Petai Empty pod
Parkia speciosa Hassk.
Fabaceae








Figure 1: Parkia speciosa. (a) Mature bisexual, small and orange flowers in densely crowded light bulb-shaped head; (b) immature, small and creamy green flowers in densely crowded light bulb-shaped heads dangling from the main stalk; (c) newly developing young pods dangling in small bundles; (d) mature pods on tree; (e) harvested mature pods bundled in the heads; (f) empty pods without seeds; (g) seeds; (h) empty pods powder 0.355 mm. (Photos courtesy of Hawa ZE Jaafar, UPM, 2022).
DEFINITION
Petai empty pod consists of the powder of dried fruits pericarp of Parkia speciosa Hassk. (Fabaceae).
SYNONYM
Parkia macropoda Miq., Parkia harbesonii Elmer, Inga pyriformis Jungh.L., Mimosa pedunculata Hunter [1].
VERNACULAR NAMES
Bitter bean, twisted cluster bean, stink bean (English); petai, petah, patai, patag, nyiring (Malay); chou dou (Chinese); yongchak (Indian) [2, 3, 4].
CHARACTER
| Colour | Brownish colour |
| Odour | Very strong foul smell |
| Taste | Garlic flavour |
IDENTIFICATION
Plant morphology
P. speciosa is a perennial tree up to 15–40 m in height and 50–100 cm in diameter of bole; branchlets are hairy. Leaves bipinnate on 2–6 cm long stalks with gland 7–15 mm above stalk base; pinnae 10–19 pairs, 5–9 cm long, each with 31–38 pairs of opposite linear leaflets, 5–9 mm long and about 2 mm wide, with rounded tip and small pointed lobe or ear at base. Flowers bisexual, small and creamy white, found in densely crowded heads; the heads are stalked, 5.1–8.9 cm long. Pods large, 35–55 cm long and 3–5 cm wide, straight, or more commonly twisted; dangling in small bundles, green becoming black; each pod contains 10–18 large, light to dark green seeds; valves swollen over seeds. Seeds elliptic, green in colour, up to 2-3 cm across; testa is soft [3].
Microscopy
Powdered empty pod consists of the fragments of abaxial epidermal cells with straight to slightly curved anticlinal walls and staurocytic stomata; parenchyma and collenchyma cells; fibre fragments; abundant of calcium oxalate crystals, solitary (prism) and druse form. Other features observed include the presence of brachysclereids cells; oil globules; spiral, pitted, and annular vessels.


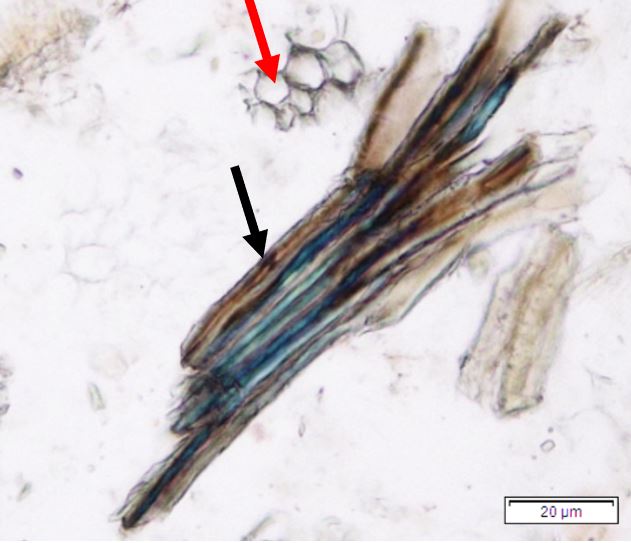
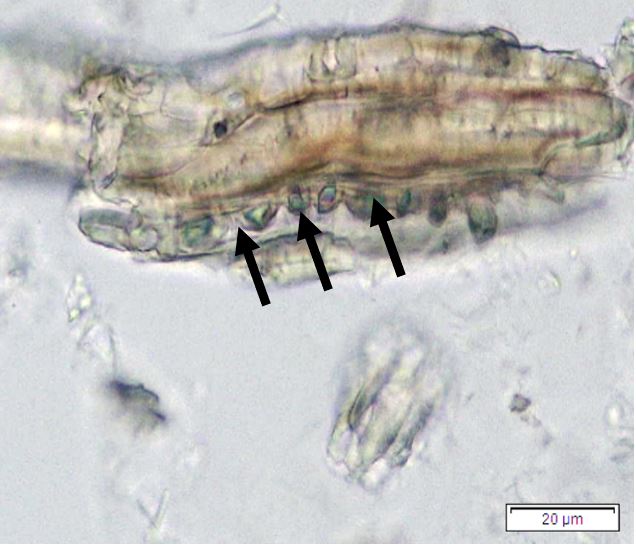
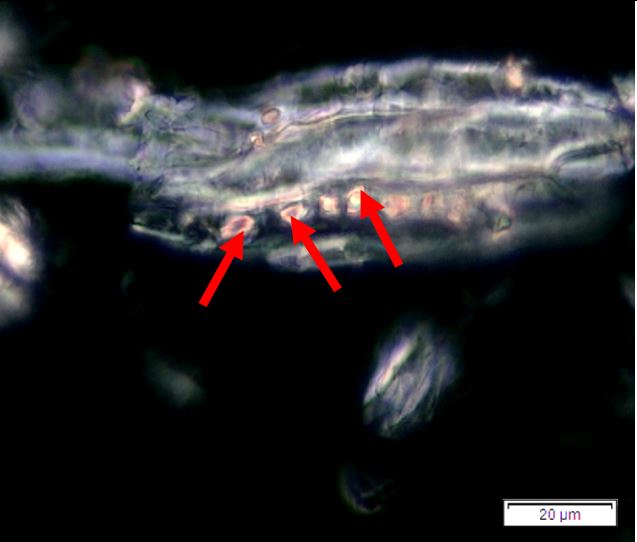
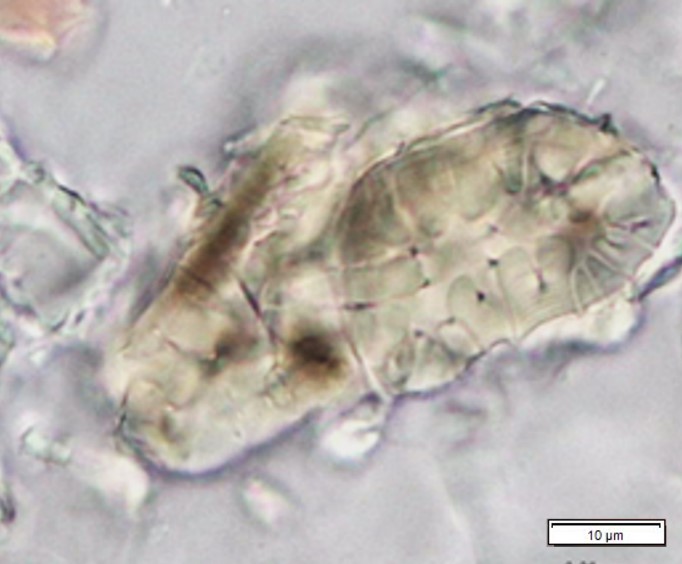
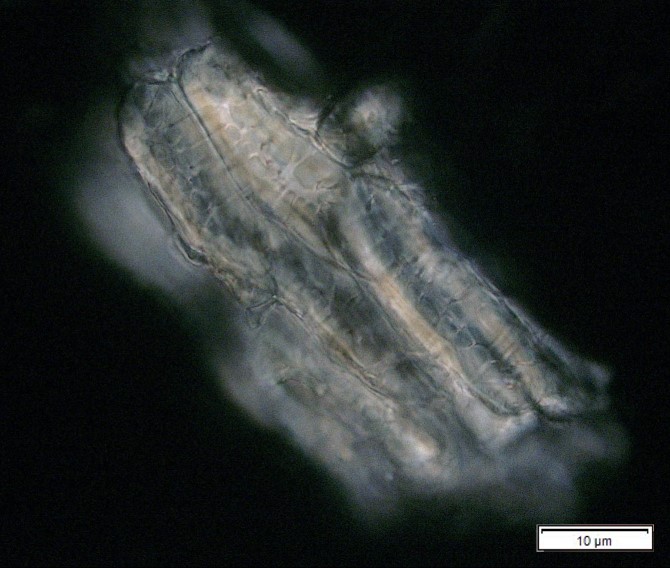


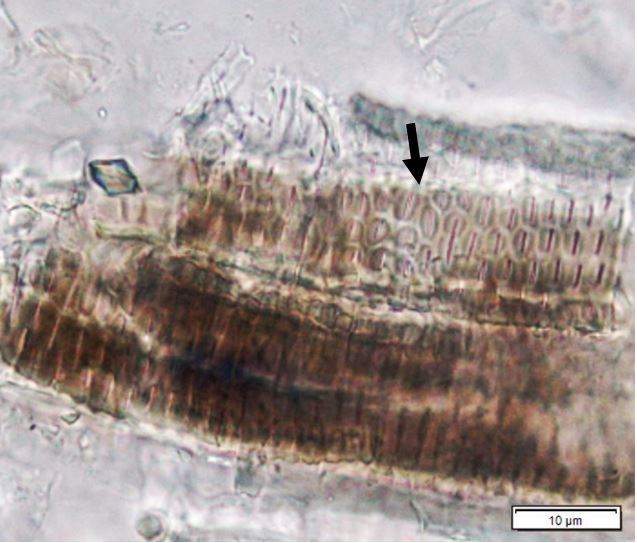


Figure 2: Microscopic characters of P. speciosa empty pods powder of 0.355 mm size. (a) Abaxial epidermal cells (lower surface) with straight to curve anticlinal walled and staurocytic stomata cells (arrow) (magnification 40x); (b) Collenchyma cells (magnification 40x); (c) fibres (black arrow) and collenchyma cells (red arrow) (magnification 20x); (d) Calcium oxalate crystals (arrow) (magnification 20x); (e) Calcium oxalate crystals under polarized filter (arrow) (magnification 20x); (f) Brachysclereids cells (magnification 40x); (g) Brachysclereids cells under polarized filter (magnification 40x); (h) Oil globules observed with Sudan red (III) (arrow) (magnification 20x); (i) Spiral vessels (red arrow) and reticulate (black arrow) (magnification 40x); (j) Pitted vessels (arrow) (magnification 40x); (k) annular vessels (magnification 40x). [Scale bars: a = 10 µm; b = 10 µm; c = 20 µm; d = 20 µm; e = 20 µm; f = 10 µm; g = 10 µm; h = 20 µm; i = 10 µm; j = 10 µm; k = 10 µm].
Colour tests
Observation of solution after treatment with various reagents:
| Test for the presence of phenolic | bluish green colour |
| Test for the presence of flavonoid | yellow colour |
| Test for the presence of saponin | presence of foam |
High Performance Thin Layer Chromatography (HPTLC)
| Test Solution | Weigh about 5.0 g of P. speciosa dried pod powder of 0.355 mm size in a conical flask. For aqueous extraction, add 50 mL of distilled water into dried pod powder and shake gently manually. Boil the solution until it reduces to half of the initial volume. Allow the mixture to cool down under room temperature. Filter the solution and freeze dry the filtrate. Dissolve the freeze-dried aqueous crude extract in methanol and use as the test solution. |
| Standard solution | Dissolve gallic acid standard [CAS no.: 149-91-7] in methanol to produce a standard concentration of 0.10 mg/mL solution. |
| Stationary Phase | HPTLC glass plate silica Gel 60 F254 10 cm X 10 cm, Merck. |
| Mobile phase | Toluene: ethyl acetate: methanol: phosphoric acid (1.5 : 1.5 : 0.4 : 0.1) (v/v/v/v) |
| Application |
|
| Development distance | 80 mm |
| Drying | Air drying |
| Detection |
|
(a)
(b)
(c)
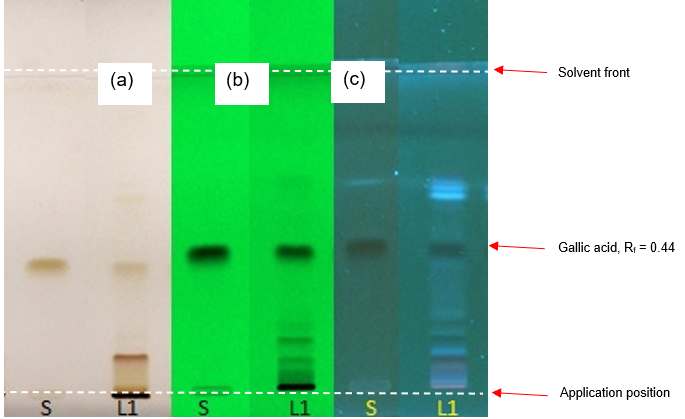
Figure 3: HPTLC chromatogram of gallic acid (S), aqueous extract of Parkia speciosa dried empty pods powder observed under (a) visible light after derivatisation, (b) UV at 254 nm before derivatisation, and (c) UV at 366 nm after derivatisation.
High Performance Liquid Chromatography (HPLC)
| Test solution |
Weigh about 5.0 g of P. speciosa dried pod powder 0.355 mm size in a conical flask. For aqueous extraction, add 50 mL of distilled water and shake gently manually. Boil the solution until it reduces to half of the initial volume. Allow the mixture to cool down at room temperature. Filter the solution and freeze dry the filtrate. Dissolve the freeze-dried aqueous crude in methanol and filter through a 0.2 µm syringe filter. Inject the filtrate into the HPLC column. |
||||||||||||||||||
| Standard solution |
Dissolve gallic acid standard [CAS no.: 149-91-7] in methanol to produce a standard concentration of 1.0 mg/mL solution. |
||||||||||||||||||
| Chromatographic system |
Detector: UV 280 nm Column: C18 (5.0 µm, 4.6 mm I.D. x 250 mm) Column oven temperature: 40°C Flow rate: 1.0 mL/min Injection volume: 10 µL |
||||||||||||||||||
| Mobile phase (gradient mode) |
|
||||||||||||||||||
| System suitability requirements |
Perform at least five replicates of injections of gallic acid (0.1 mg/mL). The requirements of the system suitability parameters are as follow:
|
||||||||||||||||||
| Acceptance criteria |
|
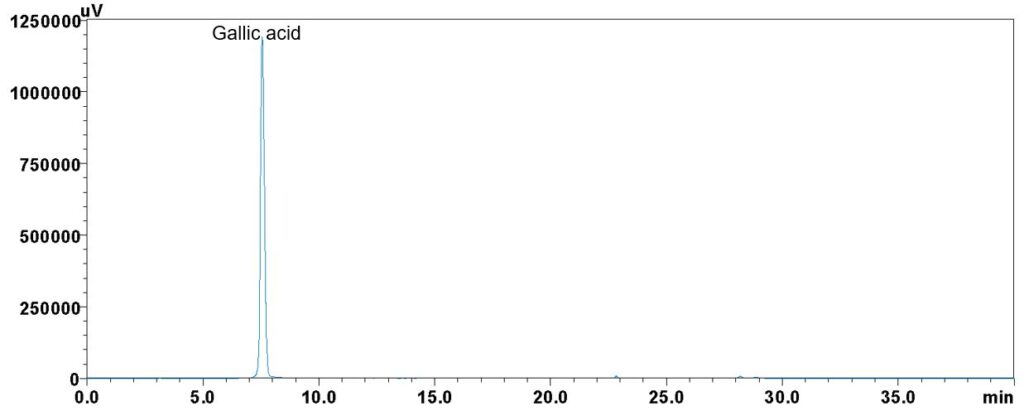

Figure 4: Whole HPLC chromatogram of (a) gallic acid standard solution (0.1 mg/mL) at peak tr = 7.55 min, and (b) aqueous extract of Parkia speciosa dried empty pods powder showing peaks corresponding to gallic acid standard solution at tr = 7.55 min.


Figure 5: HPLC chromatogram highlighting the elution region of (a) gallic acid standard solution (1.0 mg/mL) and (b) aqueous extract of Parkia speciosa dried empty pods powder showing peaks corresponding to gallic acid standard solution at tr = 7.55 min.

Figure 6: UV spectrum of gallic acid standard solution (1.0 mg/mL) and aqueous extract of Parkia speciosa dried empty pods powder.
PURITY TEST
The purity tests, except foreign matter test, are based on P. speciosa dried pods powder of 0.355 mm particle size.
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 6% |
| Acid-insoluble ash | Not more than 0.5% |
| Water soluble ash | Not less than 2% |
| Loss on Drying |
| Not more than 11 % |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 31 % |
| Cold method | Not less than 18 % |
| Ethanol-soluble extracts | |
| Hot method | Not less than 31% |
| Cold method | Not less than 19 % |
SAFETY TEST
The safety tests are based on P. speciosa dried empty pod powder of 0.355mm particle size.
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total aerobic microbial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Methanolic extract of P. speciosa empty pods was found to contain phenolics (malvidin, primulin, caftaric acid, coutaric acid, ellagic acid, and gallic acid), flavonoids (apigenin, catechin, didymin, epigallocatechin gallate, epicatechin, myricetin, nobiletin, rutin, tangeritin, quercetin, and theaflavin gallate), and tannin (punicalin) [5, 6].
Ethanol extract of P. speciosa empty pods was found to contain phenolics (gallic acid, curcumin monoglucoside, and eucommin A), flavonoids (herbacetin, gallocatechin, epigallocatechin, epigallocatechin gallate isomers, gossypetin 8-glucoside, gossypetin 8-rhamnoside isomers, myricitrin, 6-C-galactosylluteolin isomers, theaflavin-3-gallate isomers, and theasinensin A), O-glucuronide (2-phenylethanol glucuronide), glycoside (tremulacin), and benzoquinone (embelin) [7].
Ethyl acetate fraction from ethanolic extraction of P. speciosa empty pods was reported to contain flavonoid which is quercetin [8].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally, the pods of P. speciosa are consumed raw for kidney refining, detoxification, and regulating blood glucose [9,10].
The pods of P. speciosa are also consumed traditionally to control high blood pressure [10].
Biological and pharmacological activities supported by experimental data
Antimicrobial activity
Ethyl acetate (50%) extract of P. speciosa dried peel powder (50 μL) inhibited the growth of Gram-positive bacteria, Staphylococcus aureus (inhibition zone: 6.00 mm + 0.70 mm; 404.51%) compared to streptomycin (10 mg/mL; inhibition zone: 5.20 mm) using agar diffusion well [11].
Ethyl acetate (50%) extract of P. speciosa dried peel powder (50 μL) inhibited the growth of Gram-negative bacteria, Escherichia coli (inhibition zone: 7.85 mm + 0.95 mm; 279.12%) compared to streptomycin (10 mg/mL; inhibition zone: 5.78 mm) using agar diffusion well [11].
Antioxidant activity
Ethanolic (50%) extract of P. speciosa dried peel powder showed antioxidant activity with IC50 of 10.03 μg/mL compared to quercetin (IC50 = 0.45 μg/mL), and to butylated hydroxytoluene, BHT (IC50 = 3.47 μg/mL) using free radical scavenging assay, DPPH [12].
Ethanolic extract of P. speciosa pericarp (0.1 g/mL) showed antioxidant activity with IC50 of 46.29 mg/mL, respectively, compared to standard tannin (IC50 = 0.91 mg/mL) using the Heinz body induction in vitro model [13].
Antidiabetic activity
Aqueous extract of P. speciosa dried empty pods powder (1 mg/mL) demonstrated α-glucosidase inhibitory activity at 61.87+3.85% with IC50 of 0.41 mg/mL [14].
Ethanol (100%) extracts of P. speciosa dried empty pods powder (0.2 – 2.0 mg/mL) was found to inhibit (p < 0.05) α-glucosidase activity with IC50 ranged between 0.443 + 0.036 to 0.707 + 0.016 µg/mL compared to quercetin (IC50 = 3.550 + 0.130 μg/mL) [15].
A fraction of the chloroform extract (stigmast-4-en-3-one, 200 mg) of P. speciosa dried empty pods powder (100 mg/kg BW) was administered orally to 48-h-fasted diabetic Sprague Dawley rats (diabetic-induced with intravenous injection of 60 mg/kg alloxan). The administration of the fraction significantly (p < 0.0001) decreased blood glucose in diabetic rats 2 hours after ingestion from 400 mg/dL (22 mmol/L) to 170 mg/dL (9.4 mmol/L) and lasted for 24 hours compared to glibenclamide (5 mg/kg BW) that lowered blood glucose level from 400 mg/dL (22 mmol/L) to 125 mg/dL (6.9 mmol/L) [16].
Antihypertensive activity
Methanol extract of P. speciosa empty pods (800 mg/kg) and nicardipine (3 mg/kg) were administered orally to hypertension-induced Sprague Dawley rats (using N(G)-nitro-L-arginine methyl ester (L-NAME) (25mg/kg, intraperitoneally)) and both showed similar findings in which the blood pressures were prevented significantly (p<0.05) from elevations (systolic 120 mmHg at week 0 to 110 mmHg at week 8) [6].
Anti-inflammatory activity
Ethyl acetate extract of P. speciosa (500 ng/mL) significantly (p<0.05) decreased inflammatory mediators (TNF-kB P65, COX-2, and VCAM-1) which is comparable anti-inflammatory effects to quercetin (1000 µM) although quercetin was more effective in reducing iNOS activity in TNF-α induced H9c2 Cardiomyocytes [17].
Ethyl acetate extract of P. speciosa dried empty pods powder (25 µg/mL) was administered in an in vitro model of human umbilical vein endothelial cells (HUVECs) exposed to tumour necrosis factor-α (TNF-α, 10 ng/mL). The treatment significantly exhibited anti-inflammatory properties of P. speciosa empty pods with reduced iNOS activity (2.68 + 0.02 ng/mL), NO level (10.24 + 0.25 µM) and ROS level (120.0 + 5.4% of control) compared to positive control quercetin (125 µg/mL) with respective values of 2.24 + 0.30 ng/mL, 6.83 + 0.20 µM, and 97.7 + 6.9% [8].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
7 days acute toxicity
Ethanol extract of P. speciosa pod (5000 mg/kg) administered orally as a single dose to Wistar rat and observed for 7 days showed no mortality and toxic symptoms with LD50 value of > 5000 mg [18].
Others (Adverse reactions, contraindications, side effects, warning, precautions)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Temperature of storage: Store below 30 °C
Protect from light and moisture.
REFERENCES
- Plants of the World Online. [Internet] Parkia speciosa Hassk [Retrieved on 30th October 2023]. Available from https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:512231-1#synonyms.
- Musa T, Azimah AK, Zaharah H, editors. Tumbuhan Ubatan Popular Malaysia. Edisi 3. Selangor: MARDI; 2011; p.212.
- Orwa C, Mutua A, Kindt R, Jamnadass R, Simons A. [Internet] Agroforestree Database: a tree reference and selection guide. Version 4.0, 2009 (Retrieved on 30th October 2023). Available from https://www.worldagroforestry.org/publication/agroforestree-database-tree-reference-and-selection-guide-version-40.
- Nurul Izzah A, Aminah A, Md Pauzi A, Lee YH, Wan Rozita WM, Siti Fatimah D. Patterns of fruits and vegetable consumption among adults of different ethnics in Selangor, Malaysia. International Food Research Journal. 2012;19(3);1095-1107.
- Kamisah Y, Othman F, Qodriyah HMS, Jaarin K. Parkia speciose Hassk.: A Potential Phytomedicine. Evidence-Based Complement Altern Med. 2013; 2013:1-9.
- Kamisah Y, Zuhair JSF, Juliana AH, Jaarin J. Parkia speciosa empty pod prevents hypertension and cardiac damage in rats given N(G)-nitro-l-arginine methyl ester. Biomedicine & Pharmacotherapy. 2017; 96: 291-298.
- Saleh, M.S.M.; Jalil, J.; Zainalabidin, S.; Asmadi, A.Y.; Mustafa, N.H.; Kamisah, Y. Genus Parkia: Phytochemical, medicinal Uses, and pharmacological properties.Int. J. Mol. Sci. Rev.2021,22, 618.
- Mustafa NH, Ugusman A, Jalil J, Kamiah Y. Anti-inflammatory property of Parkia speciosa empty pod extract in human umbilical vein endothelial cells. Journal of Applied Pharmaceutical Science. 2018; 8(1): 152-158.
- Ismail, NA, Sabran, SF, Mohamed, M, Abu Bakar MF. Ethnomedicinal Knowledge of Plants Used for Healthcare by the Javanese-Malay Community in Parit Jelutong, Batu Pahat, Johor, Malaysia. AIP Conference Proceedings. 2018; 2002.
- Azliza, MA, Ong, HC, Vikineswary, S, Noorlidah, A, Haron, NW. Ethno-Medicinal Resources used by the Temuan in Ulu Kuang Village. Studies on Ethno-Medicine. 2012; 6: 17–22.
- Hasim DN, Faridah, Kurniawati DA. Antibacterial activity of Parkia speciosa Hassk peel to Escherichia coli and Staphylococcus aureus bacteria. Journal of Chemical and Pharmaceutical Research. 2015; 7(4):239-243.
- RamLi S, Bunrathep S, Tansaringkarn T, Ruangrungsi N. Screening for free radical scavenging activity from ethanolic extract of mimosaceous plants endemic to Thailand. Journal of Health Research. 2008;22(2):55–59.
- Tunsaringkarn T, Soogarun S, Rungsiyothin A, Palasuwan A. Inhibitory activity of Heinz body induction in vitro antioxidant model and tannin concentration of Thai mimosaceous plant extracts. Journal of Medicinal Plants Research. 2012;6(24):4096–4101.
- Tunsaringkarn T, Rungsiyothin A, Ruangrungsi N. α-Glucosidase inhibitory activity of water-soluble extract from Thai mimosaceous plants. The Public Health Journal of Burapha University. 2009;4(2):54–63.
- Saleh MSM, Jalil J, Mustafa NH RamLi FF, Asmadi AY, Kamisah Y. UPLC-MS-based metabolomics profiling for α-glucosidase inhibiting property of Parkia speciosa pods. Life 2021; 11, 78-90.
- Jamaluddin F, Mohamed S, Lajis MN. Hypoglycaemic effect of Stigmast-4-en-3-one, from Parkia speciosa empty pods. Food Chemistry. 1995; 54:9-13.
- Gui JS, Jalil J, Jubri Z, Kamisah Y. Parkia speciosa empty pod extract exerts anti-inflammatory properties by modulating NFjB and MAPK pathways in cardiomyocytes exposed to tumor necrosis factor-α. Cytotechnology. 2019;71:79–89.
- Fitrya F, Elfita E, Amriani A, Sahara D, Prasetyo PA. Toxicological assessment of Parkia Speciosa pod ethanol extract: The Acute and Sub-Chronic Oral Toxicity Test on Experimental Animal. International Journal of Pharmaceutical Research. 2021; 13(3): 130-136.


