Scientific Name
Acalypha indica L.
Synonyms
Acalypha bailloniana Müll.Arg., Acalypha chinensis Benth., Acalypha cupamenii Dragend., Acalypha decidua Forssk., Acalypha fimbriata Baill., Acalypha somalensis Pax., Acalypha somalium Müll.Arg., Acalypha spicata Forssk., Cupamenis indica (L.) Raf., Ricinocarpus baillonianus (Müll.Arg.) Kuntze, Ricinocarpus deciduus (Forssk.) Kuntze, Ricinocarpus indicus (L.) Kuntze. [1]
Vernacular Name
| Malaysia | Chika mas, kucing galak, lis-lis, rumput lis-lis, rumput lislis, tjeka mas [2] |
| English | Indian acalypha, Indian nettle, three-seeded mercury [2] |
| India | Akam, akkiniccalam, akkiniccivam, cailameni, cailamenicceti, cakkaraputpacceti, cakkaraputpam, cakkaraputpi, dadaro, dadri, harita manjari, inkaracceti, inkaram, jalamali, kalakkiniyon, khokla, lankop, motchidal, murupindi, naciyari, otuvatakki, payilikam, puti, shendri, tanivalli, tekaram, tirumeniyalaki, valakkonnai, valapparuti, vicuvakappiyam, vinaivika, vinaivikacceti [2] |
| Indonesia | Lelatang, rumput kokosongan [2] |
| Thailand | An maeo, haan maeo, lang ta kai, tam ye meao, tam ye tuapa, tamyae maeo, tamyae tua phu, tamyae tuaphuu [2] |
| Philippines | Bugos, maraotong, taptapingar [2] |
| Vietnam | Tai t[uw][owj]ng [aas]n, tai t[uw][owj]ng xanh [2] |
| Kenya | Louyongorok [2] |
| Tanzania | Myakuralusa [2]. |
Geographical Distributions
Acalypha indica is widespread in the Old World tropics from West Africa, throughout India, Indo-China to the Philippines and Java, but apparently absent in Borneo and rare in Eastern Malaysia. [3]
Botanical Description
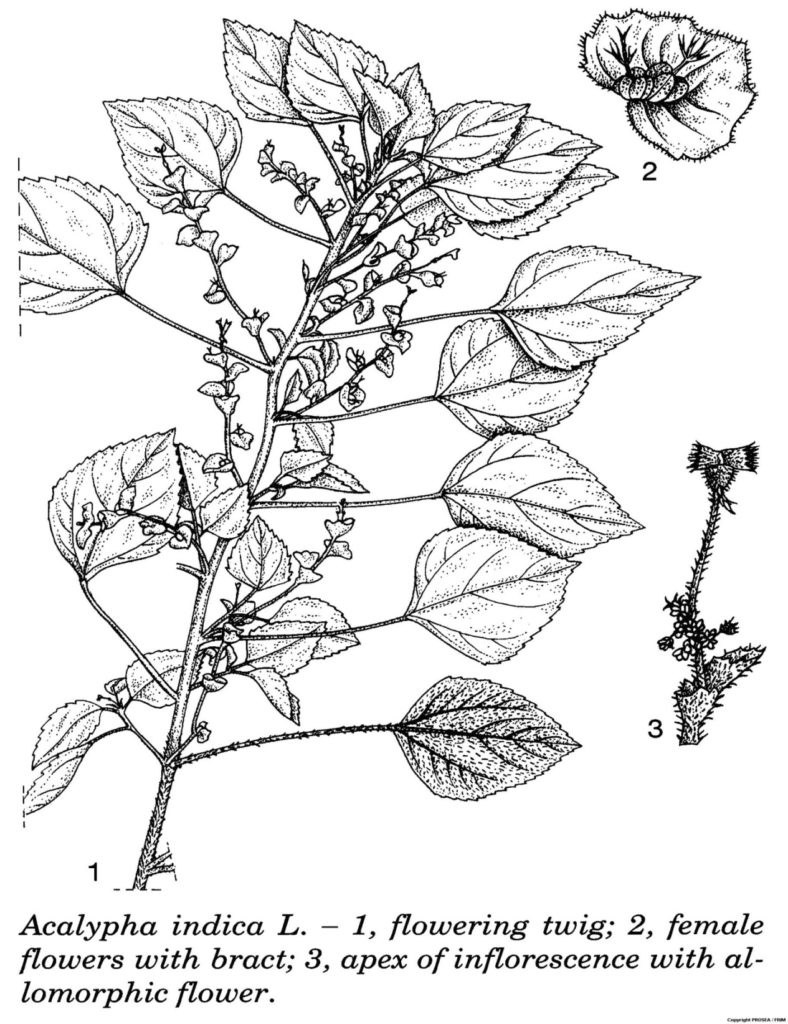
A. indica is a member of the Euphorbiaceae family. It is an erect and annual herb that can grow up to 1.5 m tall. [3]
The leaves are rhombic-ovate, measure (1-)3-5(-7) cm x (1-)2.5-3(-5) cm, wedge-shaped at base, with shallowly serrate margin and petiole 2-6 cm long. [3]
The inflorescences are bisexual, solitary and with short slender male portion while the female flowers are fewer and less crowded than in A. lanceolata. They arewith broad bracts, shallowly and obtusely toothed, which are much less closely nerved and produce allomorphic female flowers at the apex. [3]
The fruit is 2-2.5 mm in diametre. [3]
Cultivation
A. indica is found in waste places and cropped land at low altitudes, and is locally common. [3]
Chemical Constituent
No documentation
Plant Part Used
No documentation
Traditional Use
A. indica is considered by the traditional practitioners to be bitter, acrid, expectorant, purgative, emetic, gastrointestinal irritant and diuretic. [4]
A. indica leaves are known to have laxative properties and is given in the form of powder or decoction by Indians to treat constipation. In cases of obstinate constipation the leaves are grounded into paste and made into a ball and introduced per rectum. It is supposed to relax the sphincter ani and produces free motion. In Madagascar, the root decoction is used as a laxative. A. indica is also considered as a vermifuge in this case the root infusion is being used by people of Seychelles and Reunion Islands. On the other hand the Indians make use of the leaves together with garlic to expel the vermins. The leaves are considered as an emetic and most practitioners make use of the leaf sap as part of a concoction to induce vomiting. It is considered as safe even for children. [5][6]
A. indica is considered an expectorant and the juice expressed from the leaves is being given to treat croup, while in smaller doses it helps in productive cough by aiding its expectoration. The root infusion or decoction is given for asthma. [5]
A. indica leaves has been advocated for use in the treatment of various parasitic skin condition. One of the most common application is the treatment of scabies. In Madagascar crushed aerial parts are applied to skin parasites while in Mauritius the juice of the crushed leaves are mixed with salt and applied on scabitic lesions. The Indians mixed the sap with salt to treat scabies. It is also used to treat various inflammatory skin problems like maggot-infested wounds, syphilitic ulcers and some other skin infection. [5][6]
East Africans use the leaf sap to treat eye infection while in Namibia the decoction is instilled into the eye for the same purpose. In India a decoction of the leaves is instilled into the ears to treat otalgia and otitis. In Comoros, the leaf decoction is used in cream form to treat joint pains and rheumatism, while in India the leave juice mixed with oil is used instead. Poultice of the plant is applied to the head for headache. [6]
Preclinical Data
Pharmacology
Postcoital infertility activity
The petroleum ether and ethanol extracts of A. indica were found to have anti-implantation activity when they were given to female albino rats. This effect was reversible upon withdrawal of the treatment with the extracts. This effect is due to some estrogenic activity as evidenced by histological studies of the uterus. [7]
Wound healing activity
Studies found that A. indica does have wound healing ability, however, it is inferior to Heliotropium indicum which has better activity and tensile strength. [8]
Antivenom activity
A. indica is a common weed while Viper russelli russelli is amongst the deadliest snakes in the world. Shirwaika et al associated the two together when they investigated the ability of the A. indica to neutralise the snake’s venom. The viper venom induced lethality by haemorrhage, necrotisation and mast cell degranulation in rats and cardiotoxicity and neurotoxicity in frogs. Preadministration of the ethanol leaf extracts to test animals was found to significantly inhibit these effects. It was found that the extract also inhibited venom-induced lipid peroxidation in RBC, decrease GSH and catalase levels in rat kidney tissue. This indicates that the ethanol leaf extract of A. indica possesses potent snake venom neutralising properties. [9]
Antibacterial activity
A study of the antibacterial activity of 4 different extracts (hexane, chloroform, ethyl acetate and methanol) from the leaves of A. indica was carried out against Gram positive (Staphylococcus aureus, Staphylococcus epidermidis, Bacillus cereus, Streptococcus faecalis) and Gram negative (Klebsiella pneumoniae, Escherichia coli, Proteus vulgaris, Pseudomonas aeruginosa) bacteria. All the extracts exhibited antibacterial activity against the Gram positive organisms with MIC 0.156 to 2.5 mg/mL while amongst the Gram negative bacteria only Pseudomonas aeruginosa was susceptible. [10]
Toxicity
The raw herb is considered poisonous, emetic, and causes intestinal irritation. Pollens may cause allergy. [11]
Clinical Data
Clinical findings
No documentation
Precautions
No documentation
Side effects
No documentation
Pregnancy/Breast Feeding
No documentation
Age limitation
No documentation
Adverse reaction
No documentation
Interaction & Depletion
No documentation
Interaction with drug
No documentation
Interaction with other Herbs
No documentation
Contraindications
No documentation
Case Report
Acute intravascular haemolysis
It was reported in Sri Lanka that four patients developed acute intravascular haemolysis after ingesting a broth containing Acalypha indica Linn. All four patients wer found to have glucose-6-phosphate dehydrogenase deficiency. [11]
Dosage
No documentation
Poisonous Management
No documentation
Line drawing
References
- The Plant List. Ver1.1. Acalypha indica L. [homepage on the Internet]. c2013 [updated 2012 Mar 23; cited 2016 Apr 20]. Available from: http://www.theplantlist.org/tpl1.1/record/kew-713
- Quattrocchi U. CRC world dictionary of medicinal and poisonous plants: Common names, scientific names, eponyms, synonyms, and etymology. Volume I A-B. Boca Raton, Florida: CRC Press; 2012. p. 36.
- Siregar AH. Acalypha indica L. In: van Valkenburg JLCH, Bunyapraphatsara N, editors. Plant Resources of South-East Asia No. 12(2): Medicinal and poisonous plants 2. Leiden, Netherlands: Backhuys Publishers, 2001; p. 34-35.
- Warrier PK, Nambiar VPK, Ramankutty C. Indian medicinal plants: A compendium of 500 species. Volume 1. Hyderabad: Orient Longman, 1994; p. 36.
- Panda H. Herbal Soaps & Detergents Hand Book. Delhi: National Institute of Industrial Research (NIIR) Project Consultancy Services, 2011; p. 48-49.
- Schmelzer GH. Acalypha indica L. In: Schmelzer GH, Gurib-Fakim A, editors. Plant Resources of Tropical Africa 11(1): Medicinal plants 1. Wageningen, Netherlands: PROTA Foundation/Backhuys Publishers/CTA, 2008; p. 23-25.
- Hiremath SP, Rudresh K, Badami S, Patil SB, Patil SR. Post-coital antifertility activity of Acalypha indica L. J Ethnopharmacol. 1999;67(3):253-258.
- Reddy JS, Rao PR, Reddy MS. Wound healing effects of Heliotropium indicum, Plumbago zeylanicum and Acalypha indica in rats. J Ethnopharmacol. 2002;79(2):249-251.
- Shirwaikar A, Rajendran K, Bodla R, Kumar CD. Neutralization potential of Viper russelli russelli (Russell’s viper) venom by ethanol leaf extract of Acalypha indica. J Ethnopharmacol. 2004;94(2-3):267-273.
- Govindarajan M, Jebanesan A, Reetha D, Amsath R, Pushpanathan T, Samidurai K. Antibacterial activity of Acalypha indica L. Eur Rev Med Pharmacol Sci. 2008;12(5):299-302.
- Khare CP. Indian herbal remedies: Rational western therapy, ayurvedic, and other traditional usage, botany. Berlin: Springer, 2004; p. 12.

