicals (250 μg/mL; R2 = 61.24%, R3 = 61.11%, butylated hydroxyl anisole = 82.59%), nitric oxide free radical generation (100 μg/mL; R2 = 40.23%, R3 = 55.68%, curcumin = 84.27%), superoxide anion radicals (100 μg/mL; R2 = 62.23%, R3 = 70.22%, butylated hydroxyl toluene = 81.54%) and hydroxyl radicals (1000 μg/mL; R2 = 61.04%, R3 = 63.56%, catechin = 70.95%) [ 44 ].
Anti-inflammatory
Piperine isolated from P. nigrum (5-40 mg/kg) administered orally to male albino Hindustan Antibiotics strain rats (100-125 g) showed a significant anti-inflammatory activity on carageenan-induced paw edema (45-240 min), except for the dose of 5 mg/kg BW at 45 min, with values in the range of 12 to 99.2% compared to the normal control (38.3-121.2%) and positive control, oxyphenyl butazone (OPB, 50 mg/kg: 17.2-48.7%) groups. In histamine and formaline-induced paw edema experiments, piperine gave 28.3% and 32.8% anti-inflammatory activity, respectively, compared to OPB group (32.8 and 38.1%, respectively). Piperine also exhibited anti-inflammatory activity in rats induced with cotton pellet granuloma with cotton pellets weight of 20.4 mg compared to normal control (36.21 mg) and OPB (22.60 mg) groups. The weight of granuloma of croton oil-induced inflammatory assay of rated treated with P. nigrum was 0.339 mg compared to normal control (0.846 mg) and OPB (0.321 mg) groups [ 47 ].
Intercellular cell adhesion molecules binding inhibitory activity
(2E,4Z,8E)-N-[9-(3,4-methylenedioxyphenyl)-2,4,8-nonatrienoyl]piperidine, pipernonaline, piperrolein B, pellitorin and dehydropipernonaline isolated from P. nigrum fruits inhibited direct binding between sICAM-1 (intercellular cell adhesion molecules) and LFA-1 (lymphocyte function associated antigen-1) of THP-1 cells (human monocytic leukaemia cell line) with IC50 values of 10.7, 8.8, 13.4, 13.5 and 6.0 μg/mL, respectively. Inhibitors of LFA-1/ICAM-1 mediated cell adhesion are potentially useful for the treatment of inflammatory diseases [ 48 ].
Immunomodultary activity
Polysaccharides, PN-Ib and PN-IIa, isolated from P. nigrum fruits showed significant (p < 0.05) anti-complementary activity with 50% inhibition of total complement hemolysis (ITCH50) values of 96.5 ± 2.2% and 98.7 ± 1.9%, respectively, compared to the positive control, polysaccharide-K (60.2 ± 1.5%) [ 49 ].
Aqueous extract of P. nigrum fruits significantly enhance proliferation of T helper (Th)1 but suppress (Th)2 cytokine release by splenocytes dose-dependently[50 ].
Anti-cancer activity
Piperine isolated from P. nigrum was investigated for inhibition or reduction of the oxidative changes induced by chemical carcinogens in rat intestinal model. A segment of intestinal luminal of Charles foster male rats (150-200 g) was ligated and carcinogenesis-induced with 7,12-dimethyl benzanthracene (DMBA, 50 μg/mL), dimethyl amino-methyl azobenzene (DMAMAB, 20 mg/mL) and 3-methyl cholenthrene (3-MC, 3 mg/mL). Aqueous suspension of piperine in 1% carboxy methylcellulose equivalent to optimum concentration of 3.5 mM was exposed to the ligated segment. Piperine treatment with carcinogens significantly inhibited thiobarbituric reactive substances (TBARS) compared to DMBA-treated (p < 0.05), DMAMAB-treated (p < 0.01) and 3-MC-treated (p < 0.01) rat intestines. The carcinogens decreased levels of non-protein thiols (N-SH) in intestinal mucosa but exposure of piperine significantly (p < 0.01) decreased the levels. Piperine was also found to significantly reduce elevation of enzymes activities of γ-glutamyl transpeptidase (γ-GT) caused by DMAMAB and 3-MC (p < 0.001), as well as Na+-K+-ATPase induced by DMAMAB (p < 0.001), DMBA (p < 0.01) and 3-MC (p < 0.001). The study suggested a protective role of piperine against chemical carcinogen-induced oxidative stress [ 51 ].
Dried P. nigrum fruit (black pepper) powder (0.5% w/w mixed with the diet and 20% peanut oil) was administered orally daily for 30 weeks to male Wistar rats (100-120 g) with 1,2-dimethylhydrazine (DMH)-induced colon cancer. DMH-induced group treated with P. nigrum had smaller tumour size (0.5 cm) compared to DMH-induced control (2 cm). Presence of vascular granulation in the former animal group might indicate protective mechanism towards tumour development to deeper layers. Excretion of fecal bile acids and neutral sterols in 24-hour fecal samples was significantly (p < 0.05) increased in DMH-induced group treated with P. nigrum. P. nigrum given to DMH-induced group had lower (p < 0.05) cholesterol/phospholipid ratio (0.500-0.919) and increased 3-hydroxy-3-methylglutaryl (HMG-CoA) reductase activity (1.08-2.14) in the colon, intestine and liver compared to the DMH-induced control (0.818-1.567 and 0.85-1.68, respectively). The results showed that supplementation of P. nigrum prevented accumulation of lipids in tissues and optimized the excretion of fecal sterols and bile acids, suggesting the risk reduction of colon cancer in the presence of the procarcinogen DMH [ 52 ].
Antimutagenic activity
Aqueous extract of P. nigrum significantly (p < 0.05) reduced mutational events induced by promutagen agent ethyl carbamate using the wing somatic mutation and recombination test (SMART) in Drosophila melanogaster. P. nigrum reduced 38% (1% w/v) and 46% (2% w/v) of total number of spots [ 53 ].
Melanocyte proliferative activity
Aqueous extract of P. nigrum fruits (black pepper) (0.1 mg/mL) stimulated almost 300% growth of mouse melanocyte cell line, melan-a, in vitro in 8 days of experiment (p < 0.01) compared to piperine [ 54 ].
Chloroform extract of dried P. nigrum seeds (black pepper) (containing equivalent concentration of 1 μM piperine) stimulated proliferation of copiously pigmented non-tumorigenic mouse melanocyte cells (melan-a) in vitro greater than the pure piperine (1 µM piperine), suggesting that other phytochemical compounds were also responsible for the activity. Thus, piperine (p < 0.01), guineensine (p < 0.05), pipericide (p < 0.05), piperettine (p < 0.01) and piperlonguminine (p < 0.01) isolated from the chloroform extract, also stimulated proliferation of melanocytes at a concentration range of 0.1-10 µM, compared with the positive control 12-O-tetradecanoylphorbol-13-acetate (20 and 200 nM). The results suggested the potential use in vitiligo [ 55 ].
Gastrointestinal activity
Methanol (80%) extract of P. nigrum (1-10 mg/mL) possessed a spasmodic effect in guinea pig ileum at a concentration dependent manner of acetylcholine (Ach, 0.3 µM)-induced contractions with IC50 value of 0.7 mg/mL, compared to positive control loperamide and nifedipine with IC50 values of 5.9 µM and 0.3 µM, respectively. When tested against potassium (K+, 80 mM)-induced contractions, P. nigrum inhibited contraction with IC50 value of 0.6 mg/mL, compared to loperamide and nifedipine with respective IC50 values of 0.9 µM and 0.04 µM. P. nigrum extract (1000 mg/kg) produced 12.4% wet feces, compared to positive control, carbachol (47.1%). P. nigrum (500 and 1000 mg/kg) reduced castor oil-induced fluid accumulation with respective (Pi/Pm)x1,000 values of 143 and 90, compared to loperamide (78). P. nigrum extract (1000 mg/kg) exhibited 80% protective activity of castor oil-induced wet feces, compared to loperamide (10 mg/kg) as a positive control (p < 0.01) [ 56 ].
Utero-spasmolytic activity
Effect of aqueous extract of P. nigrum fruits on utero-spasmolytic activity was studied. Adult female Wistar rats (240-320 g) were injected subcutaneously with estradiol valerate (0.5 mg/kg) 24 hours prior to experiment. The rats were sacrificed and a piece (1-1.5 cm) of cervical portion of the uterus was dissected and mounted in an organ bath. The extract (0.125-2 mg/mL) dose-dependently (p < 0.01-0.001) reduced uterus contractions of KCl-induced (60 nM) greater than that of oxytocin-induced (10 mU/mL). The spasmolytic effect of extract on the KCl-induced contractions was not reduced by L-NAME (nitric oxide synthase inhibitor, 100 μM), phentolamine (α-adrenoceptor antagonist, 1 μM) and naloxone (opoid receptor antagonist, 1 μM), however the extract spasmolytic effect was reduced in the presence of propranolol (β-adrenoceptor antagonist, 1 μM) (p < 0.01-0.0001). The extract (0.0312-0.25 mg/mL) also reduced calcium chloride-induced uterus contraction dose-dependently. The study indicated that utero-spasmolytic effect of aqueous extract of P. nigrum was mediated via voltage dependent calcium channels and by inhibition of β-adrenoceptors [ 57 ].
Antidiabetic activity
Aqueous extract (0.5 mL/day) of P. nigrum seeds was administered orally to alloxan-induced diabetic on male albino Wistar rats once a day. After 4 weeks of experiment, blood glucose levels by treatment with the extract-treated group was 129 mg/100 mL compared to the insulin-treated (120 mg/100 mL), diabetic control (270 mg/100 mL) and normal control (102 mg/100 mL) groups. Total cholesterol (TC), low-density lipoprotein (LDL-C), high-density lipoprotein (HDL-C) and triglycerides (TG) values of the extract-treated group were 172.0, 90.0, 58.7 and 130 mg/100 mL, respectively, comparable to the insulin-treated group (165.5, 95.2, 48.2 and 138.6 mg/100 mL, respectively). Similarly, the activity of liver anti-oxidative stress enzymes, such as catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx) of the extract-treated group were 0.145 U/mg protein x 103, 15.68 U/mg protein and 0.131 U/mg protein, respectively, comparable to the insulin-treated group (0.138 U/mg protein x 103, 15.10 U/mg protein and 0.131 U/mg protein). The study suggested potential hypoglycemic effect of aqueous extract of P. nigrum seeds via antioxidant property [ 58 ].
Four alkamides, (2E,4Z,8E)-N-[9-(3,4-methylenedioxyphenyl)2,4,8-nonatrienoyl]piperidine, pipernonaline, piperrolein B and dehydropipernonaline, isolated from chloroform extract of P. nigrum fruits inhibited acyl CoA:diacylglycerol acyltransferase (DGAT) in vitro with IC50 values of 29.8, 37.2, 20.1 and 21.2 µM, respectively. Inhibition of DGAT is associated with improved insulin sensitivity in vivo [ 59 ].
Hepatoprotective activity
P. nigrum powder (0.5, 1 and 2% w/w mixed with diet) was fed to random-bred Swiss albino mice of either sex (aged eight weeks old) for 10 and 20 days each. Hepatic glutathione S-transferase (GST) was significantly and dose-dependently increased in both sex groups (1 and 2% w/w for 10 days: 1.22-1.38 µM 1-chloro-2-4-dinitrobenzene (CDNB)-GSH conjugate formed/min/mg protein (p < 0.05) vs. normal control, 1.12-1.13 µM CDNB-GSH conjugate formed/min/mg protein; 0.5-2% w/w for 20 days: 1.35-1.74 µM CDNB-GSH conjugate formed/min/mg protein (p < 0.05) vs. normal control, 1.26-1.31 µM CDNB-GSH conjugate formed/min/mg protein); similarly with the estimated content of acid-soluble sulfhydryl (–SH) (1 and 2% w/w for 10 days: 5.05-5.57 µM/g tissue (p < 0.05) vs. normal control, 5.03-5.24 µM/g tissue; 1 and 2% w/w for 20 days: 6.79-7.20 µM/g tissue (p < 0.05) vs. normal control, 6.48-6.49 µM/g tissue). Cytochromes b5 and P-450 were also significantly (p < 0.05) and dose-dependently elevated for all doses and both time periods and sex groups with values in the range of 0.40-0.70 (cyt. b5) and 0.65-0.96 (cyt. P-450) nM/mg protein compared to the normal control (0.35-0.36 and 0.50-0.56 nM/mg protein for the respective cytochromes). Significant reduction (p < 0.05) of lipid peroxidation (expressed in terms of malondialdehyde) was only observed for 2% w/w P. nigrum treated groups for 20 days. The study suggested that P. nigrum could be a potential inducer of hepatic detoxication system [ 60 ].
Hydrocholagoguic activity
Suspension containing P. nigrum (black pepper) powder (250 and 500 mg/kg BW) in 1 mL aqueous dispersion of soluble starch was administered by force-fed gavage to male Wistar albino rats (330-390 g) for 4 weeks. Intragastric administration of P. nigrum (250 mg/kg) significantly (p < 0.05) increased bile solids content of 2.99% compared to normal control rats (2.77%). Feeding of P. nigrum for 4 weeks significantly increased bile flow (250 mg/kg: 0.87 mL/h, p < 0.001; 500 mg/kg: 0.74 mL/h, p < 0.05 compared to normal control: 0.54 mL/h), phospholipid (250 mg/kg: 1.78 μmol/h, p < 0.02 compared to normal control: 1.12 μmol/h) and total uronic acid (250 mg/kg: 2.80 μmol/h, p < 0.001; 500 mg/kg: 2.27 μmol/h, p < 0.05 compared to normal control: 1.68 μmol/h), but reduced bile solid content (250 mg/kg: 2.21%, p < 0.001; 500 mg/kg: 2.38%, p < 0.05 compared to normal control: 2.77%). Hydrocholagoguic effect was obtained due to increased bile flow with concomitant decrease in bile solids [ 61 ].
Antianderogenic activity
Ethanol (50%) extract of P. nigrum fruits (2 mg/mL) inhibited testosterone 5-alpha-reductase in vitro with a percentage inhibition of 63.0% compared to ethinylestradiol (1 mM, 63.3% inhibition), whereas piperine isolated from the fruits had IC50 value of 0.48 mM compared to ethinylestradiol (IC50 = 0.81 mM)[62].
Insecticidal activity
A study of insecticidal properties of P. nigrum fruit extracts and essential oils against tobacco army worm, Spodoptera litura has been done using topical application bioassay on uniform weighted second instar larvae in the laboratory. The result showed that, the hexane extract was the highest toxicity at 48 h after treatment and most effective in killing the larvae. Toxicity of extracts decreased in the order of hexane (LD50 1.8 mg/g) > acetone (LD50 18.8 mg/g) > chloroform (LD50 NA, the toxicity was very low) > essential oil (no mortality). Insect development and growth index observations showed that the hexane extract had antifeedant properties resulting in severe growth inhibition of Spodoptera litura
[ 63 ].
Petroleum ether extract of P. nigrum fruits (black pepper) was active against rice moth, Corcyra cephalonica (Stainton) 3rd instar larvae with median lethal concentration (LC50) values of 12.52 μL/mL. The result also showed that the extract had strong inhibition on egg hatchabilities and adult emergence of C. cephalonica at the lowest concentration [ 64 ].
Ethanol extract of P. nigrum (black pepper) (0.3 g) resulted in 100% mortality of millipede Orthoporus fuscipes after 4 days of exposure. However, only 70% mortality was achieved after 4 days of exposure to piperine (0.3 g) [ 65 ].
Ethanol extract of P. nigrum fruits (black pepper) had LC50 values of 0.405 ppm and 0.016 ppm against fourth and third instar larvae of A. aegypti, respectively. Hexane extract of black pepper was found to be more toxic against fourth larvae of A. aegypti with LC50 0.007 ppm [ 66 , 67 ].
Isobutylmide alkaloids isolated from P. nigrum fruits were found to have insecticidal activity against third instar larvae of Culex pipiens pallens (i.e. pipercide (LC50 0.004 ppm), retrofractamide A (0.028 ppm), guineensine (0.17 ppm) and pellitorine (0.86 ppm)), Aedes aegypti (i.e. retrofractamide A (0.039 ppm), pipercide (0.1 ppm), guineensine (0.89 ppm) and pellitorine (0.92 ppm)) and Aedes togoi (i.e. retrofractamide A (0.01 ppm), pipercide (0.26 ppm), pellitorine (0.71 ppm), and guineensine (0.75 ppm)). Piperine was the least toxic with LC50 values of 3.21, 5.1 and 4.6 ppm, respectively [ 68 ].
Piptigrine, pipsaeedine, pipbinine, pipnoohine, pipyahyine and pipwaqarine isolated from P. nigrum fruits exhibited toxicity against fourth instar larvae of A. aegypti with LC50 values of 15.0, 45.0, 40.0, 35.0, 30.0 and 30.0 ppm, respectively [ 5 , 69 , 70 , 71 ].
Insecticidal activity against female C. pipiens pallens was in the following order: pellitorine (LD50 values of 0.4 μg/female), guineensine (1.9), retrofractamide A (2.4) and pipercide (3.2) compared to chlorpyrifos (0.03 μg/female); whereas activity against A. aegypti was in the order of pellitorine (0.17), retrofractamide A (1.5), guineensine (1.7) and pipercide (2.0) compared to chlorpyrifos (0.0014 μg/female) [ 72 ].
Ethanol extract, piperolein-A and piperine isolated from P. nigrum fruits were found to be toxic against pyrethroid-resistant A. aegypti larvae with LC50 values of 0.98, 1.46 and 1.53 ppm, respectively [ 73 ].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Antispermatogenic and antifertility effects
P. nigrum fruit powder (25 and 100 mg/kg BW) was administered orally to male Parkes strain mice daily for 20 and 90 days. Administration of both doses of P. nigrum for 90 days significantly (p < 0.05) reduced weight of sex organs, such as testis (25 mg/kg: 213.04 mg/100 g BW; 100 mg/kg: 183.01 mg/100 g BW compared to vehicle control: 314.49 mg/100 g BW), epididymis (25 mg/kg: 82.97 mg/100 g BW; 100 mg/kg: 77.21 mg/100 g BW compared to vehicle control: 105.22 mg/100 g BW) and seminal vesicle (25 mg/kg: 179.05 mg/100 g BW; 100 mg/kg: 171.76 mg/100 g BW compared to vehicle control: 323.69 mg/100 g BW). Mice treated with 100 mg/kg BW dose for 90 days showed degenerative changes in all seminiferous tubules, including intraepithelial vacuolation, loosening of germinal epithelium, occurrence of giant cells, and mixing of spermatids of different stages of spermatogenesis; in severe cases, the tubules were lined by mainly a layer of Sertoli cells. Treatment for 90 days also caused detectable alteration in the duct, as well as adverse effects on sperm parameters, levels of sialic acid in epididymis (54.87 μmole/100 g tissue vs. control = 128.69 μmole/100 g tissue, p < 0.05) and fructose in seminal vesicle (55.07 μmole/100 g tissue vs. control = 222.21 μmole/100 g tissue, p < 0.05) [ 74 ].
Acute toxicity
Oral single dose acute toxicity study on female Sprague Dawley rats ( aged between 8 and 12 weeks old) using aqueous extract of P. nigrum fruits showed no toxic effect on the parameters observed, including behaviours, body weight, food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. Approximate lethal dose (LD50) is more than 2,000 mg/kg body weight [ 75 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
DOSAGE
In Ayurvedic practice, the recommended dosage is 250 mg-1g of P. nigrum powder [ 76 ].
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- Malaysian Herbal Monograph Committee. Malaysian Herbal Monograph Volume 1. Kuala Lumpur: Forest Research Institute Malaysia. 1999
- Lim TK. Edible Medicinal and Non Medicinal Plants. Volume 4. Springer Dordrecht Heidelberg London New York. 2012.
- Rho M-C, Lee SW, Park, HR, Choi, J-H, Kang JY, Kim K, Lee HS, Kim YK. ACAT inhibition of alkamides identified in the fruits of Piper nigrum. Phytochemistry 2007;68:899-903.
- Siddiqui BS, Begum S, Gulvar T, Farhat, Noor F. An amide from fruits of Piper nigrum. Phytochemistry. 1997;45(8):1617-1619.
- Siddiqui BS, Gulzar T, Begum S, Afshan F, Sattar FA. Insecticidal amides from fruits of Piper nigrum Linn. Natural Product Research. 2005;19(2):143-150.
- Kiuchi F, Nakamura N, Tsuda Y, Kondo K, Yoshimura H. Studies on crude drugs effective on visceral larva migrans. IV. Isolation and identification of larvicidal principles in pepper. Chemistry and Pharmaceutical Bulletin 1998;36(7): 2452-2465.
- Su HCF, Horvat R. Isolation, identification and insecticidal of Piper nigrum amides. Journal of Agricultural Food Chemistry. 1981;29(1):115-117.
- Fijiwara Y, Naithaou K, Miyazaki T, Hashimoto K, Mori K, Yamamoto Y. 2001. Two new alkaloids, pipercyclobutanamides A and B from Piper nigrum. Tetrahedron Letters 42: 2497-2499.
- Yamaguchi I, Ozeki S. β-Caryophyllene from black pepper. Bulletin Tokyo Kasei Daigaku 1986;26:95-98.
- Srinivas PV, Rao JM. Isopiperolein B: an alkamide from Piper nigrum. Phytochemistry. 1999;52:957-958.
- Reddy SV, Srinivas PV, Praveen B, Kishore KH, Raju BC, Murthy US, Rao JM. Antibacterial constituents from the berries of Piper nigrum. Phytomedicine. 2004;11:697-700.
- Siddiqui BS, Gulzar T, Mahmood A, Begum S, Khan B, Afshan F. New insecticidal amides from petroleum ether extract of dried Piper nigrum L. whole fruits. Chemistry of Pharmaceutical Bulletin. 2004;52(11): 1349-1352.
- Ohigashi H, Nishimuro S, Koshimizu K. Larva-development inhibitors of black pepper. Bulletin of the Institute for Chemical Research, Kyoto University. 1983;61(2):104-108.
- Singh G, Marimuthu P, Catalan C, deLampasona MP. Chemical, antioxidant and antifungal activities of volatile oil of black pepper and its acetone extract. Journal of the Science of Food and Agriculture. 2004;84:1878-1884.
- Muller CJ, Creveling RK, Jennings WG. Some minor sesquiterpene hydrocarbons of black pepper. Journal of Agricultural and Food Chemistry. 1968;16(1):113-117.
- Muller CJ, Jennings CG. Constituents of black pepper. Some sesquiterpene hydrocarbons. Journal of Agricultural and Food Chemistry. 1967;15(5):762-766.
- Richard HM, Jennings WG. Volatile composition of black pepper. Journal of Food Science. 1971;36(4):584-589.
- Russell GF, Jennings WG. Constituents of black pepper. Oxygenated compounds. Journal of Agricultural and Food Chemistry. 1969;17(5):1107-1112.
- Russell GF, Jennings WG. Occurrence of cis and trans sabinene hydrate in oil of black pepper. Journal of Agricultural and Food Chemistry. 1971;18(4):733.
- Russell GF, Murray WJ, Muller CJ, Jenning WG. α-Bergamotenes in oil of black pepper. Communications. 1968;16(6):1047-1049.
- Wrolstad RE, Jennings WG. Volatile constituents of black pepper. III. The monoterpene hydrocarbon fraction. Journal of Food Science. 1968;30:274-279.
- Debrauwere J, Verzele M. Constit uents of peppers. Part VI: The oxygenated fraction of pepper essential oil. Bulletin des Sociѐtѐs Chimiques Belges. 1975;84:167-177.
- Debrauwere J, Verzele M. Constituents of peppers. Part IV: The hydrocarbon of pepper essential oil. Journal of Chromatographic Science. 1976;14:296-298.
- Hasselstrom T. Composition of volatile oil of black pepper, Piper nigrum. Pepper Analysis. 1957;5(1):53-55.
- Kumoro AC, Hasan M, Singh H. Extraction of Sarawak black pepper essential oil using supercritical carbon dioxide. Arabian Journal for Science and Engineering. 2010;35(2B):7-16.
- Sasidharan I, Menon AN. Comparative chemical composition and antimicrobial activity of berry and leaf essential oils of Piper nigrum L. International Journal of Biological and Medical Research. 2010;1(4):215-218.
- Jagella T, Grosch W. Flavour and off-flavour compounds of black and white pepper (Piper nigrum L.). I. Evaluation of potent odorants of black pepper by dilution and concentration techniques. European Food Research and Technology. 1999;209:16-21.
- Jagella T, Grosch W. Flavour and off-flavour compounds of black and white pepper (Piper nigrum L.). III. Desirable and undesirable odorants of white pepper. European Food Research and Technology. 1999;209:27-31.
- Nussbaumer C, Cadalbert R, Kraft P. Identification of m-mentha3(8),6-diene (Isosylveterpinolene) in black pepper oil. Helvetica Chimica Acta. 1999;82(1):53-58.
- Clery RA, Hammond CJ, Wright AC. Nitrogen containing compounds in black pepper oil (Pipernigrum L.). Journal of Essential Oil Research 18(1):1-3.
- Liang R, Shi S, Ma Y. 2010. Analysis of volatile oil composition of the peppers from different production areas. Medicinal Chemistry Research. 2006;19(2):157-165.
- Pino JA, Rodriguez-Feo G, Borges P, Rosado A. Chemical and sensory properties of black pepper oil. Nahrung. 1990;34(6):555-560.
- Turhene SJ, Hogg JW, Bromstein AC, Lawrence BM. Four new sesquiterpene analogs of common monoterpenes. Canadian Journal of Chemistry. 1974;53:3285-3293.
- Haranath PSRK, Akther MH, Sharif SI. Acetylcholine and choline in common species. Phytotheraphy Research. 1987;1(2):91-92.
- Lee SW, Kim YK, Kim K, Lee HS, Choi JH, Lee WS, Jun C-D, Park JH, Lee JM, Rho M-C. Alkamides from the fruits of Piper longum and Piper nigrum displaying potent cell adhesion inhibition. Bioorganic and Medicinal Chemistry Letters 2008;18:4544-4546.
- Siddiqui BS, Gulzar T, Begum S, Afshan F, Sultana R. A new natural product and insecticidal amides from seeds of Piper nigrum Linn. Natural Product Research. 2008;22(13):1107-1111.
- Siddiqui BS, Gulzar T, Begum S, Afshan F. Piptigrine, a new insecticidal amide from Piper nigrum Linn. Natural Product Research. 2004;18(5):473-477.
- Siddiqui BS, Gulzar T, Begum S, Afshan F, Sattar FA. Two new insecticidal amide dimers from fruits of Piper nigrum Linn. Helvetica Chemica Acta 2004;87:660-666.
- Daulatabad CD, Mulla GM, Mirajkar AM. Venolic and cyclopropenoic fatty acid in Piper nigrum seed oil. European Journal of Lipid Science and Technology. 1995;97:453-454
- Burkill IH. A dictionary of the economics products of the Malay Peninsular. 2nd Ed. Kuala Lumpur: Ministry of Agriculture and Cooperative. 1966.
- Pharmacopoeia Commission of People’s Republic of China. Pharmacopoeia of The People’s Republic of China (English Edition). Guangzhou: Gaungdong Science and Technology Press. 1992.
- Gulcin I. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. International Journal of Food Sciences and Nutrition.2005;56(7):491-499.
- Nahak G, Sahu RK. Phytochemical evaluation and antioxidant activity of Piper cubeba and Piper nigrum. Journal of Applied Pharmaceutical Science 2011;1(8):153-157.
- Singh R, Singh N, Saini BS, Rao HS. In vitro antioxidant activity of pet ether extract of black pepper. Indian Journal of Pharmacology2008;40(4):147-151.
- Reddy SV, Srinivas P, Praveen B, Kishore KH, Raju CU, Murthy S & Rao JM. Antibacterial constituents from the berries of Piper nigrum. Phytomedicine. 2004;11(7-8):697-700.
- Singh G, Marimuthu P, Catalan C, deLampasona MP. Chemical, antioxidant and antifungal activities of volatile oil of black pepper and its acetone extract. Journal of the Science of Food and Agriculture 2004;84:1878-1884.
- Mujumdar AM, Dhuley JN, Deshmukh VK, Raman PH, Naik SR. Anti-inflammatory activity of piperine. Japanese Journal of Medicinal Science and Biology 1990;43:95-100.
- Lee SW, Kim YK, Kim K, Lee HS, Choi JH, Lee WS, Jun CD, Park JH, Lee JM, Rho MC. Alkamides from the fruits of Piper longum and Piper nigrum displaying potent cell adhesion inhibition. Bioorganic and Medicinal Chemistry Letters. 2008;18(16):4544-4546.
- Chun H, Shin DH, Hong BS, Cho WD, Cho HY, Yang HC. Biochemical properties of polysaccharides from black pepper. Biological and Pharmaceutical Bulletin. 2002;25(9):1203-1208.
- Majdalawieh AF, Carr RI. In vitro investigation of the potential immunomodulatory and anti-cancer activities of black pepper (Piper nigrum) and cardamom (Elettaria cardamomum). Journal of Medicinal Food 2010;13(2):371-381.
- Khajuria A, Johrn RK, Zutshi U, Bedi KL. Piperine modulation of carcinogen induced oxidative stress in intestinal mucosa. Phytomedicine. 1998;6(5):351-355.
- Nalini N, Manju V, Menon VP. Effect of spices on lipid metabolism in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Journal of Medicinal Food 2006;9(2):237-242.
- Hamss RE, Idaomar M, Alonso-Moraga A, Serrano AM. Antimutagenic properties of bell and black peppers. Food and Chemical Toxicology. 2003;41:41-47.
- Lin Z, Hoult JR, Bennett DC, Raman A. Stimulation of mouse melanocyte proliferation by Piper nigrum fruit extract and its main alkaloid, piperine. Planta Medica. 1999;65(7):600-603.
- Lin Z, Liao Y, Venkatasamy R, Hider RC, Soumyanath A. Amides from Piper nigrum L. with dissimilar effects on melanocyte proliferation in-vitro. Journal of Pharmacy and Pharmacology. 2007;59:529-536.
- Mehmood MH, Gilani H. Pharmacological basis for the medicinal use of black pepper and piperine in gastrointestinal disorders. Journal of Medicinal Food. 2010;13(5):1086-1096.
- Naseri MKG, Yahyavi H. Spasmolytic activity of Piper nigrum fruit aqueous extract on rat non-pregnant uterus. Iranian Journal of Pharmacology and Therapeutics. 2007;6(1):35-40.
- Kaleem M, Sheema SH, Bano B. Protective effects of Piper nigrum and Vinca rosea in alloxan induced diabetic rats. Indian Journal of Physiology and Pharmacology 2005;49(1):65-71.
- Lee SW, Rho MC, Park HR, Choi JH, Kang JY, Lee JW, Kim K, Lee HS, Kim YK. Inhibition of diacylglycerol acyltransferase by alkamides isolated from the fruits of Piper longum and Piper nigrum. Journal of Agriculture and Food Chemistry. 2006;54(26):9759-9763.
- Singh A, Rao AR. Evaluation of the modulatory influence of black pepper (Piper nigrum L.) on the hepatic detoxication system. Cancer Letters. 1993;72(1-2):5-9.
- Ganesh Bhat B, Chandrasekhara N. Effect of black pepper and piperine on bile secretion and composition in rats. Die Nahrung. 1987;31:913-916.
- Hirata N, Tokunaga M, Naruto S, Linuma M, Matsuda H. Testosterone 5 alpha-reductase inhibitory active constituents of Piper nigrum leaf. Biological and Pharmaceutical Bulletin. 2007;30(12):2402-2405.
- Fan LS, Muhamad R, Omar D, Rahmani M. Insecticidal properties of Piper nigrum fruit extracts and essential oils against Spodoptera litura. International Journal of Agriculture & Biology. 2011;13:517-522.
- Khani M, Awang RM, Omar D, Rahmani M. Toxicity, antifeedant, egg hatchability and adult emergence effect of Piper nigrum L. and Jatropha curcas L. extracts against rice moth, Corcyra cephalonica (Stainton). Journal of Medicinal Plants Research. 2013;7(18):1255-1262.
- Romão JA, Boccardo L, de Paula VF, Chagas RJ, Moreira BO. Toxicity of extracts from Piper nigrum, piperine and piperamides to the millipede Orthoporus fuscipes under laboratory conditions. Revista Brasileira de Toxicologia. 2008;21(1):33-38.
- Kumar S, Warikoo R, Wahab N. Larvicidal potential of ethanolic extracts of dried fruits of three species of peppercorns against different instars of an Indian strain of dengue fever mosquito, Aedes aegypti L. (Diptera: Culicidae). Parasitology Research. 2010;107(4):901-907.
- Kumar S, Warikoo R, Wahab N. Relative larvicidal efficacy of three species of peppercorns against dengue fever mosquito, Aedes aegypti L. Journal of Entomological Research Society. 2011;13(2):27-36.
- Park IK, Lee SG, Shin SC, Park JD, Ahn YJ. Larvicidal activity of isobutylamides identified in Piper nigrum fruits against three mosquito species. Journal of Agriculture and Food Chemistry. 2002;50(7):1866-1870.
- Siddiqui BS, Gulzar T, Begum S, Afshan F. Piptigrine, a new insecticidal amide from Piper nigrum Linn. Natural Product Research. 2004;18(5):473-477.
- Siddiqui BS, Gulzar T, Begum S, Afshan F, Sattar FA. Two new insecticidal amide dimers from fruits of Piper nigrum Linn. Helvetica Chimica Acta. 2004;87(3):660-666.
- Siddiqui BS, Gulzar T, Mahmood A, Begum S, Khan B, Afshan F New insecticidal amides from petroleum ether extract of dried Piper nigrum L. whole fruits. Chemical and Pharmaceutical Bulletin. 2004;52(11):1349-1352.
- Park IK. Insecticidal activity of isobutylamides derived from Piper nigrum against adult of two mosquito species Culex pipiens pallens and Aedes aegypti. Natural Product Research. 2012;26(22):2129-2131.
- Simas NK, Lima Eda C, Kuster RM, Lage CL, de Oliveira Filho AM. Potential use of Piper nigrum ethanol extract against pyrethroid-resistant Aedes aegypti larvae. Revista da Sociedade Brasileira de Medicina Tropical. 2007;40(4):405-407.
- Mishra RK, Singh S. Antispermatogenic and antifertility effects of fruits of Piper nigrum L. in mice. Indian Journal of Experimental Biology. 2009;47:706-714.
- Nor Azlina Z, Emylyn M, Siau TC, Izwah H, Wan Abdul Hakim WL, Wan Mohammad Adham Afiq WZ, Teh BP, Hussin M. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute Medical for Research, Ministry of Health; 2015. Report no.: HMRC 11-045/01/PN/F/Q.
- The Ayurvedic Pharmacopoeia of India. Marica (Fruit) – Piper nigrum Linn. New Delhi: Department of ISM & H, Ministry of Health and Family Welfare, Government of India. Part I. Vol. III. 2001;117.