Scientific Name
Eurycoma longifoliaJack
Synonyms
Eurycoma latifolia Ridl., Eurycoma longifolia var. cochinchinensis Pierre, Eurycoma merguensis Planch., Eurycoma tavoyana Wall.[Invalid]. [1]
Vernacular Name
| Malaysia | Tongkat ali, penawar pahit, bedara pahit, bedara putih, bedara merah, hempedu pahit, lempedu pahit, payung ali, tongkat baginda, muntah bumi, akar jangat semang, tongkat rasul, setunjang bumi, pasak bumi, petala bumi, pokok syurga, tongkat ali hitam, pokok jelas, jelaih. [2] |
Geographical Distributions
Eurycoma longifolia is found in the jungles of Southern Burma (Myanmar), Indo-China (Cambodia, Laos and Vietnam), Thailand, Peninsular Malaysia, Sumatra, Borneo and Philippines. [3]
Botanical Description
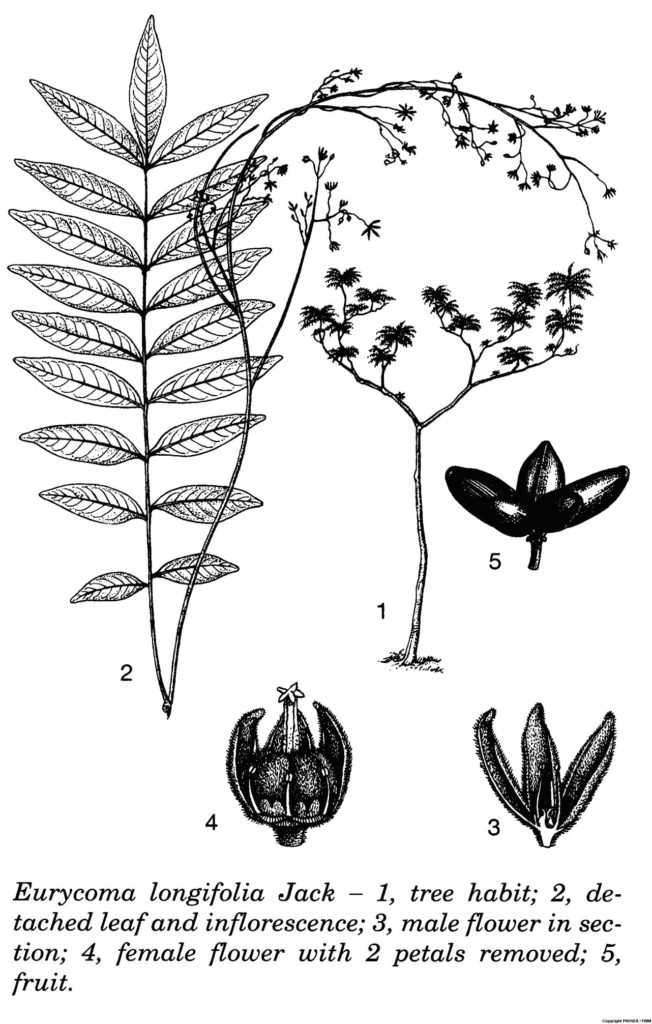
E. longifolia is a member of the Simaroubaceae family. It is a slow growing plant attaining a maximum height of 10-18 m, often spindly unbranched crowned by an umbrella-like rosette of leaves with reddish brown petioles. [3]
The leaves are compound, evenly pinnate, spirally arranged reaching 1 m in length; each compound leaf consists of 10-40 leaflets, lanceolate to obovate-lanceolate; each leaflet is about 5-20 cm long, 1-6 cm wide, much paler on the ventral side. [3]
The inflorescence is axillary, in large brownish red panicle, very pubescent with very fine, soft, glandular trichomes. Theflowers are dioecious with small, lanceolate to ovate obovate-oblong (4.5-5.5 mm long and 2-3 mm wide), and very fine pubescent petals while the styles rather long with peltate 5 (-6)-lobed stigma and elevated about 1 mm above the carpels. [3]
The drupe is about 10-20 mm long, 5-12 mm wide, hard, ovoid, yellowish brown when young and brownish red when ripe. [3]
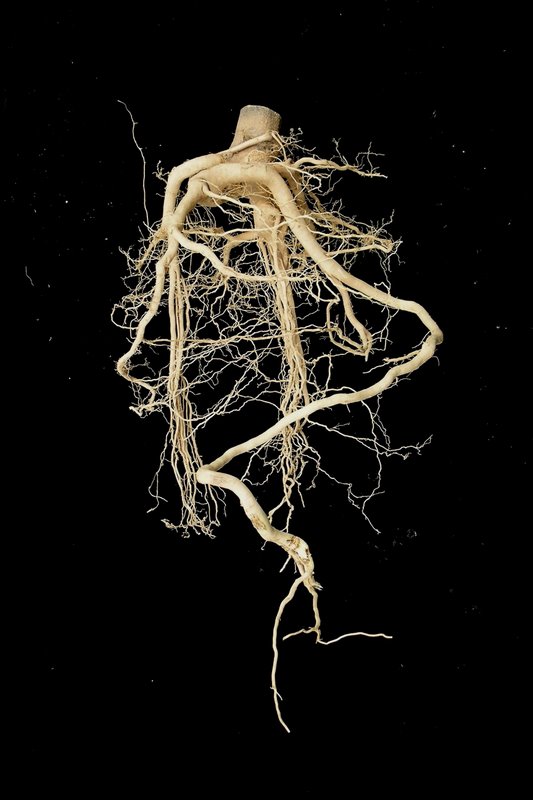
The root is pale yellowish colour; wild collected root has only tap root of 0.5-1.5 m while cultivated root has tap root with abundance of lateral roots less than 1 m. [3]
Cultivation
Soil Suitability and Climate Requirement
E. longifolia can be grown on many soil types. However, well-drained soil with high organic matter is most suitable. It requires an annual rainfall of 2,000-3,000 mm with temperature range of 25–30ºC. Although its natural habitat is under the jungle canopy, E. longifolia is found to grow well in the open without shade as long as there is enough water and nutrients. [4]
Field Preparation
Land Preparation
Prior to planting, normal field operations such as land clearing, disc ploughing and rotovation are to be conducted. Field drainage system should also be established in areas that are easily waterlogged. [4]
Production of Planting Materials
The plant can be propagated by seeds. The fully ripe fruit is deep red in colour and can be sown directly in the sand. About 90% of the seeds will germinate 6-8 weeks after sowing. The seedlings are transferred to polybags measuring 15 cm x 20 cm before field planting. The seedlings are best planted in the field not exceeding 6 months in the nursery. [4]

Field Planting
Planting holes measuring 30 cm x 30 cm x 30 cm are prepared and basal fertiliser consisting of 100 g Triple Super Phosphate (TSP), 100 g Ground Magnesium Limestone (GML) and 50 g processed chicken manure is applied. The recommended spacing for the monocropping system is 1.0 m within a row and 2.0 m between rows. This planting distance gives a planting density of 5,000 plants/ha. Planting should be done at the beginning of the rainy season. Studies have shown that the plant can also grow well as a monocrop in open planting. It can also be planted as an intercrop with young coconut, oil palm, fruit trees and in semi-cleared forest. [4][5]

Field maintenance
Fertilisation
The interim recommendation for 1-year-old plants in the field is compound fertiliser NPK (15:15:15) and processed chicken manure both at the rate of 100 g/plant. Ground Magnesium Limestone (GML) is applied at the rate of 50 g/plant. The compound fertiliser NPK is divided equally into 4 parts and applied 4 times a year, whereas the chicken manure and GML are applied twice a year in equal amounts. For plants above two years, the rate is increased to 200 g/plant respectively for compound NPK and processed chicken manure. The rate for GML is 100 g/plant. [4]
Weed Control
Weeds are controlled by spraying contact weedicide, manually or by using a grasscutter. [4]
Water management
Field planting should be conducted during the beginning of the rainy seasons in order to avoid planting stress. Supplementary irrigation by using drip system is applied during the early stages of crop growth. [4]
Pest and Disease Control
The main insect pests of E. longifolia are tiger moth (Attera sciodoxa) and scale insects. The tiger moth larva feeds on the shoot and young leaves. The insect population increases at the start of rainy season since there are a lot of young shoots start growing. The biological insecticide Bacillus thuringiensis (BT) should be used to control the infestations. The scale insects attack both the mature leaves and young shoots. The scale insects can be controlled by using white oil. The incidence of sudden death syndrome can also affect young and actively growing plants. The pathogen causing the disease has not been identified. [4]
Harvesting
The main part used is the root. In the forest, harvesting is done by pulling out the whole plant manually or by using harvesting implements developed. The optimum harvesting age is not known. However, E. longifolia plants at 2-3 years of age are found to start flowering and bearing fruit. At this age, the plant starts to mature. It is expected that cultivated E. longifolia can be harvested as early as at this age. The expected potential yield for fresh roots per plant from an open field without shade is 0.8-1.2 kg. This estimated yield is for plants harvested 4 years after planting. [4][5]
Postharvest handling
After harvesting, the roots are washed before drying. The roots can be sun-dried for a few days. They can also be dried in an oven at 40-50ºC for 72 hours. The roots are marketed in its natural form or finely sliced. Preferably the roots are first dried before they are sliced for easy processing. [4]
Estimated cost of production
The estimated production cost per hectare of ‘tongkat ali’ monocrop for 4 years is RM 36,000. This cost includes land preparation, seeds, planting, maintenance, harvesting and drying of product. With an estimated dried root yield of 3,000 kg/ha, the production cost is RM 12.04/kg. The production cost was estimated based on the cost of current inputs during writing of this article. [4]
Chemical Constituent
Aqueous extract of the E. longifolia roots has been reported to contain quassinoids (e.g. eurycolactones A-E, eurycomalides A-B, eurycomalactone, 6α-hydroxyeurycomalactone, 7α-hydroxyeurycomalactone, eurycomanone, 13α(21)-epoxyeurycomanone, 12,15-diacetyl-13α(21)-epoxy-eurycomanone,12-acetyl-13,21-dihydroeurycomanone, 15-acetyl-13α(21)-epoxyeurycomanone, 3,4ε-dihydroeurycomanone, 13,21-dihydroeurycomanone, eurycomanol, 13β,18-dihydroeurycomanol, 13β, 21-dihydroxyeurycomanol, eurycomanol-2-O-β-D-glycopyranoside, 11-dehydroklaineanone, 15β-hydroxyklaineanone, 14,15β-dihydroxyklaineanone, 5α,14β,15β-trihydroxyklaineanone,15β-O-acetyl-14-hydroxyklaineanone, 6α-acetoxy-14,15β-dihydroxyklaineanone, 6α-acetoxy-15β-hydroxyklaineanone, laurycolactones A-B, longilactone, dehydroxylongilactone, 2,3-dehydro-4α-hydroxylongilactone, ailanthone, (α/β-epoxide) ailanthone, chaparrinone (α-methyl), 3,4ε-dihydrochaparrinone, picrasinoside B, klaineanolide B, iandonoside B, eurycomaoside, 16-α-O-methylneoquassin, samaderin B and glaucarubolone), canthin-6-one alkaloids (e.g. canthin-6-one, 9-methoxycanthin-6-one, 5,9-dimethoxycanthin-6-one, 9,10-dimethoxycanthin-6-one, 11-hydroxycanthin-6-one, 1-hydroxy-11-methoxycanthin-6-one, 10-hydroxy-9-methoxycanthin-6-one, 11-hydroxy-10-methoxycanthin-6-one, 11-O-β-D-glucopyranosylcanthin-6-one, canthin-6-one-3N-oxide, 9-methoxycanthin-6-one-3N-oxide and 9-methoxy-3-methylcanthin-5,6-dione), β-carboline alkaloids (e.g. β-carboline-1-propionic acid, 7-hydroxy-β-carboline-1-propionic acid, 7-methoxy-β-carboline-1-propionic acid, and 1-methoxymethyl-β-carboline), squalene-type triterpene (e.g. eurylene and 11/14-deacetyl eurylene), biphenylneolignans (e.g. 2,2’-dimethoxy-4-(3-hydroxy-1-propenyl)-4’-(1,2,3-trihydroxypropyl) diphenyl ethers (isomer), 2-hydroxy-3,2’,6’-trimethoxy-4’-(2,3-epoxy-1-hydroxypropyl)-5-(3-hydroxy-1-propenyl)-biphenyl and 2-hydroxy-3,2’-dimethoxy-4’-(2,3-epoxy-1-hydroxypropyl)-5-(3-hydroxy-1-propenyl)-biphenyl) and others (e.g. isoleucine, calcium, magnesium and potassium). [6][11][12]
Methanol extract of the roots has been found to contain quassinoids (e.g. eurycolactones A-F, eurycomalides A-B, eurycomalactone, 6α-hydroxyeurycomalactone, 6-hydroxy-5,6-dehydroeurycomalactone, 5,6-dehydroeurycomalactone, eurycomanone, 13β,21-dihydroxyeurycomanol, 14,15β-dihydroxyklaineanone, 5α,14β,15β-trihydroxyklaineanone, laurycolactones A-B, longilactone, 6-dehydroxylongilactone, 2,3-dehydro-4α-hydroxylongilactone and pasakbumin B-C), canthin-6-one alkaloids (e.g. canthin-6-one, 1-hydroxycanthin-6-one, 9-hydroxycanthin-6-one, 5-hydroxymethylcanthin-6-one, 5-methoxycanthin-6-one, 9-methoxycanthin-6-one, 10-methoxycanthin-6-one, 1-hydroxy-9-methoxycanthin-6-one, 4-hydroxy-5-methoxycanthin-6-one, 8-hydroxy-9-methoxycanthin-6-one, 5-hydroxymethyl-9-methoxycanthin-6-one, 4,5-dimethoxycanthin-6-one, 9,10-dimethoxycanthin-6-one, canthin-6-one 9-O-β-glucopyranoside, canthin-6-one-3N-oxide, 9-hydroxycanthin-6-one-3N-oxide and 9-methoxycanthin-6-one-3N-oxide), β-carboline alkaloids (e.g. β-carboline-1-propionic acid, 7-methoxy-β-carboline-1-propionic acid, methyl β-carboline-1-carboxylate, n-pentyl β-carboline-1-propionate and picrasidine L), triterpenes (e.g. eurylene, mixture of β-sitosterol and stigmasterol, and β-sitosteryl glucoside) and others (e.g. scopoletin, fraxidin, scopolin, ρ-hydroxybenzaldehyde, syringic aldehyde, 2,4’-dihydroxy-3’-methoxyacetophenone, 2,3-dihydroxy-1-(4’-hydroxy-3’-methoxyphenyl)-propan-1-one, 3-hydroxy-1-(4’-hydroxy-3’-methoxyphenyl)propan-1-one, threo-1,2-bis-(4-hydroxy-3-methoxyphenyl)propane-1,3-diol, vanillic acid, protocatechuic acid, nicotinic acid, syringic acid, sodium syringate, sodium ρ-hydroxybenzoate, lariciresinol, erythro-1-C-syringylglycerol, threo-1-C-syringylglycerol, erythro-guaiacylglycerol, threo-guaiacylglycerol, iandonone, adenosine, guanosine, thymidine, alanine, proline, arginine, serine, glucose and fructose). [10][13][14][15][16][17][18][19]
Ethanol (50%) extract of the roots has been found to contain quassinoids (e.g. eurycomanone, 13,21-dihydroeurycomanone, 13α(21)-epoxyeurycomanone, longilactone, eurycomalactone, 14,15β-dihydroxyklaineanone, eurycomanol, eurycomanol-2-O-β-glucopyranoside) and a canthin-6-one alkaloid (e.g. 9-methoxycanthin-6-one). [23][24][25]
Volatile components were also detected in the aqueous and methanol root extracts, e.g. 3-methylbutanal, 1-butanol, 1-pentanol, 2-hexadecanol, acetol, nonanal, acetic acid, 2-methylhexanol, benzaldehyde, [S-(R*,R*)]-2,3-butanediol, butyrolactone, 2-furanmethanol, 3-methylbutanoic acid, 2(5H)-furanone, curcumene, hexanoic acid, butylated hydroxytoluene, 1-(1H-pyrrol-2-yl)-ethanone, (R)-(−)-massoilactone, 1H-pyrrole-2-carboxaldehyde, 3-phenoxy-1-propanol, octanoic acid, [1R,2S,5R]-1’-[butyn-3-one-1-yl]-menthol, 2-phenoxyethanol, ethyl p-ethoxybenzoate, nonanoic acid, 4-ethynyl-4-hydroxy-3,5,5-trimethyl-2-cyclohex-1-enone, 2,4-bis(1,1-dimethylehtyl)phenol, diethyl phthalate, benzoic acid, 2,3,6,7-tetrahydro 4a,8a-butano-[1,4]dioxino[2,3-b]-1,4dioxin. [23]
Plant Part Used
Root. [24][25]
Traditional Use
The root has been traditionally used for fever, aches, edema and gland swelling. It was also documented for boils, wounds, ulcer, bleeding gums, dysentery, sexual dysfunction and infertility. It can also be used as tonic and afterbirth medication. [24][25]
Preclinical Data
Pharmacology
Antimalarial activity
Ethanol extract of E. longifolia root showed active antimalarial activity towards both P. falciparum strain 3D7 and K1 with the IC50 of 2.16 µg/mL and 1.79 µg/mL respectively, compared to dihydroartemisinin with IC50 of 1.97 µg/mL and 1.46 µg/mL suggesting that this medicinal plant has a potential to be an antimalarial agent when compare to dihydroartemisnin, a standard antimalarial drug. [26]
A mixture of semi-purified root extract (13β,18-dihydroeurycomanol, eurycomanol-2-О-β-D-glucopyranoside, eurycomanol and eurycomanone) of 0.07-5.00 µg/mL demonstrated more than 50% inhibition of P. falciparum strain. [27][28]
7-methoxyP-carboline-1-propionic is another compound isolated from E. longifolia that possessed antimalarial activity against P. falciparum strains. [29]
Eurycomalactone, eurycomanone and eurycomanol isolated from E. longifolia exhibited potential antimalarial activity in vitro against the chloroquine resistant P. falciparum. [30]
It was also shown that eurycomanone, 13,21-dihydroeurycomanone, 13α(21)-epoxyeurycomanone, eurycomalactone, and 9-methoxycanthin-6-one displayed anti-plasmodial activity against chloroquine-resistant Gombak A isolate of Ρ. falciparum with IC50 in the range of 0.23-1.56 µg/mL. [31]
The standardised methanol extract of E. longifolia root at the concentration of 10, 30 and 60 mg/kg BW in combination with artemisinin given orally to mice for 3 days showed suppression of 63, 67 and 80% of Plasmodium yoelii infection in mice, respectively, whilst 80% suppression of P. yoelii infection was observed with subcutaneous treatment ofE. longifolia (10 mg/kg BW) combined with artemisinin. [32]
Methanol extracts of E. longifolia root at a dose of 10 mg/kg was administered intraperitoneally , given in single daily dose at midday starting from day 1 until day 4 following the inoculation (four day suppressive test) against P. berghei ANKA strain infection in male ICR mice weighing initially between 18-25 g. Day 2 observation on parasitaemia development in malarial group receiving 10 mg/kg E. longifolia extract showed a significant (p<0.05) percentage inhibition of 57% (2.75±0.37) followed by 72%(4.75±1.35) inhibition on day 3 and 78%(7.00±3.08) inhibition on day 4 compared to tween-20 treated malarial mice which showed (6.33±0.83) on day 2, (17.11±1.46) on day 3 and (32.33±4.06) on day 4. [33]
Cytotoxic activity
Eurycomanone isolated from E.longifolia dried roots showed a great inhibitory effect by significantly reduced the viability of HepG2 cells (liver cancer) by 50% inhibition at 3.8±0.12 μg/mL compared to control drug, tamoxifen which is a standard chemotherapeutic drug with the IC50 value of 3.7±0.28 μg/mL using the antiproliferative assay. However, tamoxifen is found more cytotoxic towards normal liver cells which inhibit 50% cells viability at 1.4±0.31 μg/mL. As compared to tamoxifen, eurycomanone was 12 times less toxic against normal liver cells. [6]
Longilactone that was isolated from methanol extract of E. longifolia root exhibited cytotoxic effect on human breast cancer cell line MCF-7 with IC50 of 0.53±0.19 µg/mL, but is less potent than the established anticancer agent taxol, in which the IC50 was at 0.047±0.023 µg/mL. This study demonstrated that longilactone exerted cytotoxic activity on MCF-7 associated with apoptotic cell death. The apoptotic mechanism of longilactone on MCF-7 is via activation of an extrinsic or receptor-mediated pathway but does not involve intrinsic or mitochondria pathway.[7]
A standardized quassinoids composition (SQ40) containing 40% of the total quassinoids in E. longifolia root induced selective cytotoxicity on human prostate cancer cells and inhibited the growth of LNCaP cells (human prostate cancer cell line) at IC50 value of 5.97 μg/mL by down-regulate the expression levels of G1-to-S phase transition regulatory proteins, Cyclin D1, CDK4 and CDK2 and up-regulate Cyclin inhibitor protein, p21 Waf1/Cip1 which subsequently led to cell cycle arrest in G0/G1 phase. [8]
Eurycomanone isolated from E. longifolia root showed potent cytotoxic effect towards K-562 leukemic cell line with IC50 of 6±1 µg/mL compared to Imatinib, a potent cytotoxic drug used in the treatment of chronic myelocytic leukemia (CML) with IC50 of 0.2±0.05 µg/mL using colony-forming assay. However, the cytotoxic activity of eurycomanone was 30 times less than Imatinib, respectively. [9]
9-methoxycanthin-6-one, 9-methoxycanthin-6-one-N-oxide, 9-hydroxycanthin-6-one and 9-hydroxycanthin-6-one-N-oxide isolated from E. longifolia roots exhibited cytotoxic effects against human cancer cell types including breast, colon, fibrosarcoma, lung, melanoma, KB and murine lymphocytic leukemia (P-388) with ED50 in the range of 1.4 to 11.7 µg/mL. Eurycomanone was active against all cells above including vincristine-resistant KB cells (ED50 0.2-11.3 µg/mL), except P-388 cells. [10]
Eurycomanone, 21-dihydroeurycomanone and 14,15β-dihydroxyklaineanone from E. longifolia roots showed potent cytotoxic effect against KB cells with IC50 values of 0.98, 0.81 and 0.96 µM, respectively. [13]
Aphrodisiac activity
The aqueous dried extract of E. longifolia showed significant increase in epididymal sperm counts and percentage of motile sperms. Adult male Sprague-Dawley rats weighing 200–250 g were divided into four groups of six rats each. Group A (control) was given solvent in the same manner as the treated groups were given E. longifolia. Group B was treated with E. longifolia (8 mg/kg body weight) orally. Group C was treated with estradiol (E2) (intramuscular dose of 500 µg/kg body weight), and group D received a combined treatment of oral E. longifolia and intramuscular E2. After fourteen consecutive days of treatment, rats from all groups were sacrificed and subjected to epididymal sperm counts. Rats treated with E. longifolia (Group B) showed marked increase in the spermatogenic cell count (65%) and highest percentage of motile sperms (60-70%), respectively. [34]
The proprietary standardized, water-soluble extract of E. longifolia root exhibited significant improvement in semen volume, sperm concentration, sperm motility and the percentage of morphologically normal sperm in men with idiopathic infertility. A total of 350 patients were given 200 mg of the extract daily and follow-up semen analyses were performed every 3 months for 9 months. The semen volume (mL) showed increasing in trend up till the third cycle, 3.52 ± 0.35 as compared to baseline of 2.95 ± 0.14. As for the semen concentration (million per mL) it showed highest value at third cycle which was 17.53 ± 5.04, compared to baseline of 10.59 ± 2.06. The normal sperm morphology showed the highest percentage at the second cycle, 10.41 ± 1.28 compared to the baseline of 5.28 ± 0.66. The last parameter which was the sperm motility (%) showed the highest percentage at the first cycle, 49.99 ± 2.81 as compared to the baseline of 44.68 ± 2.44. Consequently, it is noteworthy that 11 (14.7%) spontaneous pregnancies were achieved after the treatment of infertile men presenting with idiopathic infertility in the setting of decreased sperm concentration, poor sperm motility and abnormal sperm morphology, using the proprietary standardized water soluble extract of E. longifolia. [35]
The root powder of E. longifolia showed increase percentages of mounting and ejaculating rats in sluggish animals. It was orally administered to adult Sprague–Dawley male rats (250–300 g b.w.) classified as sexually sluggish or impotent taking in account their behavior in pre-experimental tests. Groups of 8 animals each were submitted to three different types of treatment: (1) acute at 3 dose levels (250, 500 and 1000 mg/kg); (2) subacute (daily for 6 days) at the dose of 500 mg/kg and (3) subchronic (daily for 12 days) at the same dose (500 mg/kg). The sexual behavior of impotent rats was not affected by the acute treatment with the plant root powder even if administered at the highest dose: no control or treated animal showed mount or intromission behavior in the presence of a receptive female (therefore the data are omitted in the paper). On the other hand the repeated administration of 500 mg/kg daily for 6 and 12 days produced an increase in the percentage of mounting rats from 0% assessed in control group to 25% observed in rats treated for 6 days and 37.5% observed in rats treated for 12 days. The percentage of ejaculating animals increased from 0% (control rats) to 25% in both groups of rats treated with E. longifolia. [36]
Chloroform, methanol, butanol and water fractions of E. longifolia root given orally to 3-4 month old male Sprague Dawley albino rats for 10 days showed a dose-dependent increase in mounting frequency from 5.3 (400 mg/kg) to 5.4 (800 mg/kg), 4.9 to 5.4, 4.8 to 5.2 and 5.2 to 5.3, respectively [37]. These fractions (500 mg/kg) contributed towards sexual motivation activity in adult, middle-aged male albino mice and in retired breeders [38], as well as enhanced sexual qualities of middle-aged male rats [39].
Methanol, chloroform, water and butanol fractions of E. longifolia root (800 mg/kg) given via oral gavage to 9-month old Sprague Dawley rats for 10 days showed changes in sexual behaviour such as increased orientation activities towards the receptive female rats (anogenital sniffing, licking and mounting), increased genital grooming towards themselves and restricted movements to a particular area of the cage [40]. The fractions (800 mg/kg) were also found to promote sexual arousal in sexually sluggish old male rats [41]. Additionally, these fractions (800 mg/kg) given orally for 12 weeks to testosterone-stimulated castrated intact male rats revealed pro-androgenic property as evidenced by the enhanced growth of the laevator ani muscle [42].
Antidiabetic activity
Aqueous extract ofE. longifolia root showed antihyperglycaemic activity at a dose of 150 mg/kg body weight which was observed in streptozotocin-induced hyperglycaemic Sprague Dawley rats after 10 days of treatment. [43]
Anxiolytic activity
Chloroform, methanol, butanol and water fractions of E. longifolia root (0.3 g/kg) were administered by oral gavage to inbred adult albino mice (35-40 g) exerted anxiolytic effect. [44]
Antiosteoporotic activity
Supplementation of E. longifolia root aqueous extract given to 12-month old orchidectomised Sprague Dawley rats for 6 weeks showed maintenance of bone calcium level. [45]
A freeze-dried standardized extract of E. longifolia root with combination of testosterone could reduce bone turnover much better than treatment with E. longifolia or testosterone alone in androgen-deficient osteoporosis in men. 40 male Sprague-Dawley rats (aged 10 to 12 months) were divided into: shamoperated (SHAM), orchidectomized-control (ORX), orchidectomized with testosterone (ORX + T), orchidectomized with EL(ORX + EL), and orchidectomized with combined T and EL therapy (ORX + T + EL). EL was administered via oral gavages daily at the dose of 15 mg/kg. T was injected intramuscularly at 8 mg/kg and 4 mg/kg for the ORX + T and ORX + T + EL groups, respectively. Following 6 weeks of treatment, the osteocalcin levels of ORX + T and ORX + T + EL groups were significantly lower than the SHAM group (P<0.05). The post treatment CTX levels of ORX + T and ORX + T + EL groups were significantly lower than their pretreatment levels (P<0.05). [46]
Antimicrobial activity
Methanol, acetone, ethyl acetate, chloroform and petroleum ether extracts of E. longifolia stem were found highly active against gram positive bacteria. All the stem extracts exhibited high to moderate activity against Staphylococcal aureus at the lowest tested concentration of 12.5 µg/µL with ZI of 7.67±0.58 (acetone extract), 9.33±0.58 (ethyl acetate extract), 7.33±0.58 (chloroform extract), 8.67±1.15 (petroleum ether extract) while methanol extract showed very high activity at 200 µg/µL with ZI, 17.00±6.56 mm. Moderate activity was observed by all the stem extracts against Bacillus cereus at lowest concentration of 12.5 µg/µL with ZI of 7.67±0.58 (acetone extract), 8.33±0.58 (ethyl acetate extract ), 7.33±0.58 (chloroform extract ), 7.33±0.58 (petroleum ether extract) and methanol extract was found more potent than the other four extracts, showed high activity at concentration of 200 µg/µL with ZI, 14.00±1.00. This was tested by using the disc diffusion method. [47]
Toxicity
Acute toxicity
Oral administration of E. longifolia aqueous extract at 5000 mg/kg body weight via oral gavage on male Sprague Dawley rats within 24 hours did not produce any physical, behavioural and pathological changes in the liver, testes and kidneys. The oral LD50 for the extract was found to be more than 5000 mg/kg body weight [48]. A similar study demonstrated that oral administration of E. longifolia aqueous extract (2000 mg/kg body weight) to male and female Wistar rats also produced neither mortality nor changes in behavior or any other physiological activities [49].
Aqueous ethanolic extract (50%) and its diethyl ether, n-butanol and water fractions were orally administered as a single dose to the Sprague Dawley mice. Observation was made after 48 hours after administration. The LD50 values ranged from 1.36-5.23 g/kg. The most toxic n-butanol fraction was found to be due to eurycomanone. [21]
Oral single dose acute toxicity study on female Sprague Dawley rats (aged between 8 and 12 weeks old) using aqueous mixture of E. longifolia roots showed no toxic effects on the parameters observed, including behaviors, body weight, food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. LD50 value was determined as > 2000 mg/kg. [50]
Sub-acute toxicity
Aqueous extract of E. longifolia root (up to 2400 mg/kg body weight) orally administered to Sprague Dawley rats for 28 days did not show significant changes in body weight, toxic symptoms such as piloerection, salivation and lacrimation, as well as in the biochemical and haematological parameters. No pathological changes were observed in the testes and kidneys. However, the livers from animals treated with extract of 1200 and 2400 mg/kg body weight exhibited hydropic changes in the livers. [48]
Male and female Wistar rats treated with up to 1000 mg/kg body weight of E. longifolia aqueous root extract for 28 days did not produce any mortality or toxic effect. No changes were observed in body and organ weights, histopathological analysis, blood biochemical parameters (glucose, creatinine, urea, aspartate transminase, potassium, sodium, total billirubin, total cholesterol, total protein, albumin and clotting time) and haematological parameters (white blood count, platelet and haemoglobin estimation) of both sexes. [49]
Alcohol extract of E. longifolia showed signs of toxicity such as increased weights of liver, kidneys, spleen and testes on animals treated with 600 mg/kg body weight extract. [51]
Sub-chronic toxicity
Aqueous extract of E. longifolia root (up to 1000 mg/kg body weight) orally administered for 90 days to both females and male Wistar rats for 90 days did not cause any mortality, clinical abnormalities and neurotoxicity effects. [49]
Mutagenicity
Methanol-chloroform (1:1) extract ofE. longifolia root (250 μg/mL) did not show any mutagenic effect on Salmonella typhimurium strains, TA 98 and TA 100. [52]
Reproductive toxicity
Adult male Sprague Dawley rats exhibited no toxicity effects in testicular histology after oral treatment with 8 mg/kg body weight of aqueousE. longifolia root extract for 14 days. [53]
Clinical Data
Clinical findings
Anxiolytic activity
Sixty-four (64) subjects (32 men and 32 women) were randomized to receive tongkat ali (TA; 200 mg/day of Physta™, Biotropics Malaysia Berhad; 32 subjects) or look-alike placebo (PL; 32 subjects) for 4 weeks using a screening survey to detect moderate stress (scoring 6 and above). Mood state parameters showed mixed results with no effect observed between supplementation groups for indices of depression, vigor, or fatigue, whereas significant improvements were found in the TA group for tension (−11%), anger (−12%), and confusion (−15%) compared to placebo. [54]
Men’s fertility
Male patients suffered from late onset of hypogonadism aged between 28 to 70 years old were given daily oral dosage of 200 mg of standardized aqueous extract of E. longifolia root for 1 month and exhibited significant improvement in testosterone level and scores of the Ageing Male’s Symptoms. [55]
Precautions
No documentation.
Side effects
No documentation.
Pregnancy/Breast Feeding
No documentation.
Age limitation
No documentation.
Adverse reaction
Fresh E. longifolia root and E. longifolia-based products potentially cause allergic reaction based on an in vitro specific immunoglobulin E (IgE) immunoblotting test on sera of a patient with prawn allergy. [56]
Interaction & Depletion
No documentation.
Interaction with drug
Co-administration ofE. longifolia root water extract containing 0.0272±0.0026% of eurycomanone and propanolol (80 mg) was found to reduce absorption of propanolol and decrease its bioavailability in healthy non-smoker young males. [57]
Interaction with other Herbs
No documentation
Contraindications
No documentation
Case Report
No documentation
Dosage
No documentation.
Poisonous Management
No documentation.
Line drawing
References
- The Plant List. Ver1.1. Eurycoma longifolia Jack. [homepage on the internet]. c2013 [updated 2012 March 26; cited 2015 Dec 14]. Available from: http://www.theplantlist.org/tpl1.1/record/kew-2805148
- Burkill IH. A dictionary of the economic products of the Malay Peninsula. Volume 1. London: Published on behalf of the governments of the Straits settlements and Federated Malay states by the Crown agents for the colonies, 1935; p. 984-986.
- Malaysian Herbal MonographCommittee. Malaysian Herbal Monograph 2015. Kuala Lumpur: Institute for Medical Research, 2015; p. 176.
- Mohd Noh J, Muhamad Ghawas M, Musa Y. Tongkat ali (Eurycoma Longifolia Jack). In: Musa Y, Muhammad Ghawas M, Mansor P, editors. Penanaman tumbuhan ubatan & beraroma. Selangor, Malaysia: MARDI, 2005; p. 95-101.
- Musa Y, Mansor P, Ramli M. Pertumbuhan dan prestasi hasil akar tongkat ali di bawah sistem tanaman terbuka di KESEDAR Mengkebang. Buletin Teknol. Tanaman. 2005;2:27-33.
- Zakaria Y, Rahmat A, Pihie AH, Abdullah NR, Houghton PJ. Eurycomanone induce apoptosis in HepG2 cells via up-regulation of p53. Cancer Cell Int. 2009;9:21.
- Muhamad S, Pihie AH, Latif J, Rha C, Sambandan TG. Induction of apoptosis in MCF-7 via the Caspase pathway by longilactone from Eurycoma longifolia Jack. Res Pharm Biotechnol. 2011;3(1):1-10.
- Tong KL, Chan KL, AbuBakar S, Low BS, Ma HQ, Wong PF. The in vitro and in vivo anti-cancer activities of a standardized quassinoids composition from Eurycoma longifolia on LNCaP human prostate cancer cells. PLoS ONE. 2015;10(3):e0121752.
- Al-Salahi OS, Ji D, Majid AM, et al. Anti-tumor activity of Eurycoma longifolia root extracts against K-562 cell line: In vitro and in vivo study. PLoS One. 2014;9(1):e83818.
- Kardono LB, Angerhofer CK, Tsauri S, Padmawinata K, Pezzuto LM, Kinghorn ADJ. Cytotoxic and antimalarial constituents of the roots of Eurycoma longifolia. J Nat Prod. 2004;54(5):1360-1367.
- Chua LS, Mohd-Amin NA, Neo JCH, Lee TH, Lee CT, Sarmidi MR, Abdul-Aziz R. LC–MS/MS-based metabolites of Eurycoma longifolia (Tongkat Ali) in Malaysia (Perak and Pahang). J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(32):3909-3919.
- Chua LS, Abdul-Rahman N, Rosidi B, Lee CT. Plant proteins, minerals and trace elements of Eurycoma longifolia (Tongkat Ali). Natural Product Research. 2013;27(4-5):314-318.
- Chan KL, Lee SP, Sam TW, Tan SC, Noguchi H, Sankawa U. 13β,18-dihydroeurycomanol, a quassinoid from Eurycoma longifolia. Phytochemistry. 1991;30(9):3138-3141.
- Chan KL, Iitaka Y, Noguchi H, Sugiyama H, Saito I, Sankawa U. 6α-Hydroxyeurycomalactone, a quassinoid from Eurycoma longifolia. Phytochemistry. 1992;31(12):4295-4298.
- Ang HH, Hitotsuyanagi Y, Takeya K. Eurycolactones A-C, novel quassinoids from Eurycoma longifolia. Tetrahedron Lett. 2000;41(35):6849-6853.
- Ang HH, Hitotsuyanagi Y, Fukaya H, Takeya K. Quassinoids from Eurycoma longifolia. Phytochemistry. 2002;59(8):833-837.
- Kuo PC, Shi LS, Damu AG, et al. Cytotoxic and antimalarial β-carboline alkaloids from the roots of Eurycoma longifolia. J Nat Prod. 2003;66(10):1324-1327.
- Teh CH, Morita H, Shirota O, Chan KL. 2,3-Dehydro-4α-hydroxylongilactone, a novel quassinoid and two known phenyl propanoids from Eurycoma longifolia Jack. Food Chem. 2010;120(3):794-798.
- Kuo PC, Damu AG, Wu TS. [Characterization of the water soluble fraction from the root extract of Eurycoma longifolia]. Zhonghua yaoxue zazhi. 2003;55(4):257-265. Chinese.
- Chan KL, Lee S, Sam TW, Han BH. A quassinoid glycoside from the roots of Eurycoma longifolia. Phytochemistry. 1989;28(10):2857-2859.
- Chan KL, Choo CY. The toxicity of some quassinoids from Eurycoma longifolia. Planta Med. 2002;68(7):662-624.
- Chan KL, Choo CY, Abdullah NR, Ismail Z. Antiplasmodial studies of Eurycoma longifolia Jack using the lactate dehydrogenase assay of Plasmodium falciparum. J Ethnopharmacol. 2004;92(2-3):223-227.
- Shafiqul Islam AKM, Ismail Z, Saad B, Othman AR, Ahmad MN, Shakaff AYM. Correlation studies between electronic nose response and headspace volatiles of Eurycoma longifolia extracts. Sens Actuators B Chem. 2006;120(1):245–251.
- Gimlette JD, Burkhill IH. The Medical Book of Malayan Medicine. The Gardens’ Bulletin Straits Settlements Volume 6. Singapore: Botanic Gardens, 1930; p. 329.
- Burkill IH, Haniff M. Malay village medicine. The Gardens’ Bulletin Straits Settlement 2. Singapore: Botanic Garden, 1930; p. 182.
- Katib S, Ruangrungsi N, Chaijaroenkul W, Rungsihirunrat K. Standardization parameters, internal transcribed spacer nucleotide sequence and their anti-malarial activity of Eurycoma longifolia Jack. Int J Adv Biol Chem. 2015;4(1):3-5.
- Kuo PC, Damu AG, Lee KH, Wu TS. Cytotoxic and antimalarial constituents from the roots of Eurycoma longifolia. Bioorg Med Chem. 2004;12(3):537-544.
- Ang HH, Chan KL, Mak JW. Effect of 7-day daily replacement of culture medium containing Eurycoma longifolia Jack constituents on the Malaysian Plasmodium falciparum isolates. J Ethnopharmacol. 1995;49(3):171–175.
- Kardono LBS, Abgerhofer CK, Tsauri S, Padmawinata K, Pezzuto LM, Kinghorn ADJ. Cytotoxic and antimalarial constituents of the roots of Eurycoma longifolia. J Nat Prod. 1991;54(5):1360-1367.
- Chan KL, O’neill MJ, Phillipson JD, Warhurst DC. Plants as sources of antimalarial drugs, part 3. Eurycoma longifolia. Planta Med. 1986;52(2):105-107.
- Chan KL, Choo CY, Abdullah NR, Ismail Z. Antiplasmodial studies of Eurycoma longifolia Jack using the lactate dehydrogenase assay of Plasmodium falciparum. J Ethnopharmacol. 2004;92(2-3):223-227.
- Mohd Ridzuan MA, Sow A, Noor Rain A, Mohd Ilham A, Zakiah I. Eurycoma longifolia extract-artemisinin combination: Parasitemia suppression of Plasmodium yoelii-infected mice. Trop Biomed. 2007;24(1):111-118.
- Basir R, Chan KL, Yam MF, et al. Antimalarial activity of selected Malaysian medicinal plants. Phytopharmacology Inforesights Publishing UK. 2012, 3(1) 82-92.
- Wahab NA, Mokhtar NM, Halim WN, Das S. The effect of Eurycoma longifolia Jack on spermatogenesis in estrogen treated rats. Clinics. 2010;65(1):93-98.
- Tambi MI, Imran MK. Eurycoma longifolia Jack in managing idiopathic male infertility. Asian J Androl. 2010;12(3):376-380.
- Zanoli P, Zavatti M, Montanari C, Baraldi M. Influence of Eurycoma longifolia on the copulatory activity of sexually sluggish and impotent male rats. J Ethnopharmacol. 2009;126(2):308–313.
- Ang HH, Sim MK. Eurycoma longifolia Jack enhances libido in sexually experienced male rats. Exp Anim. 1997;46(4):287–290.
- Ang HH, Lee KL, Kiyoshi M. Eurycoma longifolia Jack enhances sexual motivation in middle-aged male mice. J Basic Clin Physiol Pharmacol. 2003;14(3):301–308.
- Ang HH, Ngai TH, Tan TH. Effects of Eurycoma longifolia Jack on sexual qualities in middle aged male rats. Phytomedicine. 2003;10(6-7):590–593.
- Ang HH, Lee KL. Effect of Eurycoma longifolia Jack on orientation activities in middle-aged male rats. Fundam Clin Pharmacol. 2002;16(6): 479–483.
- Ang HH, Lee KL, Kiyoshi M. Sexual arousal in sexually sluggish old male rats after oral administration of Eurycoma longifolia Jack. J Basic Clin Physiol Pharmacol. 2004;15(3-4):303–309.
- Ang HH, Cheang HS. Effects of Eurycoma longifolia Jack on laevator ani muscle in both uncastrated and testosterone -stimulated castrated intact male rats. Arch Pharm Res. 2001;24(5):437-440.
- Husen R, Hawariah A, Pihie L, Nallappan M. Screening for antihyperglycaemic activity in several local herbs of Malaysia. J Ethnopharmacol. 2004;95(2-3):205-208.
- Ang HH, Cheang HS. Studies on the anxiolytic activity of Eurycoma longifolia Jack roots in mice. Jpn J Pharmacol. 1999;79(4):497-500.
- Shuid AN , Abu Bakar MF, Abdul Shukor TA, Muhammad N, Mohamed N, Soelaiman IN. The anti-osteoporotic effect of Eurycoma longifolia in aged orchidectomised rat model. Aging Male. 2011;14(3):150-154.
- Saadiah Abdul Razak H, Shuid AN, Naina Mohamed I. Combined effects of Eurycoma longifolia and testosterone on androgen-deficient osteoporosis in a male rat model. Evid Based Complement Alternat Med. 2012;2012:872406.
- Khanam Z, Wen CS, Bhat IU. Phytochemical screening and antimicrobialactivity of root and stem extracts of wild Eurycoma longifolia Jack (Tongkat Ali). J King Saud Uni Sci. 2015;27(1):23-30.
- Shuid AN, Siang LK, Chin TG, Muhammad N, Mohamed N, Soelaiman IN. Acute and subacute toxicity studies of Eurycoma longifolia in male rats. International J Pharmacol. 2011;7(5):641-646.
- Yogendra KC, Prareen B, Yee KM, Noraisyah Z. Acute, subacute and subchronic 90-days toxicity of Eurycoma longifolia aqueous extract (PHYSTA) in Wistar rats. Int J Pharm Pharm Sci. 2012;4(3):232-238.
- Teh BP, Hamzah NF, Rosli SNS, Yahaya MAF, Zakiah I, Murizal Z. Acute oral toxity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2012. Report No.: HMRC 11-045/01/EL/R/P.
- Satayavivad J, Soonthornehareonnon N, Somanabandhu A, Thebtaranonth Y. Toxicological and antimalarial activity of eurycomalactone and Eurycoma longifolia Jack extracts in mice. Thai J Phytopharm. 1998;5(2):14-27.
- Razak MF, Aidoo KE, Candlish GG. Mutagenic and cytotoxic properties of three herbal plants from Southeast Asia. Tropical Biomedicine. 2007;24(2):49-59.
- Norhazlina AW, Norfilza MM, Wan Nurul Heriza AH, Srijit D. The effect of Eurycoma longifolia Jack on spermatogenesis in estrogen-treated rats. Clinics. 2010;65(1):93-98.
- Talbott SM, Talbott JA, George A, Pugh M. Effect of Tongkat Ali on stress hormones and psychological mood state in moderately stressed subjects. J Int Soc Sports Nutr. 2013;10(1):28.
- Tambi MI, Imran MK, Henkel RR. Standardised water-soluble extract of Eurycoma longifolia, Tongkat Ali, as testosterone booster for managing men with late-onset hypogonadism?. Andrologia. 2012;44(1):226-230.
- Faizal B, Noormalin A, Zailatul HMY, et al. Allergic reaction to Eurycoma longifolia Jack- a case report. Med J Malaysia. 2010:65(Supplement A).
- Salman SA, Amrah S, Wahab MS, et al. Modification of propranolol’s bioavailability by Eurycoma longifolia water-based extract. J Clin Pharm Ther. 2010;35(6):691-696.

