Halia hitam Rhizome
Kaempferia parviflora Wallich. ex Baker
Zingiberaceae







Figure 1 : K. parviflora (a)whole plant; (b) leaves, (c) pseudostem, (d) flower, (e) field plantation, (f) rhizome with dark purple inside and (g) rhizome (Photos courtesy of MARDI, 2023).
DEFINITION
Halia hitam rhizome consist of the powder of dried rhizomes of Kaempferia parviflora (Zingiberaceae).
SYNONYM
Kaempferia rubromarginata (S.Q.Tong) R.J.Searle; Stahlianthus rubromarginatus S.Q.Tong [1]
VERNACULAR NAMES
Black ginger, Thai black ginger, Thai ginseng (English); kunyit hitam Thai (Malay), xiǎo huā shān nài (Chinese) and kalahalood (Tamil) [2].
CHARACTER
| Colour | Purple |
| Odour | Pungent, smells like spicy camphor |
| Taste | Bitter taste and spicy |
IDENTIFICATION
Plant morphology
K. parviflora is an herbaceous plant. Pseudostem erect and elongate from the ground, tall up to 25 cm. Rhizome dark blue or dark purple inside, bearing several roots in a fascicle. Bladeless sheaths 2, up to 1.5 cm long, glabrous, reddish. Leaf 4–6, leaf sheath up to 4–7 cm long, glabrous, green-reddish, petiole up to 5 cm long with glabrous, green and reddish, ligule inconspicuous, blade horizontal near the ground, elliptic, 10–12 × 9–10 cm, both surface glabrous and upper surface green and lower surface pale green, margin undulate, apex acute. Inflorescence exerted from the leaf sheath, peduncle ca. 9 cm long; enclosed by leaf-sheath, white with light pale green at the tip, glabrous. Bract one per flower, lanceolate, the outermost 2.0–2.2 × 1.0–1.5 cm, inner ones lanceolate, smaller than the outer ones, both surfaces glabrous, white towards the base, greenish towards the apex. Bracteoles folded, lanceolate, ca. 1.8 × 1.2 cm, glabrous, translucent white. Flowers 7–10, exerted from bracts. Calyx tubular, 1.0–1.5 cm long, glabrous, translucent white, unilaterally slit at apex, slit ca. 0.7 cm long. Corolla tube 1.5–1.8 cm long, glabrous, white, dorsal corolla lobe lanceolate, 2.7–3.0 × 0.5–0.6 cm, mucronate at apex, hooded, translucent white, lateral corolla lobes linear, 2.5–2.6 × 0.4–0.5 cm, glabrous, translucent white. Labellum longer than broad, 3.5–3.7 × 3.0–3.2 cm, bilobed, lobes overlapping, each lobe obovate, apex obtuse to rounded, whitish or pale purple with dark purple patches towards the base and pure white spot at base, further inside purple with sparsely white spots. Lateral staminodes obovoid, 3.0–3.2 × 1.0–1.2 cm, white. Anther thecae ca. 5 mm long, parallel, white, anther-crest ca. 8×8 mm, deeply bilobed, white. Stigma was subglobose with lateral ciliated. Ovary cylindrical, ca. 7 × 3–4 mm, glabrous, creamy white, 3-locule, placentation axile, ovules many, styles 2, filiform, ca. 5 mm long [3,4].
Microscopy
Powdered material consists of K. parviflora rhizome varies from rounded to oval-shaped grain starches; spiral, reticulate and annular vessels, phylloderm cells, prismatic crystals with rhombus shape, and also parenchyma cells [3,4].
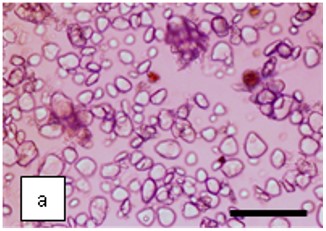
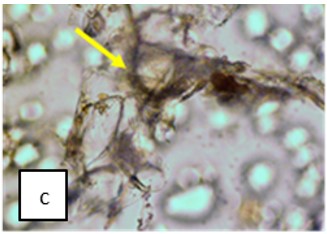
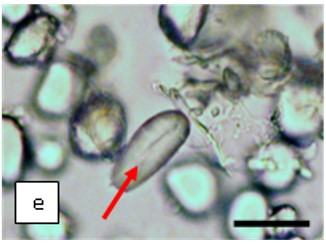
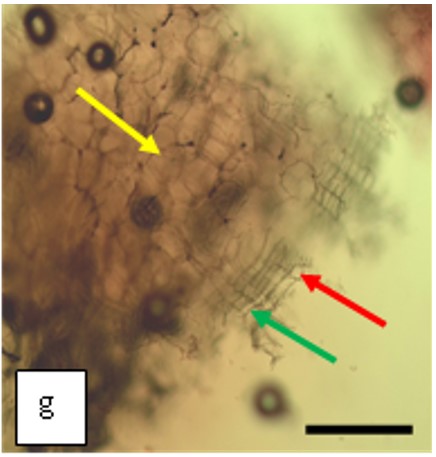
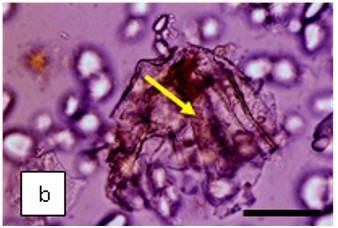
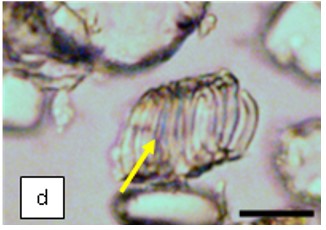
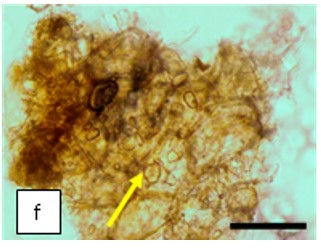
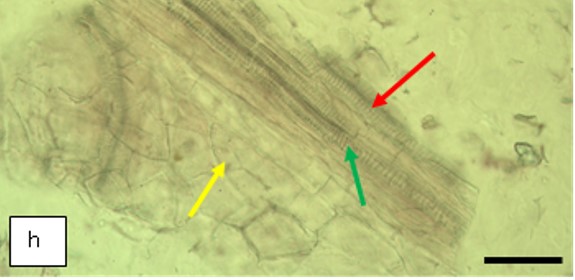
Figure 2 : Microscopic characters of K. parviflora rhizome powder of 0.355 mm size. (a) Variation of starch grains (magnification 40×); (b) vessels (yellow arrow) (magnification 40×); (c) parenchyma cells (yellow arrow) (magnification 40×); (d) spiral vessel (yellow arrow) (magnification 40×); (e) oval-shaped starch grain (red arrow) (magnification 40×); (f) parenchyma cells with starch grains (yellow arrow) (magnification 40×); (g) parenchyma (yellow arrow) and periderm (green arrow), prismatic crystal (rhombus shape) in red arrow (magnification 40×); (h) patches of reticulate vessel (red arrow), annular vessel (green arrow) and parenchyma cell (yellow arrow) [scale bars: a = 50 µm; b = 100 µm, c = 100 µm; d = 100 µm; e = 100 µm; f = 50 µm; g = 100 µm; h = 100 µm].
Chemical Tests
Observed colour of solution after treatment for the presence of:
| Test for the presence of alkaloids | Green |
| Test for the presence of streoids | Red |
| Test for the presence of phenolics | Bluish green |
| Test for the presence of flavonoids | Colourless |
Thin Layer Chromatography (TLC)
| Test Solution | Weigh about 1.0 g of K. parviflora dried rhizome powder of 0.355 mm size in a 20mL vial. Add 10 mL of methanol : water (70 : 30, v/v) and sonicate the mixture for 30 min at 40°C . Use the filtrate as test solution and dilute (10×) with methanol : water (70 : 30, v/v) before use. |
| Standard Solution | Dissolve the5,7-dimethoxyflavone standard [CAS no.: 21392-57-4] in methanol to give a standard concentration of 0.2 mg/mL. Dissolve the 5,7,4’-trimethoxyflavone standard [CAS no.: 26964-29-4] in methanol to give a standard concentration of 0.2 mg/mL. |
| Stationary Phase | HPTLC silica gel pre-coated plate 60 F254, 10 × 10 cm |
| Mobile Phase | Toluene : chloroform : acetone : acetic acid (48 : 40 : 10 : 2, v/v/v/v). |
| Application | a. 5,7-dimethoxyflavone (S1) standard solution; 1 µL, as a band b. 5,7,4’-trimethoxyflavone solution (S2) standard solution; 1 µL, as a band. c. Methanol extract of K. parviflora dried rhizome powder (L); 1 µL, as a band. |
| Development distance | 8cm |
| Drying | Air drying |
| Detection | UV at 366 nm |
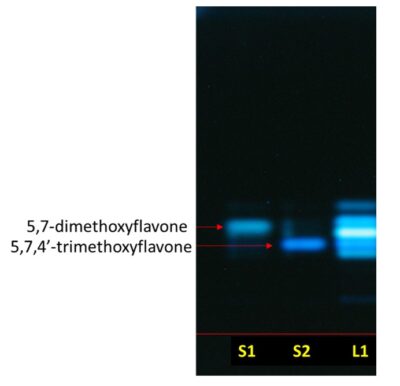
Figure 3 : TLC chromatogram of 5,7-dimethoxyflavone (S1) and 5,7,4’-trimethoxyflavone (S2) and methanol extract of K. parviflora dried rhizome powder (L1) observed under UV at 366 nm.
High Performance liquid Chromatography (HPLC)
| Test Solution | Weigh about 1.0 g of K. parviflora dried rhizome powder of 0.355 mm size in a 20-mL vial. Add 10 mL of methanol : water (70 : 30, v/v) and sonicate the mixture for 60 min at 50°C. Filter the mixture with 0.22 µm nylon membrane syringe filter into vials. Use the filtrate as test solution | |||||||||||||||||||||
| Standard solution | Dissolve the 5,7-dimethoxyflavone standard [CAS no.: 21392-57-4] and 5,7,4’-trimethoxyflavone standard [CAS no.: 26964-29-4] in methanol to give a standard concentration of 1.0mg/mL. |
|||||||||||||||||||||
| Chromatographic System | Detector: UV 310 nm Column: C18 column (2.5 µm, 3.0 mm I.D × 150 mm) Column oven temperature: 40°C Flow rate: 0.8 mL/min Injection volume: 1 µL |
|||||||||||||||||||||
| Mobile Phase (gradient mode) |
|
|||||||||||||||||||||
| System suitability requirements | Perform at least five replicate injections of the standard solutions (1 mg/mL). The requirements of the system suitability parameters are as follow:
|
|||||||||||||||||||||
| Acceptance criteria |
|
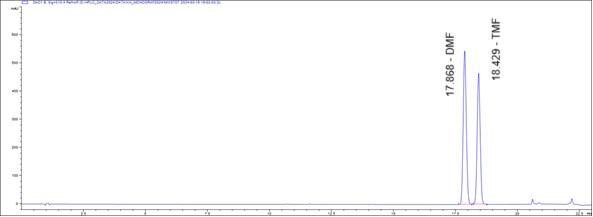
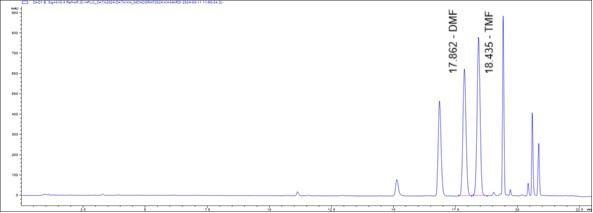
Figure 4 : Whole HPLC chromatogram of (a) 5,7-dimethoxyflavone (DMF) and 5,7,4’-trimethoxyflavone (TMF) standard solution (1 mg/mL) at tr = 17.87 and 18.43 min and (b) methanol extract of K. parviflora dried rhizome powder showing peaks corresponding to 5,7-dimethoxyflavone (DMF) at tr = 17.86 min and 5,7,4’-trimethoxyflavone (TMF) at tr = 18.44 min.
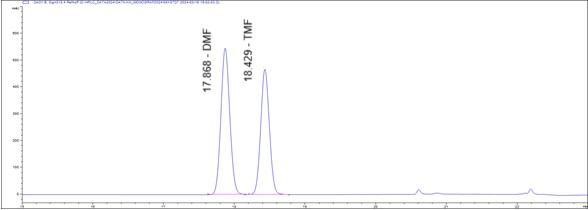
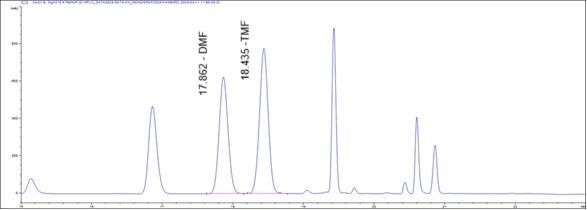
Figure 5 : HPLC chromatogram highlighting the elution region of 5,7-dimethoxyflavone (DMF) and 5,7,4’-trimethoxyflavone (TMF) in (a) standard solution (1 mg/mL) at tr = 17.87 and 18.43 min and (b) methanol extract of K. parviflora dried rhizome showing peak corresponding to 5,7-dimethoxyflavone (DMF) at tr = 17.86 min and 5,7,4’-trimethoxyflavone (TMF) standard solution at tr = 18.44 min.
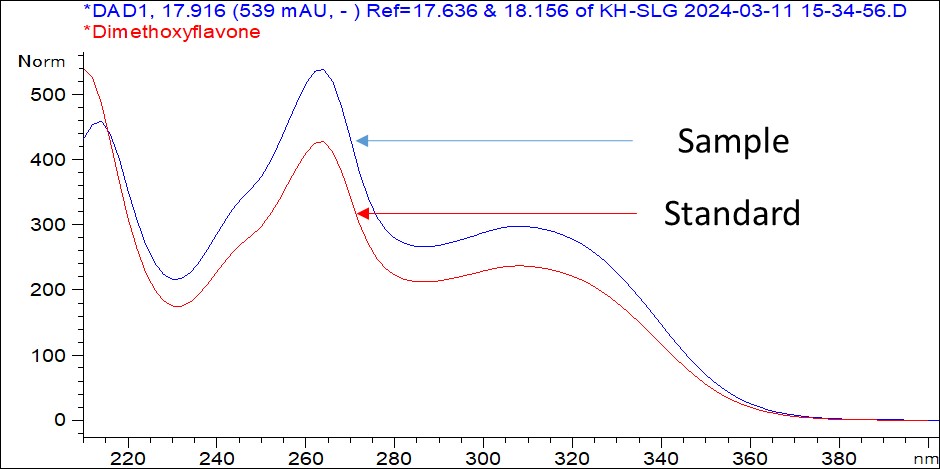
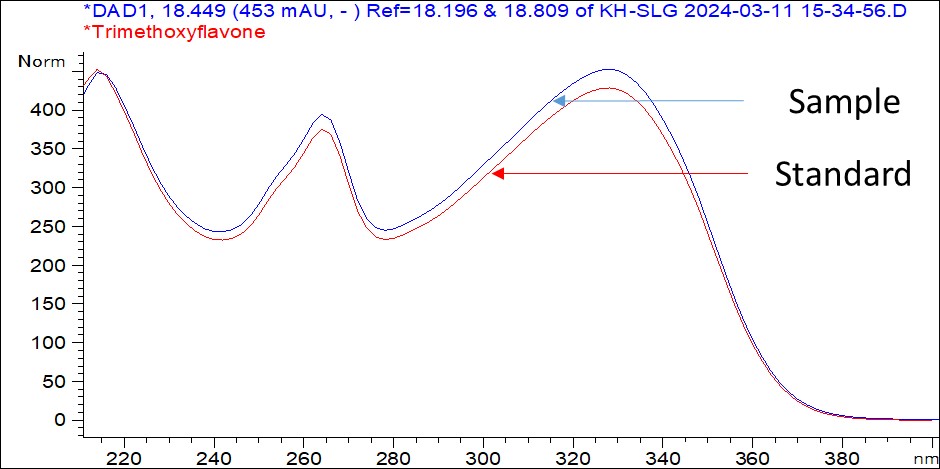
Figure 6 : UV spectrum of (a) 5,7-dimethoxyflavone and (b) 5,7,4’-trimethoxyflavone standard solution (1 mg/mL) and methanol extract of K. parviflora dried rhizome powder.
Table 1: The Relative Retention Time (RRT) of the characteristic peaks for all of the standards used
| Standard | RRT (min) |
| 5,7-dimethoxyflavone (standard) | 1 min |
| 5,7,4’-trimethoxyflavone (internal standard) | 1.03 min |
PURITY TEST
The purity tests except foreign matter test are based on K. parviflora dried rhizome powder of 0.355 mm particle size.
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 6% |
| Acid-insoluble ash | Not more than 2% |
| Loss on Drying |
| Not more than 12% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 17% |
| Cold method | Not less than 10% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 8% |
| Cold method | Not less than 5% |
SAFETY TEST
The safety tests are based on K. parviflora dried rhizome powder of 0.355 mm particle size.
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
The methanolic extracts (80%) with 5% formic acid of K. parviflora rhizome had been found to contain methoxyflavone aglycones (e.g. 6-hydroxy-7,4’-dimethoxyflavone, 5,7,3’,4’-tetramethoxyflavone; 3,5,7,3’,4’-pentamethoxyflavone; 5,7-dimethoxyflavone; 5,7,4’-trimethoxyflavone; 3,5,7-trimethoxyflavone; 3,5,7,4’-tetramethoxyflavone; 5-hydroxy-7,3’,4’-trimethoxyflavone; 5-hydroxy-3,7,3’,4’-tetramethoxyflavone; 5-hydroxy-7-methoxyflavone; 5-hydroxy-7,4’-dimethoxyflavone; 5-hydroxy-3,7-dimethoxyflavone and 5-hydroxy-3,7,4’-trimethoxyflavone) [5].
The methanolic extracts of K. parviflora rhizome had been found phenolic glycosides (e.g. kaempferiaoside A, kaempferiaoside B, tamarixetin 3-O-rutinoside, syringetin 3-O-rutinoside and 2,4-di-O-β-D-glucopyranoside) and flavonoids (e.g 5-hydroxy-3,7-dimethoxyflavone, 5-hydroxy-7-methoxyflavone, 5-hydroxy-3,7,4′-trimethoxyflavone, 5-hydroxy-7,4-dimethoxyflavone, 5,3-dihydroxy-3,7,4-trimethoxyflavone, 5,4′-dihydroxy-7-methoxyflavone, 5-hydroxy-7,3,4-trimethoxyflavone, 3,5,7,4-tetramethoxyflavone, 3,5,7-trimethoxyflavone, 5,7-dimethoxyflavone, 5,7,4-trimethoxyflavone, 5,7,3,4-tetramethoxyflavone and 3,5,7,3,4-pentamethoxyflavone and tilianine) [6].
The ethanolic extract of K. parviflora rhizome had been found flavonoids (e.g. 3,5,7-trimethoxyflavone, 3,5,7,3’,4’-pentamethoxyflavone and 5,7,4’-trimethoxyflavone) [7].
The dichloromethane extracts of K. parviflora rhizome has been found to contain polyoxygenated cyclohexanes (e.g. (-)-4-benzoyloxymethyl-3,8-dioxatricyclo[5.1.0.0]octane-5,6-diol 6-acetate and (+)-4-benzoyloxymethyl-3,8-dioxatricyclo[5.1.0.0]octane-5,6-diol 5-acetate), flavonoids (e.g. 5-hydroxy-7-methoxyflavone; 5-hydroxy-3,7-dimethoxyflavone; 5,7-dimethoxyflavone; 3,5,7-trimethoxyflavone; 5-hydroxy-7,4’-methoxyflavone; 5-hydroxy-3,7,4’-methoxyflavone; 5,7,4’-trimethoxyflavone; 3,5,7,4’-tetramethoxyflavone; 5-hydroxy-3,7,3’,4’-tetramethoxyflavone; 3,5,7,3’,4’-pentamethoxyflavone and 5,7,4’-trimethoxyflavonone) and sterol (e.g. β-sitosterol) [8].
The hexane extract K. parviflora rhizome was found to contain flavonoids (e.g. 5,7-dimethoxyflavone, 3,5,7-trimethoxyflavone, di-O-methylpinocembrin, bisdemethoxycurcumin and aloe-emodin) [9].
The volatile oil of Kaempferia parviflora rhizome contains several monoterpenes, including α-pinene, β-pinene, camphene, limonene, linalool, borneol, and bornyl acetate. The oil also consists of sesquiterpenes such as α-copaene, β-elemene, (E)-caryophyllene, α-humulene, γ-gurjunene, β-salinene, Δ-cadinene, spathulenol, caryophyllene oxide, epi-α-muurolol, and α-cadinol. It also includes diterpenes like dauca-5,8-diene and longiborneol acetate [10].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Rhizomes of K. parviflora is traditionally used for goiter, dysentery, diarrhea with vomiting. Leaf and rhizome paste applied as poultice for poisonous insect bite [2]. It also acts as tonic and have carminative effect [11].
Biological and pharmacological activities supported by experimental data
Anti-allergy activity
The isolated compounds from ethanolic extract of K. parviflora dried rhizome showed an inhibitory effect on the allergic reaction caused by β-hexosaminidase release from RBL-2H3 cells (histamine-releasing cell line), with a IC₅₀ value of 5-hydroxy-3,7,3,4- tetramethoxyflavone (8 µM), 5-hydroxy-7-methoxyflavone (20.6 µM) and 5-hydroxy-7,4-dimethoxyflavone (26 µM) compared to ketotifen fumarate (47.5 µM), using β-hexosaminidase inhibitory activity [12].
Anti-microbial activity
Ethyl acetate extracts of K. parviflora dried rhizomes inhibited the growth of Cutibacterium acnes with minimum inhibitory concentrations (MIC) value of 0.015 mg/mL compared to gentamycin, 0.004 mg/mL using brain heart infusion agar (BHI agar) under anaerobic conditions [13].
Anti-inflammatory activity
Ethyl acetate extracts of K. parviflora dried rhizomes inhibited nitric oxide production with the IC50 value of 12.59 ± 0.35 µg/mL compared to indomethacin, 23.13 ± 1.51 µg/mL using nitric oxide inhibition assay [13].
Anti-obesity activity
Ethanol extract of K. parviflora (100 mg/kg) administered orally to C57BL/6J HFD-fed mice (4 weeks old male) demonstrated a significant p value reduction in weight gain (20.81 ± 2.29 g) and total white adipose tissue (4.89 ± 0.35 g) compared to metformin (lower weight gain of 16.75 ± 1.22 g; total white adipose tissue of 4.29 ± 0.26 g) [14].
Clinical studies
A randomised, single-blind, crossover study involving 28 male volunteers (21-29 years old) was conducted to examine the effects of K. parviflora (KP) ingestion on energy expenditures (EE) by indirect calorimetry and analyzed its relation to brown adipose tissue assessed by fluorodeoxyglucose FDG-positron emission tomography PET with computed tomography CT. Initially the subjects fasted overnight and underwent FDG-PET/CT examination after being in cold conditions at 19˚C for two hours to determine their EE. Four weeks after, subjects fasted overnight and underwent respiratory gas analysis before ingesting placebo or 100 mg KP extract. Results showed that 100 mg of KP extract significantly (p < 0.05) increase EE with maximal rise of 226 ± 69 kJ/d at 60 minutes compared to placebo group (-1 ± 56kJ/d). In high brown adipose tissue (BAT) group, KP extract showed significant (p < 0.05) maximal increase of EE 351 ± 50 kJ/d at 60 minutes while no significant change; while no significant changes for both groups in low BAT and respiratory quotient [15].
A randomised controlled trial involving eighty healthy adult participants with moderate stress level was conducted to study the effects of K. parviflora on heart rate variabilities. The treatment group was given K. parviflora extract at doses of one capsule twice daily (360 mg/day) for 14 days (K. parviflora group) or placebo capsules (placebo group) 360 mg/day for 14 days and were followed-up on day 7 and 14 after taking the capsules. The results indicated the treatment group reduced the low frequency per high frequency ratio (LF/HF) and root mean square of successive differences (RMSSD) index levels compared to placebo group (p < 0.05) [16].
A randomised, double-blind, placebo-controlled, parallel group study involving 45 healthy elderly volunteers (male & female, aged over 60 years) was conducted to investigate the effects of K. parviflora extract on health-related physical fitness and oxidative status. The treatment group was directed to consume K. parviflora extract at doses of 25 or 90 mg once daily for 8 weeks. After 4 weeks of consumption, the treatment group receiving 90 mg showed significant improvements in the 30-second chair stand test and the 6-minute walk test compared to the placebo group (p < 0.05). Additionally, there was a significant increase in the activities of antioxidant enzymes, including superoxide dismutase (SOD) and catalase (CAT), and a decrease in serum malondialdehyde (MDA) levels (p < 0.01). After 8 weeks of consumption, the treatment group at 90 mg exhibited sustained significant increases in SOD, CAT, and GSH-Px activities, along with a further decrease in MDA levels compared to the placebo group (p < 0.01). The findings suggest that K. parviflora may enhance physical fitness and reduce oxidative stress in healthy elderly individuals [17].
A randomised, double blind, placebo-controlled, parallel group study involving 69 healthy volunteers (male & female, 19–60 years old, 9 dropout) was conducted to study the effect of K. parviflora extract (formulated with five other excipients) on cardiorespiratory fitness and physical flexibility. The treatment group was directed to consume a functional drink containing K. parviflora extract at doses of 90 or 180 mg per serving per 80 mL within 5 minutes for 12 weeks. After 6 weeks of consumption, the levels of lactate, catalase, oxidative stress markers (superoxide dismutase (SOD), serum malondialdehyde (MDA)), and maximal aerobic capacity (VO2 max) in both treatment groups were significantly higher than placebo group (p < 0.05). While after 12 weeks of consumption, only the levels of SOD and MDA of the treatment groups were significantly higher than the placebo group (p <0.05). The higher dose of treatment showed higher values in VO2 max after 12-week treatment and longer 5-min run distance test, compared to placebo group (p <0.05) [18].
A randomised, double-blind, placebo-controlled clinical trial involving 76 participants (both males and females aged 20 to under 65, with a body mass index between 24 and 30 kg/m²) was conducted. The participants were randomly assigned to two groups, with each group receiving either a placebo or capsule (containing 150 mg of K. parviflora extract) once daily for 12 weeks. The treatment group showed a significant reduction in abdominal fat area (including visceral, subcutaneous, and total fat) and triglyceride levels compared to the placebo group after 12 weeks (p <0.05). Subgroup analyses revealed that healthy subjects in the treatment group also experienced significant reductions in abdominal fat area and triglyceride levels compared to the placebo group (p < 0.05). Neither group reported adverse events related to the test foods, nor were there any clinically relevant abnormal changes in physical, biochemical, or hematologic parameters, urinalysis results, or medical interviews [19].
A randomised controlled study involving 40 volunteers (18–60 years old) was conducted to study the anti-inflammatory effect of transdermal patch of K. parviflora extract. The volunteers were randomised into two groups: placebo or herbal patches (2.5-10%). The patch area of 70cm2 was applied to the pain site (shoulder or lower back) once a day for 7 days. For the treatment group, the pain scores according to the numerical rating scale decreased from 5.84 ± 1.57 to 4.00 ± 1.33 and 2.74 ± 1.37 on Day 3 and Day 7 respectively. In contrast, the pain scores of the control group did not change after the patch application. The pressure pain threshold (PPT) for the treatment group significantly increased from 1.79 ± 0.51 N to 2.55 ± 0.41 N after 7 days of application, indicating an improvement in pain tolerance, while the control group showed no significant changes in PPT throughout the study period. It can be concluded from the findings of the skin irritation study that the patches were found to be compatible with the skin and safe for human volunteers [20].
A pilot, open-label study involving 14 generally healthy male volunteers aged 50–68 years (with 1 dropout) was conducted to investigate the effects of KaempMax™, an ethanol extract of K. parviflora, on erectile function. Participants were instructed to take 100 mg of KaempMax™ daily for 30 days. After the treatment period, statistically significant improvements were observed in erectile function, intercourse satisfaction, and total scores on the International Index of Erectile Function (IIEF) questionnaire, with a reported 6.3% increase in the total IIEF score (p < 0.05). Additionally, 61.5% of participants reported improvements in their erections based on the Global Assessment Question (GAQ). KaempMax™ was well tolerated, with no serious adverse events reported, and no significant changes in metabolic parameters, indicating a favorable safety profile [21].
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Sub-chronic and mutagenicity studies
Hydroethanolic extract of K. parviflora rhizome at concentration range of 39.1–1250 µg/plate did not induce gene mutations in selected bacteria. This extract exhibits no toxicity in male or female Sprague Dawley rats after 90 days of repeated oral administration at high dose with no-observed-adverse-effect level (NOAEL) greater than 249 mg/kg/day, equivalent to 747 mg K. parviflora extract. There were no changes in the microscopic and histopathological examinations of the organs. The mean lethal dose was more than 2000 mg/kg. These results indicate that the extract is not genotoxic and translating to human consumption, the safe daily intake of the extract is about 150 mg/day [22].
Others (Adverse reactions, contraindications, side effects, warning, precautions)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The World Flora Online Plant List. [Internet] Kaempferia parviflora 2024 [cited Nov 21 2024]. Available from: https://www.worldfloraonline.org/search?query=Kaempferia+parviflora
- Quattrocchi Umberto. CRC World Dictionary of Medicinal and Poisonous Plants: Common Names, Scientific Names, Eponyms, Synonyms and Etymology. Vol III E-L. Florida: CRC Press. 2012; Vol. III 653.
- Picheansoonthon, C. and S. Koonterm. 2008. Notes on the genus Kaempferia L. (Zingiberaceae) in Thailand. J. Thai Traditional Altern. Med., 6: 73-93.
- Ridley, H.N. 1922. Flora of Malay Peninsular. Volume 1. pp. 569. London, L. Reeve & Co.
- Asamenew, G., Kim, H.W., Lee, M.K., Lee, S.H., Kim, Y.J., Cha, Y.S., Yoo, S.M. and Kim, J.B. 2019. Characterization of phenolic compounds from normal ginger (Zingiber officinale Rosc.) and black ginger (Kaempferia parviflora Wall.) using UPLC-DAD-QToF-MS. European Food Research and Technology 245:653-665. https://doi.org.10.1007/s00217-018-3188-z
- Chaipech, S., Morikawa, T., Ninomiya, K., Yoshikawa, M., Pongpiriyadacha, Y., Hayakawa, T. and Muraoka, O. 2012. Structures of two new phenolic glycosides, kaempferiaosides A and B, and hepatoprotective constituents from the rhizomes of Kaempferia parviflora. Chem. Pharm. Bull. 60(1);62-69. Doi: 10.1248/cpb.60.62
- Sookkhee, S., Sakonwasun, C., Mungkornasawakul, P., Khamnoi, P., Wikan, N. and Nimlamool, W. 2022. Synergistic effects of some methoxyflavones extracted from rhizome of Kaempferia parviflora combined with gentamicin against carbapenem-resistant strains of Klebsiella pneumonia, Pseudomonas aeruginosa and Acinetobacter baumannii. Plants. 11:3128.
- Sun, S., Kim, M.J., Dibwe, D.F., Omar, A.M., Athikomkulchai, S., Phrutivorapongkul, A., Okada, T., Tsuge, K., Toyooka, N. and Awale, S. 2021. Anti-austerity activity of Thai Medicinal Plants: Chemical Constituents and Anti-pancreatic cancer activities of Kaempferia parviflora. Plants 10: 229.
- Mai, D.T., Nguyen, T.P., Pham Q.T. & Tran H.K. 2021. Chemical constituents of the rhizomes of Kaempferia parviflora. Can Tho University Journal of Science 57(1): 45-50.
- Pitakpawasutthi, Y., Palanuvej, C. and Ruangrungsi, N. 2018. Quality evaluation of Kaempferia parviflora rhizome with reference to 5,7-dimethoxyflavone. J. Adv. Pharm. Technol. Res. 2018 Jan-Mar; 9(1): 26-31.
- Thailand. Krom Witthayāsāt Kānphǣt, Subcommittee on The Establishment of The Thai Herbal Pharmacopoeia. Thai herbal pharmacopoeia 2018. Nonthaburi, Thailand: Department of Medical Sciences, Ministry of Public Health; 2018.
- Tewtrakul, S. & Subhadhirasakul, S. 2007. Anti-allergic activity of some selected plants in the Zingiberaceae family. Journal of ethnopharmacology, 109(3), 535-538.)
- Sitthichai, P., Chanpirom, S., Maneerat, T., Charoensup, R., Tree-Udom, T., Pintathong, P., Laphookhieo, S. and Sripisut, T. 2022. Kaempferia parviflora rhizome extract as potential anti-acne ingredient. Molecules, 27(14): 4401. https://doi.org/10.3390/molecules27144401
- Park, S. H., Park, J., Lee, M., Kim, J., Eun, S., Jun, W., Kim, O. K., & Lee, J. 2023. Antiobesity effect of Kaempferia parviflora accompanied by inhibition of lipogenesis and stimulation of lipolysis. Food & nutrition research, 67, 10.29219/fnr.v67.9374
- Matsushita, M., Yoneshiro, T., Aita, S., Kamiya, T., Kusaba, N., Yamaguchi, K., Takagaki, K., Kameya, T., Sugie, H., & Saito, M. 2015. Kaempferia parviflora extract increases whole-body energy expenditure in humans: roles of brown adipose tissue. Journal of nutritional science and vitaminology, 61(1), 79–83.
- Wichai, E., Uraiwan, C., Bung-orn, S.S.A and Warangkana, C. 2018. Effects of Kaempferia parviflora on physical and psychological stresses in adults. International Journal of GEOMATE. 15(50): 26-31.
- Wattanathorn, J., Muchimapura, S., Tong-Un, T., Saenghong, N., Thukhum-Mee, W., Sripanidkulchai, B. 2012. Positive modulation effect of 8-week consumption of Kaempferia parviflora on health-related physical fitness and oxidative status in healthy elderly volunteers. Evidence-Based Complementary Altern Med. 2012;2012:732816. doi:10.1155/2012/732816
- Wattanathorn, J., Tong-Un, T., Thukham-Mee, W. and Weerapreeyakul, N. 2023. A functional drink containing Kaempferia parviflora extract increase cardiorespiratory fitness and physical flexibility in adult volunteers. Foods. 12: 3411.
- Yoshino, S., Awa, R., Miyake, Y., Fukuhara, I., Sato, H. Ashino, T., Tomita, S. and Kuwahara, H. 2018. Daily intake of Kaempferia parviflora extract decreases abdominal fat in overweight and preobese subjects: a randomized, double-blind, placebo-controlled clinical study. Diabetes Metab Syndr Obes. 11:447-458. Doi: 10.2147/DMSO.S169925
- Tuntiyasawasdikul, S. and Sripanidkulchai, B. 2022. Development and clinical trials on anti-inflammatory effect of transdermal patch containing a combination of Kaempferia parviflora and Curcuma longa extracts. J. Drug Deliv. Sci. Technol. 2022:68:103093.
- Stein, R.A., Schmid, K., Bolivar, J., Swick, A.G., Joyal, S.V., Hirsh, S.P. 2018. Kaempferia parviflora ethanol extract improves self-assessed sexual health in men: a pilot study. J Integr. Med. 2018:16(4):249‐254.
- Yoshino, S., Awa, R., Ohto, N., Miyake, Y. and Kuwahara, H. 2019. Toxicological evaluation of standardized Kaempferia parviflora extract: Sub-chronic and mutagenicity studies. Toxicology Reports 6: 544-549.


