Global Information Hub on Integrated Medicine.2016.pg 1-14.
Rahizan Issa*1, June Lee Chelyn2, Nor Syaidatul Akmal Mohd Yousof2
1 Bacteriology Unit, Infectious Disease Research Centre, Institute for Medical Research, Jalan Pahang, 50588 Kuala Lumpur, Malaysia
2 Phytochemistry Unit, Herbal Medicine Research Centre,Institute for Medical Research, Jalan Pahang, 50588 Kuala Lumpur, Malaysia
Contact Address
June Lee Chelyn, Phytochemistry Unit, Herbal Medicine Research Centre (HMRC), Institute for Medical Research, Jalan Pahang, 50588 Kuala Lumpur, Malaysia.
[email protected]
Editorial Group
GlobinMed, Traditional and Complementary Medicine Division
Publication status and date: 2016
Citations
Rahizan I, June LCL, Akmal NSMY. Anthocyanin extracts: management of hypercholestrolemia disease. Global Information Hub on Intergrated Medicine.2016.pg 1-14.
ABSTRACT
Importance: Modification of serum cholesterol levels is an important strategy for the prevention and control of hypercholesterolemia disease. Anthocyanins have been promoted for reducing cholesterol levels, but its reported impact on cholesterol levels has been inconsistent. The aim of the study is to assess systematically the evidence and quality of current research on anthocyanins extracts (AE) for the management of hypercholesterolemia disease.
Objective: The objective of this review is to assess the effectiveness of AE for the management of patients with hypercholesterolemia disease.
Methods: Electronic databases were searched up to April 2014 for relevant randomised controlled trials (RCTs) (4-12 weeks). The quality of the selected trials was assessed using the Cochrane Risk of Bias Assessment Tool. The same comparators of similar studies were pooled. The outcomes examined were levels of total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C) and triglycerides (TG).
Results: Our inclusion criteria included five studies involving 473 subjects. In these studies, the types of interventions and duration of trials were noted. Overall, AE only produces significant effects on TC.
Conclusions and Relevance: The available evidence from RCTs only supports the efficacy of AE in lowering TC in serum cholesterol levels. Further rigorously designed trials with larger sample sizes are warranted to confirm the effects of AE for managing patients with hypercholesterolemia disease.
Introduction
Cardiovascular disease (CVD) is a leading cause of death globally. The World Health Organisation (WHO) has estimated that in 2008 alone, approximately 17.3 million deaths occurred due to CVD, and this is estimated to increase to 23.3 million deaths by 2030 [1]. In Malaysia, lifestyle changes have increased the incidence of coronary heart disease (CHD) and is one of the leading cause of CVD in this country [2]. Hypercholesterolemia has been a known risk factor for CHD, a condition that is characterised by elevated serum cholesterol levels in blood [3]. Lifestyle practices are recommended for lowering cholesterol levels such as not smoking, exercising regularly, maintaining a healthy weight and adopting good dietary habits [4][5][6]. However, drug intervention is often prescribed to individuals who could not attain satisfactory serum lipid levels through lifestyle changes [7]. Statin is the most commonly prescribed cholesterol-lowering agent, but due to statin-associated myopathy many patients may not tolerate long-term use of statin [2][8]. Therefore, effective drugs with improved safety profiles are still needed for the treatment of elevated serum lipid levels in patients at risk of CHD and CVD.
Several studies have linked high intake of fruits and vegetables to the reduced risk of CVD and it is a subject of considerable interest to many researchers [9]. One of the dietary compounds of interest is the anthocyanins. Anthocyanins are naturally occurring plant pigments that can be found abundantly in many coloured fruits and vegetables such as grapes, cranberries, blueberries and raspberries [9]. Anthocyanins are shown to reduce the risk of CVD through several mechanisms including protection against oxidative processes such as lipid peroxidation [10][11], upregulation of the nitric oxide production [12] and also improving endothelium function [13][14]. Moreover, a meta-analysis of 16 cohort studies have revealed that the increase of polyphenol consumption such as anthocyanins decreases CHD mortality significantly [15]. Similarly, a multivariate prospective study conducted on CVD-free postmenopausal women (n= 34 489) in Iowa Women’s Health Study has shown that the intake of flavonoids such as anthocyanins were associated with reduced risk of death from CVD and CHD [16].
A number of clinical trials using anthocyanin extracts have been conducted on various groups such as healthy adults, hypertensive subjects and hypercholesterolemic subjects [17][18][19]. However, the results from these studies have been either contradictory to animal studies or insignificant either due to the small sample size of the clinical studies or the lower amount of anthocyanins due to low bioavailability of the compound in humans compared to rats. The aim of this study is therefore to systematically review the clinical evidence on the effect of anthocyanin extracts (AE) for the management of hypercholesterolemia patients which could shed a new light on the benefits of AE for the management of CVD.
MATERIALS AND METHODS
Search strategy
The searches for relevant trials involved multiple strategies such as publication year, database, keywords and language. The following electronic databases were searched from January 2000 to April 2014: PubMed, MEDLINE via Ovid, OneSearch via EBSCOhost, The Cochrane Database of Systematic Reviews (CDSR) and The Cochrane Central Register of Controlled Trials (CENTRAL). The main keywords that were used in the search strategy were anthocyanins, CVD and clinical trials. The references of published papers on clinical trials and published review papers were scrutinised for additional articles. There were restrictions on the basis of language (English only) and publication status (2000 to April 2014) for trial inclusion in the review.
Study Selection
Studies involving patients above the age of 20 years old and were described as having elevated serum cholesterol levels (normal total cholesterol level ≤200 mg/dL) were included. We included all trials comparing the effectiveness of AE (cranberry, bilberry, blackcurrant, whortleberry, grape seed) used as a single active substance against any control such as placebo, diet, or drinks. There was no restriction on the duration of the trials. Findings from trials were included in the analysis only if they reported primary outcomes of serum cholesterol levels such as total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C) and triglycerides (TG). Data on adverse effects were also reported. Two reviewers (JLC, NSAMY) independently screened titles and abstracts of studies identified from the searches. We obtained full-text articles if they appeared to satisfy, or to potentially satisfy, the inclusion criteria and then independently checked full papers to identify those trials that were eligible for inclusion. Any disagreement between the two reviewers was resolved through discussion.
Data Extraction and Synthesis
Two reviewers (JLC, NSAMY) independently undertook data extraction using a uniform data extraction form. The third reviewer (RI) checked for accuracy of the data extracted. We extracted details of the study design, participant characteristics, intervention (i.e. dosage form, dose, frequency and duration), and outcome data. We assessed the potential for risk of bias in the included studies by examining the quality of the random sequence generation, allocation concealment, description of dropout and withdrawal and blinding (participants, personnel and outcome assessment). Two reviewers (JLC, NSAMY) independently assessed the risk of bias of the included studies and each domain was rated as having a ‘low risk of bias’, a ‘high risk of bias’ or an ‘unclear risk of bias”. Unclear risk of bias were due to either lack of information or uncertainty over the potential of bias.
We conducted the meta-analysis to determine the effect of AE on TC, HDL-C, LDL-C and TG. We used the weighted mean difference method in RevMan (version 5.2, London, UK), inputting the number of participants and the means and SDs of endpoint cholesterol levels for the comparison groups. The treatment effect was estimated as the mean difference in endpoint values between comparison groups. We compared final measurements because theoretically, in a randomised trial, the final measurements estimate the same quantity as the comparison of changes from baseline [20]. If data were available, we planned to use the mean change from baseline values instead of endpoint values.
Statistical significance was set at P<0.05 for all outcomes. Studies that evaluated similar comparisons were assessed for the presence of statistical heterogeneity by using the Ι2 tests and the amount of heterogeneity was estimated using the I2 statistic (which indicates the percentage of variation between studies that is due to heterogeneity rather than chance). We used a fixed-effect model if pooling seemed appropriate in view of clinical and methodological similarities between studies where I2 was below 25%.
Results
Description of studies
The search strategy in selected databases had identified a total of 282 hits (Fig 1, Table 1). From the 282 hits, duplicates were removed and the remaining articles were further scrutinised for only randomised, double-blinded and placebo controlled clinical trials which resulted in only 11 trials. From these 11 trials, five trials were included in this systematic review in which all five were randomised trials. In four trials, the patients consisted of hypercholesterolemia subjects [21][22][23][24]. However, in one of the trials (Dohadwala 2011) the subjects had relatively low lipid concentration from 180-207 mg/dL and it was a cross-over study [25]. Six trials were removed for the following reasons; trials with prehypertensive subjects, trials not assessing the effects on lipids, trials measuring anthocyanins in urine and a short acute trial (1 day).
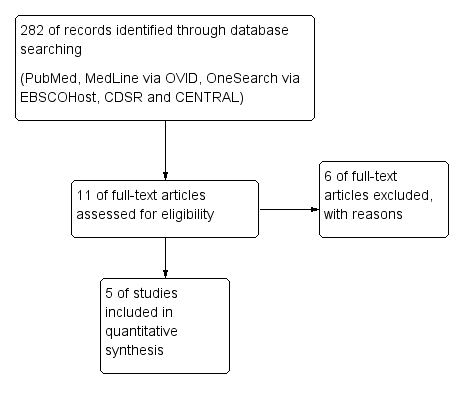
Figure 1:The Steps and Number of Trials Identified and Selected for Inclusion
| No | Databases | Years searched | Search key used | No studies in search |
| 1 | Pubmed | 2000-2014 | Anthocyanin AND cardiovascular | 143 |
| 2 | Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews, Cochrane Methodology Register, Database of Abstracts of Reviews of Effects, Health Technology Assessments, NHS Economic Evaluation Database, eBook collection (EBSCOhost); Medline with Full Text (2000- 30 April 2014) | Anthocyanin AND cardiovascular AND randomized control trial | 0 | |
| 3 | Cochrane Central Register of Controlled Trials (CENTRAL) (2000- 30 April 2014) | Anthocyanin AND cardiovascular AND randomized control trial | 0 | |
| 4 | Scopus | Anthocyanin AND cardiovascular AND Hyperlipidemia | 15 | |
| 5 | Ovid | Anthocyanin AND cardiovascular AND cholesterol | 124 |
Table 1 : Search Strategies
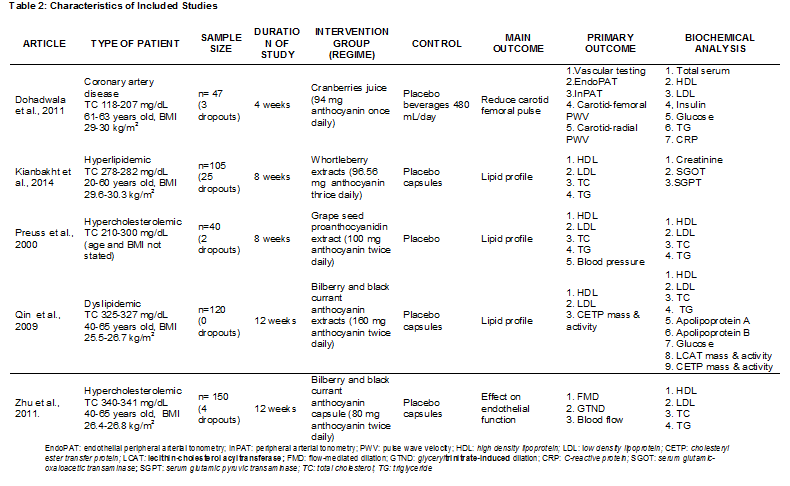
Table 2: Characteristic of included studies
The characteristics of the five trials included in this systematic review (patient type, sample size, trial duration, intervention, outcome and biochemical analysis) are summarised in Table 2. These trials had involved a total of 473 subjects (with 34 dropouts) with reported data on TC, LDL-C, HDL-C and TG values at baseline and end of trials. Studies by Dohadwala 2011 [25] and Zhu 2011 [26] did a pre-trial to determine the acute effects of anthocyanins. The trial by Dohadwala 2011 [25] was conducted in the United States and subjects had consumed anthocyanins in cranberry juice that exceeding the average intake. Meanwhile, Preuss 2000 [22] did the trial in the US and subjects were given chromium (Cr) and/or proanthocyanidins extracted from grape seeds. A trial in China by Zhu 2011 [24] had given encapsulated anthocyanins from berries. The trial by Qin 2009 [23] was conducted in China and the subjects were given anthocyanin capsules from bilberry and blackcurrant. Kianbakht 2014 [21] did a trial in Iran and the subjects had whortleberry capsules. The anthocyanins content for each studies included in this review are listed in Table 3.
Risk of bias in included studies
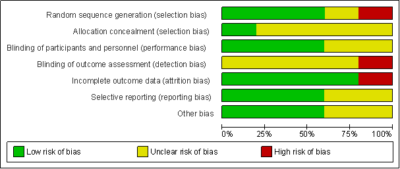
The assessment of risk of bias is summarised in Fig. 2 and 3. For three trials [21][22][25], random sequence generation were assessed as low risk of bias while one trial was unclear risk of bias [24] and one trial was considered high risk of bias [23]. In the allocation concealment, one trial was assessed as low risk of bias [21]. It was reported that the participants were randomly distributed to an intervention or control group according to sequential numbered containers. All the other trials had unclear risk of bias in the allocation concealment.
Blinding of participants and personnel were considered to be adequate for all five trials. Three studies were assessed as low risk of bias [21][22][25] and the other two trials were unclear risk of bias [23][24]. For blinding of outcome assessment, one trial [21] was considered high risk of bias while the other trials had unclear risk of bias. In judging the risk of bias for incomplete outcome data, only one trial [22] was assessed as high risk of bias as there was one dropout due to adverse events during the trial. The four other trials were of low risk of bias. We defined three trials [21][23][25] as having low risk of bias to selective reporting as all expected primary outcomes were clearly reported while two other trials were unclear risk of bias.
Other potential sources of bias were either low risk or unclear risk of bias. In the data analysis, although the participants were randomised in the intervention and control groups, we defined trials as having conducted intention-to-treat (ITT) analysis. The ITT analysis was conducted for primary outcomes in judging the risk of bias for this item, however none of the trials carried out an ITT analysis. We also focused on baseline comparability and the trial’s financial support as other potential sources of bias. In all trials the intervention groups were reported or appeared to be comparable at baseline for the cholesterol levels. Three trials [21][24][25] were of low risk of bias. These trials clearly reported that the studies received financial support from non-profitable organisations such as the university research grant.
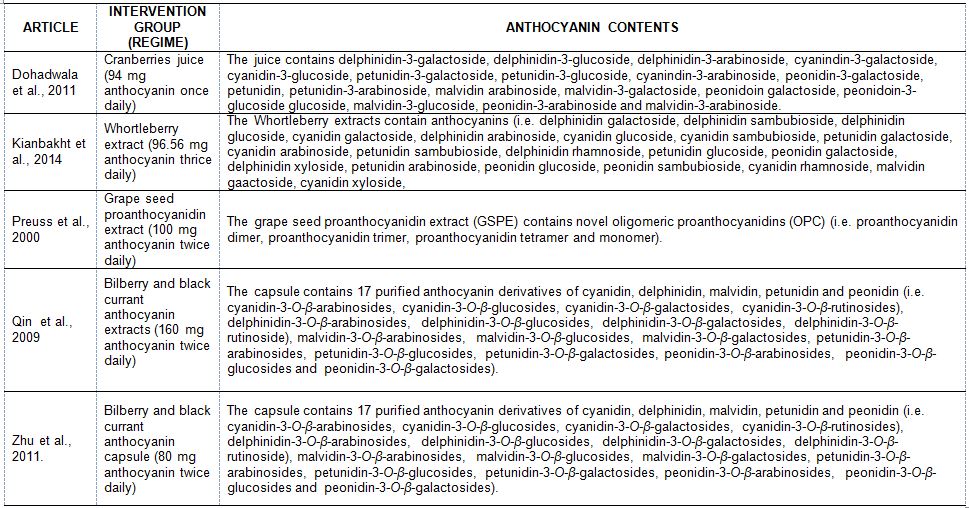
Table 3: Anthocyanin content of juices, capsules and extracts
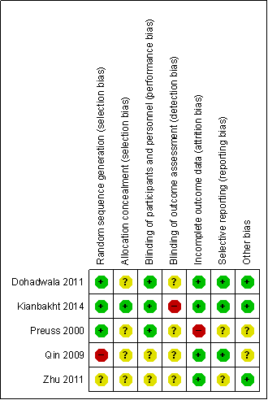 |
Figure 2: Risk of bias summary reviews authors judgement about each risk of bias item for each included study
Figure 3: Risk of Bias Graph Review Authors’ Judgements About Each Risk of Bias Item Presented as Percentages Across All Included Studies.
EFFECTS OF INTERVENTIONS
The forest plots were organised according to the following outcomes:
TC
Significant differences between groups in total cholesterol were found for comparisons between AE and placebo (Fig 4). One trial [21] that compared AE showed a significant difference between the treatment favouring AE [mean differences = -68.16 (95% CI:-82.00, -54.36) mg/dL, P<0.001].
LDL-C
No significant differences were found in LDL-C between groups for all comparisons (Fig 5). Meanwhile two trials [21][23] showed significant differences between the treatment favouring AE [mean differences = -20.79 (95% CI:-30.64, -10.94) mg/dL, P<0.0001].
HDL-C
No significant differences were found in HDL-C between groups for all comparisons (Fig 6).
TG
For three trials [21][23][25], the reduction of TG level was greater for AE compared to control [mean differences = -6.63 (95% CI:-22.89, 9.63) mg/dL]. However, it did not reach statistical significance (P=0.40) (Fig 7).
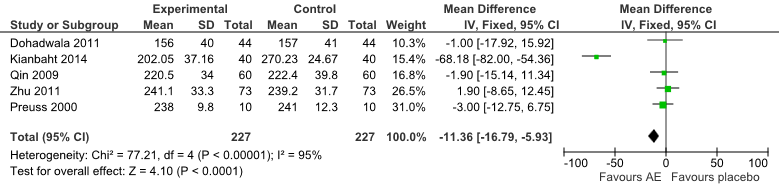
Fig 4: Forest Plot of Comparison of Anthocyanin Extracts versus Placebo (Outcome: TC).
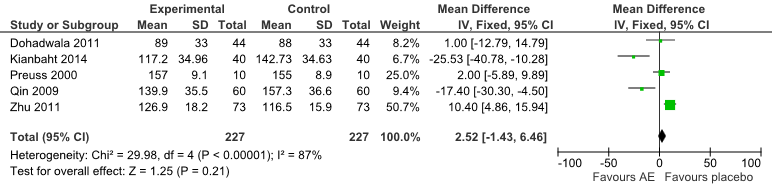
Fig 5: Forest Plot of Comparison of Anthocyanin Extracts versus Placebo (Outcome: LDL-C).
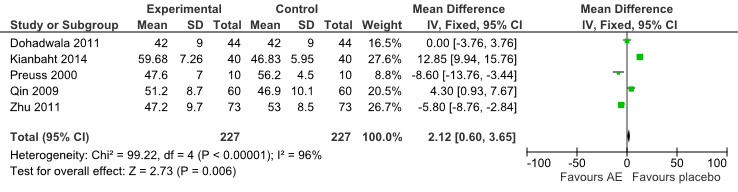
Fig 6: Forest Plot of Comparison of Anthocyanin Extracts versus Placebo (Outcome: HDL-C).
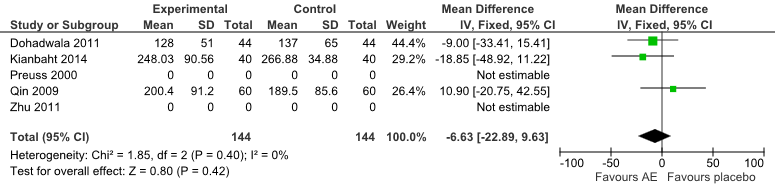
Fig 7: Forest Plot of Comparison of Anthocyanin Extracts versus Placebo (Outcome: TG).
DISCUSSION
Summary of main results
In this systematic review, five RCTs involving a total of 473 subjects were included. One of the trial was a cross-over study but was included in this review as the study design was deemed suitable with appropriate analysis and wash out period [20]. The findings from one trial [21] showed that AE (whortleberry extracts) had significant effects on all measured parameters. This may be due to the higher dosage given to patients (96.56 mg anthocyanins thrice daily); nevertheless no serious adverse effects were reported. The overall estimated effect of AE from the five RCTs showed effectiveness in reducing TC levels. However, no significant effect was observed for all the other parameters such as LDL-C, HDL-C and TG. Despite the fact, the possibility of its beneficial effects cannot be ruled out as the included trials were small and had methodological problems. The trials were also of short duration (4-12 weeks) and therefore the long-term benefits of AE on cholesterol levels remained unknown.
One of the major limitations of this current review is that not all papers provided full taxonomic information on the extracts used and the composition of the preparation tested. AE, like any other natural products, vary in their chemical composition depending on several factors such as subspecies, time of harvest, methods of preparations and batch-to-batch variation. This variability can produce significant differences in the effects tested [27]. As for the safety of AE, this review found AE to be tolerable for short-term use. In the included trials, adverse events were reported by Preuss et al. 2000 [22] as low and consisted predominantly of gastrointestinal symptoms. Additionally, there were no reported changes in laboratory parameters such as creatinine, urea and liver enzymes. However, precaution should be taken in co-medication of AE with other drugs taken in management of hypercholesterolemia disease as AE-drug interactions were not considered in these trials.
Quality of the evidence
Overall, the studies included in this review were at some risk of bias or there was insufficient information to judge the risk of bias. The method of random sequence generation was unclear in one (24) of the five included studies, while in three other studies the result was of low risk. Nevertheless, one study was of high risk [23] because subjects were allocated by age. Similarly the method of allocation concealment was unclear in most of the included studies except for one study [21] which was clear and therefore judged to be at low risk of bias.
The blinding of participants and personnel were unclear in two of the five trials [23][24], but three studies [21][22][25] were blinded from intervention and therefore were judged to be at low risk of bias. Blinding of outcome assessment was also unclear in most trials, however in one study [21], the person assessing the outcome was not blinded to the intervention and therefore is of high risk of bias. For incomplete outcome data, we judged three studies [21][22][25] at low risk of attrition bias as the reasons for exclusions and loss-of-follow-up were provided. The method of selective reporting was clear for three studies [21][23][25] as the primary outcomes were stated and the results were reported. The other two studies [22][24] were unclear. There was insufficient information provided to assess the risk of bias in other sources of bias not covered above.
Strengths and limitations
A quantitative result and assessment of risk of bias are included in this systematic review. The Cochrane Collaboration for systematic reviews of interventions [20] was used as a study method and this is the strength of this review. Efforts were made to include all trials that investigated the effect of AE on cholesterol levels. In this regard, the search strategy included both low and elevated cholesterol level.
An extensive search for published articles were undertaken, however it is not possible to include both studies of non English language publications and studies with negative findings. The small number of included studies had limited our study to generate a funnel plot to assess the degree of publication bias in this systematic review [20]. Furthermore, subgroup analysis by dosage of AE, duration of trials and baseline cholesterol was not undertaken due to the small number of included studies.
CONCLUSIONS
Implications for practice
Currently, there is limited trial evidence, especially on the effectiveness of AE. Therefore, recommending AE for the management of hypercholesterolemia disease is not justifiable based on current evidence. Nevertheless, the available trial and observational evidence is promising and generally supportive of the favourable effects of AE on hypercholesterolemia disease. This evidence is also supported by the biological plausibility of several mechanisms which explains the beneficial effect on hypercholesterolemia disease [28][29][30].
Implications for research
There are currently very few randomised controlled trials that meet our inclusion criteria to examine AE for the management of hypercholesterolemia disease. Furthermore, the included trials were small with methodological problems and further rigorously designed trials with larger sample sizes are needed. The available evidence suggests that while larger trials over longer intervention periods, particularly in patients with high cholesterol levels, may be appropriate, any effects are likely to be modest.
ACKNOWLEDGEMENT
This study was funded by the Ministry of Health (MOH), Malaysia. The authors express their deepest gratitude to the Director General of Health, and the Director of Institute for Medical Research for permission to publish this article.
REFERENCES
- Organization WH. Cardiovascular Disease Fact Sheet 2013 [21 October 2014]. Available from: http://www.who.int/mediacentre/factsheets/fs317/en/.
- Malaysia MoH. Management of Dyslipidemia 2011 [22 April 2014]. Available from: http://www.moh.gov.my/attachments/6632.pdf.
- Khot UN, Khot MB, Bajzer CT, Sapp SK, Ohman EM, Brener SJ, et al. Prevalence of conventional risk factors in patients with coronary heart disease. The journal of the American Medical Association. 2003;290(7):898-904.
- Kennedy ET, Bowman SA, Spence JT, Freedman M, King J. Popular diets: correlation to health, nutrition, and obesity. Journal of the American Dietetic Association. 2001;101(4):411-20.
- Gepner AD, Piper ME, Johnson HM, Fiore MC, Baker TB, Stein JH. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. American heart journal. 2011;161(1):145-51.
- Wedick NM, Pan A, Cassidy A, Rimm EB, Sampson L, Rosner B, et al. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. The American journal of clinical nutrition. 2012;95(4):925-33.
- Weintraub H. Update on marine omega-3 fatty acids: management of dyslipidemia and current omega-3 treatment options. Atherosclerosis. 2013;230(2):381-9.
- Law M, Rudnicka AR. Statin safety: a systematic review. The American journal of cardiology. 2006;97(8A):52C-60C.
- Wallace TC. Anthocyanins in cardiovascular disease. Advances in nutrition (Bethesda, Md). 2011;2(1):1-7.
- Chang YC, Huang KX, Huang AC, Ho YC, Wang CJ. Hibiscus anthocyanins-rich extract inhibited LDL oxidation and oxLDL-mediated macrophages apoptosis. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2006;44(7):1015-23.
- Chang JJ, Hsu MJ, Huang HP, Chung DJ, Chang YC, Wang CJ. Mulberry anthocyanins inhibit oleic acid induced lipid accumulation by reduction of lipogenesis and promotion of hepatic lipid clearance. Journal of Agricultural and Food Chemistry. 2013;61(25):6069-76.
- Xu JW, Ikeda K, Yamori Y. Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension. 2004;44(2):217-22.
- Nakamura Y, Matsumoto H, Todoki K. Endothelium-dependent vasorelaxation induced by black currant concentrate in rat thoracic aorta. Japanese journal of pharmacology. 2002;89(1):29-35.
- Andriambeloson E, Magnier C, Haan-Archipoff G, Lobstein A, Anton R, Beretz A, et al. Natural dietary polyphenolic compounds cause endothelium-dependent vasorelaxation in rat thoracic aorta. The Journal of nutrition. 1998;128(12):2324-33.
- Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Archives of internal medicine. 1995;155(4):381-6.
- Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. The American journal of clinical nutrition. 2007;85(3):895-909.
- Hassellund SS, Flaa A, Kjeldsen SE, Seljeflot I, Karlsen A, Erlund I, et al. Effects of anthocyanins on cardiovascular risk factors and inflammation in prehypertensive men: a double-blind randomized placebo-controlled crossover study. Journal of human hypertension. 2013;27(2):100-6.
- Hassellund SS, Flaa A, Sandvik L, Kjeldsen SE, Rostrup M. Effects of anthocyanins on blood pressure and stress reactivity: a double-blind randomized placebo-controlled crossover study. J Hum Hypertens. 2012;26(6):396-404.
- Asgary S, Sahebkar A, Afshani MR, Keshvari M, Haghjooyjavanmard S, Rafieian-Kopaei M. Clinical Evaluation of Blood Pressure Lowering, Endothelial Function Improving, Hypolipidemic and Anti-Inflammatory Effects of Pomegranate Juice in Hypertensive Subjects. Phytotherapy Research. 2014;28(2):193-9.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions: Wiley; 2011.
- Kianbakht S, Abasi B, Hashem Dabaghian F. Improved Lipid Profile in Hyperlipidemic Patients Taking Vaccinium arctostaphylos Fruit Hydroalcoholic Extract: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Phytotherapy Research. 2014;28(3):432-6.
- Preuss HG, Wallerstedt D, Talpur N, Tutuncuoglu SO, Echard B, Myers A, et al. Effects of niacin-bound chromium and grape seed proanthocyanidin extract on the lipid profile of hypercholesterolemic subjects: a pilot study. Journal of medicine. 2000;31(5-6):227-46.
- Qin Y, Xia M, Ma J, Hao Y, Liu J, Mou H, et al. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. The American journal of clinical nutrition. 2009;90(3):485-92.
- Zhu Y, Xia M, Yang Y, Liu F, Li Z, Hao Y, et al. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clinical chemistry. 2011;57(11):1524-33.
- Dohadwala MM, Holbrook M, Hamburg NM, Shenouda SM, Chung WB, Titas M, et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr. 2011;93(5):934-40.
- Jin Y, Alimbetov D, George T, Gordon MH, Lovegrove JA. A randomised trial to investigate the effects of acute consumption of a blackcurrant juice drink on markers of vascular reactivity and bioavailability of anthocyanins in human subjects. Eur J Clin Nutr. 2011;65(7):849-56.
- Aziz Z, Wong SY, Chong NJ. Effects of Hibiscus sabdariffa L. on serum lipids: a systematic review and meta-analysis. Journal of ethnopharmacology. 2013;150(2):442-50.
- Bell DR, Gochenaur K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. Journal Of Applied Physiology (Bethesda, Md: 1985). 2006;100(4):1164-70.
- Bunea A, Rugină D, Sconţa Z, Pop RM, Pintea A, Socaciu C, et al. Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16-F10 metastatic murine melanoma cells. Phytochemistry. 2013;95:436-44.
- Graf D, Seifert S, Jaudszus A, Bub A, Watzl B. Anthocyanin-Rich Juice Lowers Serum Cholesterol, Leptin, and Resistin and Improves Plasma Fatty Acid Composition in Fischer Rats. PloS one. 2013;8(6):e66690-e.