Manggis Rind
Garcinia mangostana Linn.
Clusiaceae
DEFINITION
Manggis rind consists of the powder of dried rind of Garcinia mangostana Linn.
SYNONYM
Mangostana garcinia Gaertn [ ].
VERNACULAR NAMES
Mangosteen (English); manggis, semetah, semontah (Malay); dao nian zi (Chinese); sulambali (Tamil) [ 2 , 3 ].
CHARACTER
| Colour | Yellowish-light brown |
| Odour | Nutty flavour |
| Taste | Acidic and bitter |
IDENTIFICATION
Plant Morphology
Garcinia mangostana is a small or medium height tree with 6–25 m, evergreen, with a straight trunk, symmetrically branched to form a conical crown. Leaves are opposite, entire and cuspidate at the apex, 19–23 cm long and 4–10 cm wide, shining and coriaceous, dark green, rarely yellow green, glabrous above, dull pale green or yellow green beneath. Flowers 4–5 cm wide, fleshy, may be male or hermaphrodite on the same tree; the former are in clusters of 3–9 at the branch tips; 4 sepals and 4 ovate, thick, fleshy petals, green with red spots on the outside, yellowish-red inside; stamens many, fertile and sterile; the hermaphrodite are borne singly or in pairs at the tips of young branchlets; petals may be yellowish-green edged with red or mostly red, and are quickly shed; ovary is broadly ellipsoid to globose, sessile and 4–8 celled; stigma is sessile, 4–8 radiate and large in diameter. Fruit a subglobose berry, capped by the prominent calyx at the stem end, with 4–8 triangular, flat remnants of the stigma in a rosette at the apex, dark-purple to red-purple and smooth externally, 3.4–7.5 cm in diameter; the rind is 6–10 mm thick, red in cross-section, purplish-white on the inside; 4–8 triangular segments of snow-white, juicy, soft flesh (actually the arils of the seeds); the fruit may be seedless or have 1–5 fully developed seeds. Seed ovoid oblong, somewhat flattened, 2.5 cm long and 1.6 cm wide that cling to the flesh; flesh slightly acid, mild to distinctly acid in flavour [ 4 ].
Microscopy
Powdered material consists of fragment of brachysclereids cells, scalariform vessel, annular vessels, microspore of the seed, druse calcium oxalate crystal, parenchyma cells, oil globule, spiral vessels and fragment fibre.







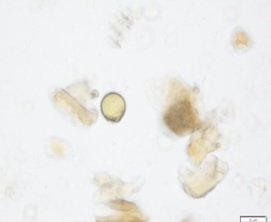



Figure 2 : Microscopic characters of Garcinia mangostana dried rind powder of 0.355 mm size. (a) Fragment of brachysclereids cells (magnification 40x); (b) scalariform vessel (magnification 40x); (c) annular vessels (magnification 40x); (d) microspore of the seed (magnification 40x); (e) druse calcium oxalate crystal (magnification 40x); (f) druse calcium oxalate crystal under polarizing filter (magnification 40x); (g) parenchyma cells (magnification 40x); (h) oil globule (magnification 40x); (i) spiral vessels (magnification 20x); (j) brachysclereids cells (magnification 40x); (k) fragment fibre (magnification 40x). [Scale bars: a – f, h-j = 20 µm; g, k = 20 µm].
Chemical Tests
Observation of solution after treatment with various reagents:
| Test for the presence of phenolic | Bluish green |
| Test for the presence of flavonoid | Yellow to colourless |
Thin Layer Chromatography (TLC)

Figure 3 : TLC chromatogram of ferulic acid (S), methanol extract of Garcinia mangostana dried rind powder (L) observed under (a) UV at 254 nm and (c) UV at 366 nm before derivatisation.
| Test Solutions | Weigh about 2.5 g of G. mangostana dried rind powder of 0.355 mm size in a 50 mL flask and add 20 mL of methanol in to the flask and shake gently (manually) for 1 min. Reflux the sample for 20 min and allow to cool at room temperature. Filter the mixture and use the filtrate as the test solution. |
| Standard solution | Dissolve 5.0 mg of ferulic acid standard [CAS no: 537-98-4] in 5 mL methanol to produce a standard concentration of 1.0 mg/mL. |
| Stationary Phase | HPTLC Glass Silica Gel 60 F254, 10 x 10 cm |
| Mobile phase | Toluene : ethyl acetate : formic acid; (5 : 4 : 1) (v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
High Performance Liquid Chromatography (HPLC)
| Test solution | Weigh about 2.5 g of G. mangostana dried rind powder of 0.355 mm size in a 50 mL flask and add 20 mL of methanol in to the flask and shake gently (manually) for 1 min. Reflux the sample for 20 min and allow to cool at room temperature. Filter the mixture and use the filtrate as the test solution. | ||||||||||||||||||
| Standard solution | Dissolve 5.0 mg of ferulic acid standard [CAS no: 537-98-4] in 5 mL methanol to produce a standard concentration of 1.0 mg/mL. | ||||||||||||||||||
| Chromatographic system |
Detector: UV 280 nmColumn: C18 (5.0 µm, 4.6 mm I.D x 250 mm) (ZORBAX Eclipse Plus C18 if necessary) Column oven temperature: 35˚C Flow rate: 1.0 mL/min Injection volume: 1.0 µL |
||||||||||||||||||
| Mobile Phase (gradient mode) |
|
||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the standard solutions (1.0 mg/mL). The requirements of the system suitability parameters are as follow:
|
||||||||||||||||||
| Acceptance criteria |
|

(a)

(a)
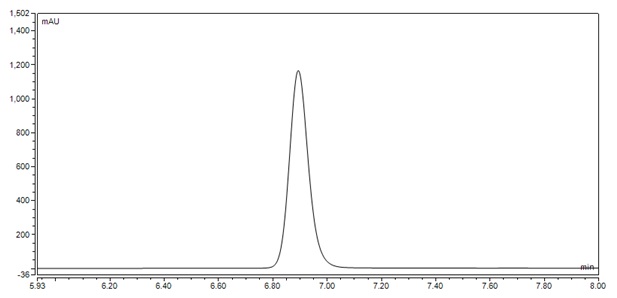
Figure 4 : Whole HPLC chromatogram of (a) ferulic acid standard solution (1.0 mg/mL) at tr = 6.907 min and (b) methanol extract of Garcinia mangostana dried rind powder showing peak corresponding to ferulic acid standard solution at tr = 6.900 min.
(a)

(a)
Figure 5 : HPLC chromatogram highlighting the elution region of (a) ferulic acid standard solution (1.0 mg/mL) at tr = 6.907 min and (b) methanol extract of Garcinia mangostana dried rind powder showing peak corresponding to ferulic acid standard solution at tr = 6.900 min.

Figure 6 : UV spectrum of ferulic acid standard solution (1.0 mg/mL) and methanol extract of Garcinia mangostana dried rind powder.
PURITY TESTS
The purity tests except foreign matter test are based on G. mangostana dried rind powder of 0.355 mm particle size.
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 5% |
| Acid-insoluble ash | Not more than 1% |
| Loss on Drying |
| Not more than 9% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 24% |
| Cold method | Not less than 16% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 22% |
| Cold method | Not less than 17% |
SAFETY TESTS
The safety tests are based on G. mangostana dried rind powder of 0.355 mm particle size.
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 5.0 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 3.0 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Methanol water (85 : 15) extract of G. mangostana pericarp was found to contain phenolic acids (e.g. ferulic acid, p-coumaric acid, veratric acid t-cinnamic acid, vanillic acid, cinnamic acid, caffeic acid, mandelic acid, gentisic acid, sinapic acid) and flavonoids (e.g. epicatechin and quercetin) [ 5 , 6 ].
Methanol and water extract of G. mangostana rind was found to contain tartaric esters, phenolics, flavonols and tannins [ 7 ].
Ethanol extract of G. mangostana pericarp was found to contain mangostin (α, β, γ), 1,5-dihydroxy-2-(3-methylbut-2-enyl)-3-methoxy-xanthone, 1,5-dihydroxy-2-isopentyl-3-methoxy xanthone, 1,7-dihydroxy-2-(3-methylbut-2-enyl)-3-methoxy-xanthone, 1,7-dihydroxy-2-isopentyl-3-methoxy xanthone 1-isomangostin, 1-isomangostin hydrate, 2-(γ,γ-dimethylallyl)-1,7-dihydroxy-3-methoxyxanthone, 3-isomangostin, 3-isomangostin hydrate, 8-deoxygartanin, 8-hydroxycudraxanthone, BR-xanthone, cudraxanthone G, garcimangosone (B, C, D), garcinone (B, D, E), gartanin, mangostanin, mangostenol, mangostenone (A, B), mangostinone, smeathxanthone A, tovophyllin (A and B), trapezifolixanthone, maclurin, kolanone, epicatechin, chrysanthemin cyanidin-3-O-sophoroside [ 8 ].
Ethanol (50%) extract of G. mangostana pericarp was found to contain phenolics (e.g. gallic acid) and anthocyanins (e.g. cyanindin-3-glucoside) [ 9 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Pericarp of G. mangostana was used as a cure for chronic intestinal inflammation, cure for dysentry and use as lotion [ 10 ], healing skin infections and relief of diarrhoea [ 11 ] and astringent [ 12 ].
Biological and pharmacological activities supported by experimental data
Antimicrobial activity
Ethanol extract of G. mangostana pericarp (200 μg) inhibited the growth of Staphylococcus aureus (24 mm) compared to amoxicillin (30 mcg/disc) (22 mm) using agar well diffusion method [ 13 ].
Ethanol extract of G. mangostana pericarp (200 μg) inhibited the growth of Escherichia coli with inhibition zone of 20 mm, Salmonella typhi (20 mm), Shigella dysenteriae (25 mm), Klebsiella pneumoniae (17 mm) and Vibrio cholerae (24 mm) compared to ciprofloxacin (30 mcg/disc) (21 – 24 mm) using agar well diffusion method [ 13 ].
Antioxidant activity
Butanol fraction of aqueous extract of G. mangostana rind showed antioxidant activity with inhibition concentration at 50% (IC50) of 6.56 µg/mL compared to alpha-mangostin (IC50 = 66.63 µg/mL) using 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assay (15). Alpha mangostin isolated from methanol extract of G. mangostana dried rind showed antioxidant activity with IC50 of 7.4 µg/mL compared to ascorbic acid (IC50 = 4.5 µg/mL) using DPPH assay [ 7 ].
Methanol (85%) extract of G. mangostana rind showed antioxidant activity with IC50 of 58 µg/mL compared to beta hydroxy acid (BHA) (IC50 = 62 µg/mL) using thiobarbituric acid assay [ 5 ].
Methanol (85%) extract of G. mangostana rind showed antioxidant activity with IC50 of 30 µg/mL compared to BHA (IC50 = 20 µg/mL) using DPPH assay [ 5 ].
Methanol extract of G. mangostana dried hulls showed antioxidant activity with IC50 of 20.50 µg/mL compared to alpha-tocopherol (IC50 = 34.01 µg/mL) using DPPH assay [ 14 ].
Methanol extract of G. mangostana dried hulls showed antioxidant activity with IC50 of 14.7 µg/mL compared to vitamin E (IC50 = 26.2 µM) and epicatechin (IC50 = 13.5 µM) using DPPH assay [ 15 ].
Water extract of G. mangostana dried hulls showed antioxidant activity with IC50 of 11.0 µg/mL compared to vitamin E (IC50 = 26.2 µM) and epicatechin (IC50 = 13.5 µM) using DPPH assay [ 15 ].
Antiproliferative activity
Methanol extract of G. mangostana rind (12.5 and 25.0 μg/mL) decreased the proliferation of breast cancer cells (SKBR3) (≈2.5% and ≈25.0% cell viability) with median effective dose (ED50) of 9.25 ± 0.64 μg/mL compared to quercetin (≈ 35.0%) and paclitaxel (25.0%) using MTT assay [ 16 ].
Methanol extract of G. mangostana pericarp decreased the proliferation of murine colon adenocarcinoma cells (NL-17) (IC50 = 17 µg/mL) after 24 hr of treatment compared to non-treated cells using 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-1) assay [ 17 ].
Methanol extract of G. mangostana pericarp decreased the proliferation of NL-17 (IC50 = 84 µg/mL) after 24 hr of treatment compared to non-treated cells using lactate dehydrogenase leakage (LDH) assay [ 17 ].
Antidiabetic activity
Ethanol extract of G. mangostana pericarp (200 mg/kg body weight) was administered orally as a single dose to male Sprague–Dawley rats (weighed 150 – 250 g; aged 16 – 19 weeks) and observed for duration of 14 days. The blood glucose levels of normoglycaemic rats were significantly (p < 0.05) reduced at two- and eight-hour of pre-treatment in extract-treated group (≈ 107.5 mg/dL and ≈ 100.0 mg/dL) compared to vehicle-control group (≈ 120.0 mg/dL and ≈ 115.0 mg/dL). Whilst the blood glucose levels of streptozocin-induced diabetic rats were significantly (p < 0.05) reduced at four- and eight-hour of pre-treatment in extract-treated group (≈ 330.0 mg/dL and ≈ 320.0 mg/dL) compared to vehicle-control diabetic group (≈ 337.5 mg/dL and ≈ 335.0 mg/dL) [ 18 ].
Ethanol extract of G. mangostana pericarp (50 – 200 mg/kg body weight) was administered orally once daily to male streptozocin-induced Sprague–Dawley rats (weighed 150 – 250 g; aged 16 – 19 weeks) for a duration of 28 days. The blood glucose levels of the rats were significantly (p < 0.05) reduced on 14-day to 28-day of pre-treatment in extract-treated group (≈ 300 – 350 mg/dL) compared to vehicle-control diabetic group (≈ 500 mg/dL). However, the body weights of the diabetic rats were significantly (p < 0.05) increased on 14-day to 28-day of pre-treatment in extract-treated group (≈ 250 – 262 g) compared to vehicle-control diabetic group (≈ 200 g) [ 18 ].
Antitumour activity
Methanol extract of G. mangostana rind (200 mg/kg) was administered intraperitoneally once daily to NL-17 cells-implanted female BalB/c mice (18 – 20 g body weight, six-week old) for 14 days. The extract inhibited the growth of NL-17 cells in the mice (73.0% antitumour activity; 47.4 ± 5.6 median survival days; 97.5% increase in life span) compared to 5-fluorouracil-control group (60.3%; 31.8 ± 4.4 days; 32.5%). The same extract of 500 mg/kg was administered intraperitoneally once daily to female BalB/c mice (18-20 g body weight, six-week old) for a duration of 14 days. The levels of serum glutamic oxaloacetic transaminase (SGOT = 87.6 ± 6.1 IU/L), serum glutamic pyruvic transaminase (SGPT = 32.9 ± 0.2 IU/L) and blood urea nitrate (BUN = 25.5 ± 1.1 mg/dL) significantly increased compared to non-treated group (SGOT = 50.0 ± 5.7 IU/L; SGPT = 19.2 ± 0.1 IU/L; BUN = 20.1 ± 0.0 mg/dL) [ 19 ].
Ethanol (70%) extract of G. mangostana dried rind (3.125 – 25 mg/kg) showed cytotoxicity activity against human acute promyelotic (89.7 – 109.1% mortality), human chronic myelogenous (46.23 – 93.14% mortality) compared to normal lymphocyte cells (16.17 – 44.86%) [ 17 ].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute toxicity
Methanol extract of G. mangostana rind (50 – 2000 mg/kg) was administered intraperitoneally as a single dose to female BalB/c mice (18 – 20 g body weight, six-week old) and observed for 14 hr. The median lethal dose (LD50) and approximate lethal dose (ALD) were found at 1000 mg/kg. However, transient hypoactivity, loss of appetite and piloerection were observed at 100 and 250 mg/kg dose [ 17 ].
Ethanol extract of G. mangostana pericarp (2000 mg/kg body weight) administered orally as a single dose to male Sprague–Dawley rats (weighed 150 – 250 g; aged 16 – 19 weeks) and observed for 14 days showed no toxic effect [ 18 ].
Ethyl acetate fraction of ethanol (75%) extract of G. mangostana dried pericarp (8 and 18 mg/kg) was administered orally as a single dose to female Sprague-dawlay rats. The toxicity effect was observed for 14 days and showed no toxic effect. The LD50 was found at 15,480 mg/kg [ 20 ].
Methanolic extract of G. mangostana rind (1 – 3 g/kg) administered orally as a single dose to specific-pathogen-free bred Wistar rats (6-week old, 175 g body weight) and observed 14 days showed no toxic effect. The brain weight for 1 and 2 g/kg extract-treated group was significantly (p < 0.05) differently (0.65 ± 0.04 g and 0.69 ± 0.03 g) from distilled-water control group (0.79 ± 0.06 g). There was a significant (p < 0.05) increased in packed cell volume (PCV; 45 ± 1.1% ), white blood cells (WBC; 8633 ± 513.3 cells/cu.mm; 46 ± 4.1%), platelet count (2.1 x 105 ± 0.14 cells/cu.mm), mean corpuscular haemoglobin (84.6 ± 5.6 FI/red cell) and mean corpuscular haemoglobin concentration (30.15 ± 2.3 pg/red cell) compared to distilled-water control group (42.11 ± 1.05%; 7375 ± 440.17 cells/cu.mm; 39.08 ± 1.42%; 1.52 x 105 ± 0.05 cells/cu.mm; 78.5 ± 2.6 FI/red cell; 27.3 ± 1.6 pg/red cell) [ 21].
Oral single dose acute toxicity study on female Sprague Dawley rats ( aged between 8 and 12 weeks old) using aqueous extract of G. mangostana rinds showed no toxic effect on the parameters observed, including behaviors, body weight, food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. Approximate lethal dose (LD50) is 2,000 mg/kg body weight [ 22 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The Plant List. [Internet] Garcinia mangostana L; 2012. (cited on 10th April 2017). Available from http://www.theplantlist.org/tpl1.1/record/kew-2816978?ref=tpl1.
- Philippine Medicinal Plants. [Internet] Garcinia mangostana Linn; (cited on 10th April 2017) . Available from http://www.stuartxchange.com/Mangosteen.html.
- Upaganlawar AB, Badole SL. Mangosteen (Garcinia mangostana Linn.): Role in Prevention of Skin Disorders. Bioactive Dietary Factors and Plant Extracts in Dermatology: Springer. 2013;451-462.
- Osman MB, Milan AR. Fruits for the future: Mangosteen Garcinia mangostana: Crops for the Future; 2006.
- Zarena A, Sankar KU. Phenolic acids, flavonoid profile and antioxidant activity in mangosteen (Garcinia Mangostana L.) pericarp. Journal of Food Biochemistry. 2012;36(5):627-33.
- Zadernowski R, Czaplicki S, Naczk M. Phenolic acid profiles of mangosteen fruits (Garcinia mangostana). Food Chemistry. 2009;112(3):685-9.
- Pradeep Kumar SV, Puranik SB, Nandini BN. Evaluation of alpha-mangostin, isolated and purified from the crude extract of Garcinia mangostana for the anti-diabetic, anti-inflammatory and antioxidant activity. International Journal of Pharmacy and Pharmaceutical Research. 2017;8(2):75-95.
- Hemshekhar M, Sunitha K, Santhosh MS, Devaraja S, Kemparaju K, Vishwanath B, et al. An overview on genus Garcinia: phytochemical and therapeutical aspects. Phytochemistry Reviews. 2011;10(3):325-51.
- Hiranrangsee L, Kumaree KK, Sadiq MB, Anal AK. Extraction of anthocyanins from pericarp and lipids from seeds of mangosteen (Garcinia mangostana L.) by Ultrasound-assisted extraction (UAE) and evaluation of pericarp extract enriched functional ice-cream. Journal of Food Science and Technology. 2016;53(10):3806-13.
- Burkill IH. A Dictionary of the Economics Products of the Malay Peninsular. 2nd Ed. Kuala Lumpur: Ministry of Agriculture and Cooperative. 1966.
- Yaacob O, Tindall HD. Mangosteen cultivation: Food and Agriculture Organization; 1995.
- Macmillan HF. Tropical Planting and Gardening with Sepcial Reference to Ceylon: Asian Educational Services; 1935.
- Geetha R, Roy A, Lakshmi T. Evaluation of antibacterial activity of fruit rind extract of Garcinia mangostana Linn on enteric pathogens–an in vitro study. Asian Journal of Pharmaceutical and Clinical Research. 2011;4:115-8.
- Kosem N, Han Y-H, Moongkarndi P. Antioxidant and cytoprotective activities of methanolic extract from Garcinia mangostana hulls. Science Asia. 2007;33(1):283-92.
- Ngawhirunpat T, Opanasopi P, Sukma M, Sittisombut C, Kat A, Adachi I. Antioxidant, free radical-scavenging activity and cytotoxicity of different solvent extracts and their phenolic constituents from the fruit hull of mangosteen (Garcinia mangostana). Pharmaceutical Biology. 2010;48(1):55-62.
- Moongkarndi P, Kosem N, Kaslungka S, Luanratana O, Pongpan N, Neungton N. Antiproliferation, antioxidation and induction of apoptosis by Garcinia mangostana (mangosteen) on SKBR3 human breast cancer cell line. Journal of Ethnopharmacology. 2004;90(1):161-6.
- Kosem N, Ichikawa K, Utsumi H, Moongkarndi P. In vivo toxicity and antitumor activity of mangosteen extract. Journal of Natural Medicines. 2013;67(2):255-63.
- Taher M, Zakaria TMFST, Susanti D, Zakaria ZA. Hypoglycaemic activity of ethanolic extract of Garcinia mangostana Linn. in normoglycaemic and streptozotocin-induced diabetic rats. BMC Complementary and Alternative Medicine. 2016;16(1):135.
- Novilla A, Djamhuri DS, Fauziah N, Maesaroh M, Balqis B, Widowati W. Cytotoxic activity of mangosteen (Garcinia mangostana L.) peel extract and α-mangostin toward leukemia cell lines (HL-60 and K-562). Journal of Natural Remedies. 2016;16(2):52-9.
- Rahmayanti F, Suniarti DF, Mas`ud ZA, Bachtiar BM, Wimardhani YS, Subita GP. Acute oral toxicity testing of ethyl acetate fraction from Garcinia mangostana Linn extract in sprague-Dawley rats. Research Journal of Medicinal plant. 2016;10(3):261-4.
- Vishnu Priya V, Jainu M, Mohan SK, Karthik B, Saraswathi P, Chandra Sada G. Toxicity study of Garcinia mangostana Linn. pericarp extract in rats. Asian Journal of Experimental Biological Sciences. 2010;1(3):633-7.
- Norzahirah A, Umi Rubiah SZ, Bazilah J, Nurul Syahira S, Dalnitha Lea N, Tavinraj R, Teh BP. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2019. report no.: NON-GLP/2019/12/01.







