Kucing galak aerial
Acalypha indica L.
Euphorbiaceae
DEFINITION
Kucing galak aerial consist of the powder of dried leaves, stems and flowers of Acalypha indica L (Euphorbiaceae).
SYNONYM
Acalypha bailloniana Mull.Arg., Acalypha chinensis Benth., Acalypha cupamenii Dragend., Acalypha decidua Forssk., Acalypha fimbriata Baill., Acalypha somalensis Pax, Acalypha somalium Müll.Arg., Acalypha spicata Forssk., Cupamenis indica (L.) Raf., Ricinocarpus baillonianus (Müll.Arg.) Kuntze, Ricinocarpus deciduus (Forssk.) Kuntze, Ricinocarpus indicus (L.) Kuntze [ 1 ].
VERNACULAR NAMES
Indian acalypha, India nettle, three-seeded mercury (English); chika mas, kucing galak, lis-lis, rumput lislis, tjeka mas (Malay); tie xian cai (Chinese); kupaaimeni (Tamil) [ 2 , 3 , 4 ].
CHARACTER
IDENTIFICATION
Plant Morphology
A. indica is an erect and annual herb, 0.3–1.5 m in height. Stems are brownish when mature and younger parts are green, sparsely hairy, terete, 2–10 mm in thickness. Leaves are simple and alternate, dull to dark green to brownish; brittle when dry; petiole 1–7 cm, lower leaves with longer petiole, pubescent; lamina 2–5 cm long and 1–4 cm broad, ovate to rhombic ovate, tip acute, base cuneate, pale green below and dark green above, margin serrate and hairy; veins 5–7 pairs, generally alternate, usually 3 veins arising from the base, prominent and hairy below; midrib slightly raised on the upper surface, and prominent on the lower surface. Inflorescence are axillary, stalked, spike, 1–7 cm long. Flowers unisexual, green, subsessile and encircled by a leafy, orbicular serrate bract of about 4 mm long and 5–8 mm broad. Female flowers 5 to 15, basal, 2 mm across. Male flowers numerous, minute; spike usually terminating in an allomorphic flower. Fruits capsules, small and green. Seeds minute, ovoid and pale brown. Roots are vertical and branched; 2–8 mm in thickness, tortuous, rough; colour varies from grey to brown when dry, broken surface creamy yellow; fracture giving rise to a cloud of dusty particles; no characteristic smell; bitter [ 4 , 5 ].
Microscopy
Powdered material consists of fragments of pitted, annular, spiral and scalariform vessels; covering trichomes consist of whole unicellular and multicellular with thin and smooth walls; calcium oxalate consist of numerous type of solitary and druses crystals; fragment of thin-walled parenchyma cells; fragment of idioblast tannin cells; fragments of lower epidermis in surface view with wavy to sinuous anticlinal walls and abundant paracytic type of stomata; fragment of lignified fibre [ 4 , 5 ].

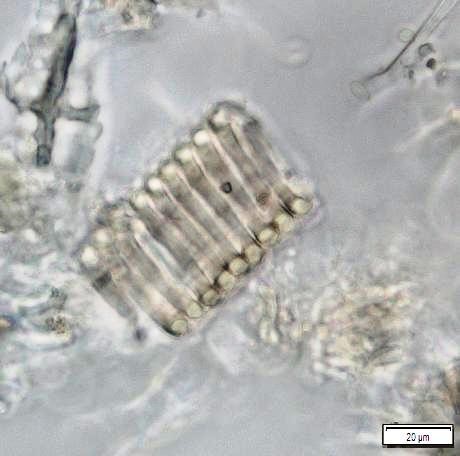
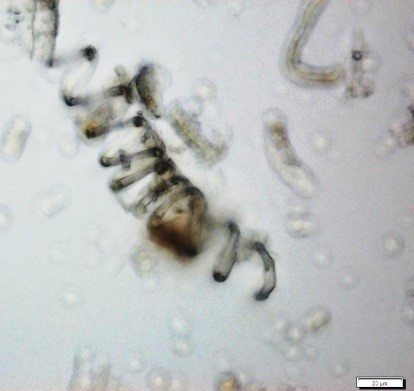
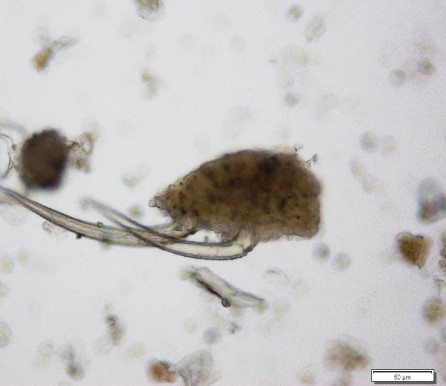
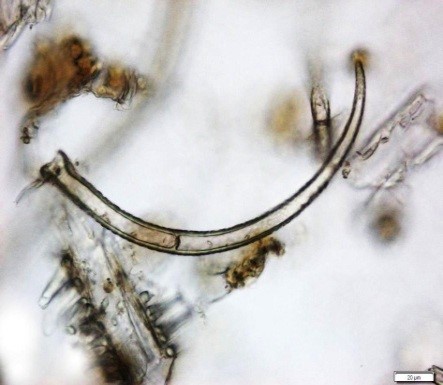
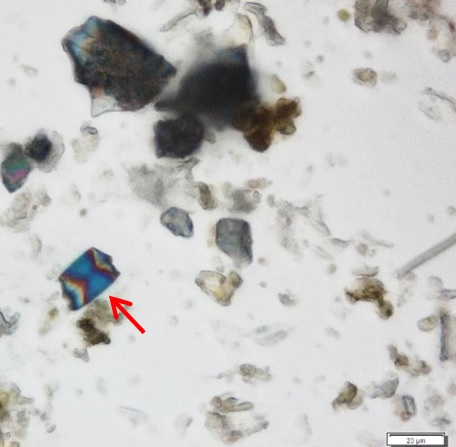
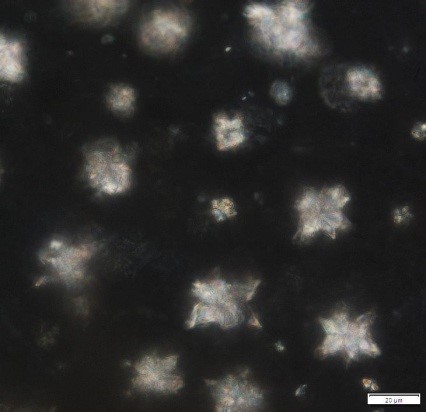
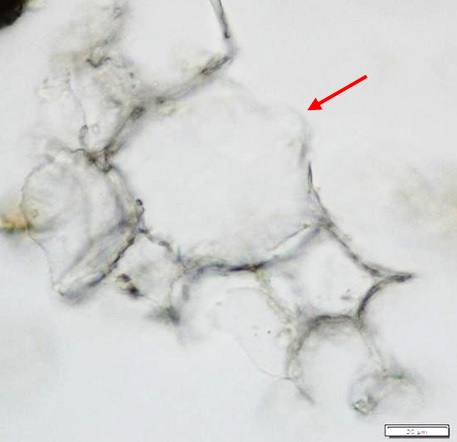
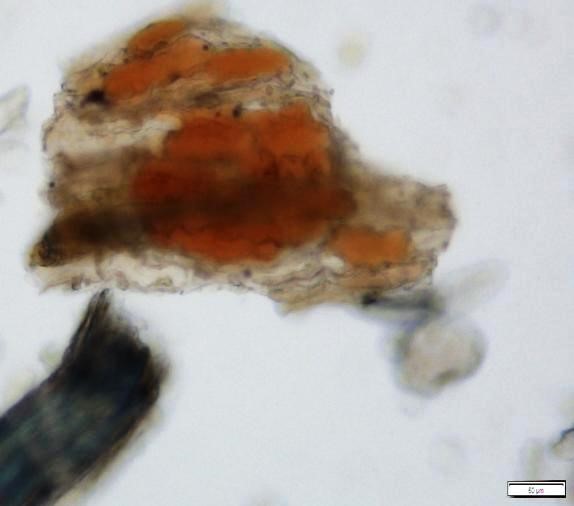
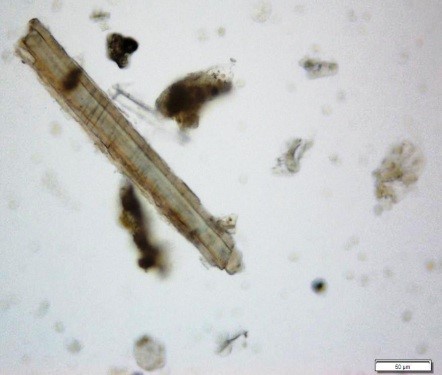
Figure 2 : Microscopic characters of Acalypha indica aerial powder of 0.106 mm size. (a) Pitted vessel (magnification 40x); (b) spiral vessel (magnification 40x); (c) spiral vessel (magnification 40x); (d) simple unicellular trichome (magnification 20x); (e) simple multicellular trichome (magnification 40x); (f) solitary crystal (arrow) (magnification 40x); (g) druses crystal (magnification 40x); (h) idioblast cell (arrow) (magnification 40x); (i) idioblast tannin cell (magnification 40x); (j) paracytic stomata (arrow) (magnification 40x); (k) lignified parenchyma (magnification 40x). [Scale bars: a = 200 µm; b, c, f, g, h, I, k = 20 µm; d, e, j, l = 50 µm]
Chemical Tests
Observed colour of solution after treatment for the presence of:
| Test for the presence of steroids | Bluish green |
| Test for the presence of phenolic | Green |
Thin Layer Chromatography (TLC)
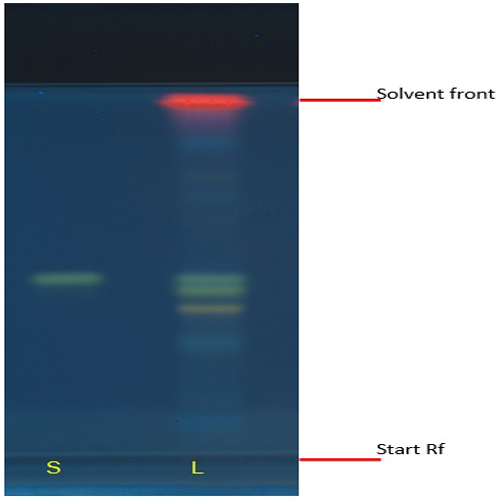
Figure 3 : TLC chromatogram of biorobin (S), methanol extract of Acalypha indica dried aerial parts (L) observed under (a) UV at 366 nm after derivatization
| Test Solutions | Weigh about 1.0 g of A. indica dried aerial powder of 0.106 mm size in 50 mL of conical flask. Add 10 mL of methanol. Sonicate the mixture for 15 min. The mixture was then centrifuged and supernatant was collected. |
| Standard solution | Dissolve biorobin standard [CAS no. 1729-56-2] in methanol to give concentration of 0.5 mg/mL. [Vortex standard for 15 sec]. |
| Stationary Phase | HPTLC Silica gel 60 F254 10 x 10 cm |
| Mobile phase | Ethyl acetate : water : formic acid : acetic acid; (34 : 7 : 2.5 : 5.5) (v/v/v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
High Performance Liquid Chromatography (HPLC)
| Test solution | Weigh about 1.0 g of A. indica dried aerial powder of 0.106 mm size in 50 mL of conical flask. Add 10 mL of methanol. Sonicate the mixture for 15 min. The mixture was then centrifuged and supernatant was collected. | |||||||||||||||||||||
| Standard solution | Dissolve biorobin standard [CAS no. 1729-56-2] in methanol to give a standard concentration of 0.05 mg/mL. Vortex standard for 15 sec. | |||||||||||||||||||||
| Chromatographic system |
Detector: 350 nm Column: C18 (150 X 4.6 mm, 3.5 µm) (Zorbax SB-C18 if necessary) Column oven temperature: 25oC Flow rate: 1 mL/min Injection volume:20 µL |
|||||||||||||||||||||
| Mobile Phase (gradient mode) |
|
|||||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the standard solutions (0.05 mg/mL). The requirements of the system suitability parameters are as follow:
|
|||||||||||||||||||||
| Acceptance criteria |
|
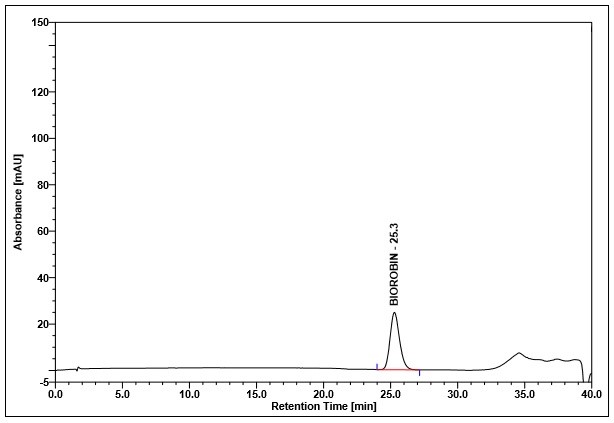
(a)
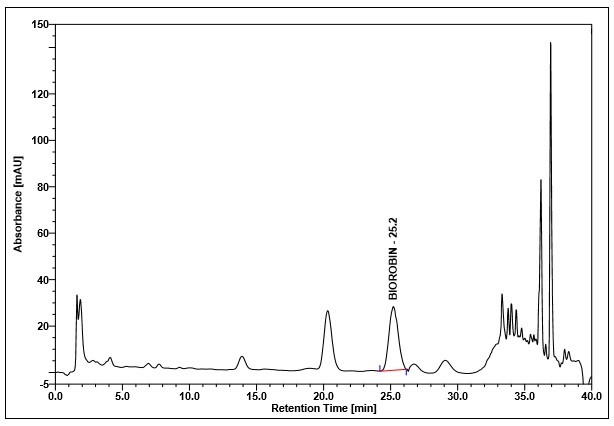
(b)
Figure 4 : Whole HPLC chromatogram of (a) biorobin standard solution (0.05 mg/mL) at tr= 25.3 min and (b) methanol extract of Acalypha indica dried aerial powder showing peak corresponding to biorobin standard standard solution at tr= 25.2 min.
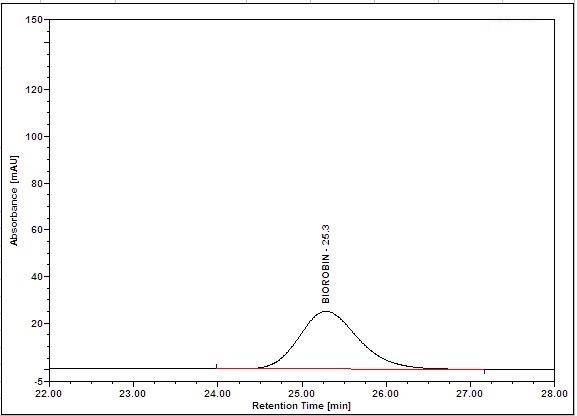
(a)
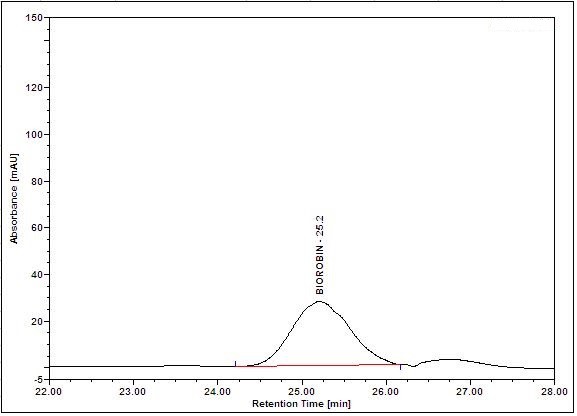
(b)
Figure 5 : HPLC chromatogram highlighting the elution region of (a) biorobin standard standard solution (0.05 mg/mL) at tr= 25.3 min and (b) methanol extract of Acalypha indica dried aerial powder showing peak corresponding to biorobin standard standard solution at tr= 25.2 min.
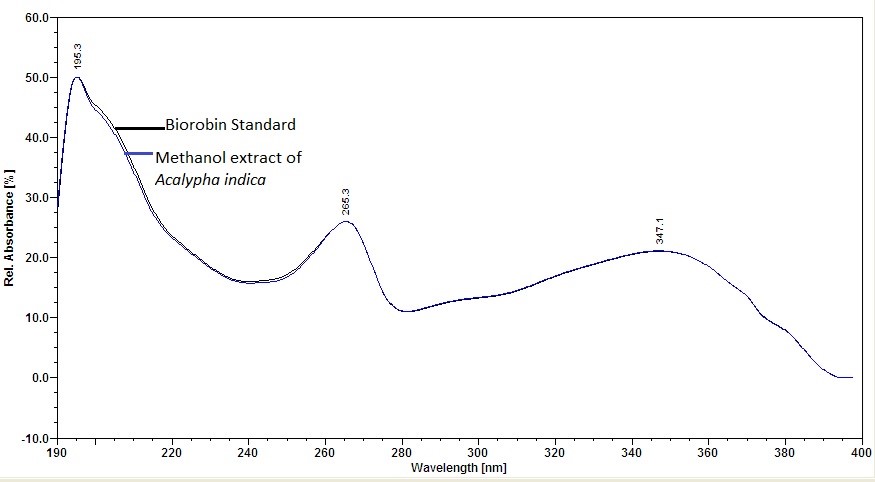
Figure 6 : UV spectrum of biorobin standard solution (0.05 mg/mL) and methanol extract of Acalypha indica dried aerial powder.
PURITY TESTS
The purity tests, except foreign matter test are based on A. indica dried aerial powder of 0.106 mm particle size.
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 15% |
| Acid-insoluble ash | Not more than 1% |
| Water soluble ash | Not less than 4 % |
| Loss on Drying |
| Not more than 8% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 16% |
| Cold method | Not less than 12% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 5% |
| Cold method | Not less than 2% |
SAFETY TESTS
The safety tests are based on A. indica dried aerial powder of 0.106 mm particle size.
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total aerobic microbial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Methanol extract of A. indica dried flowers and leaves were found to contain kaempferol glycosides (mauritianin, clitorin, nicotiflorin and biorobin) [ 6 ].
Methanol extract of A. indica dried aerial parts were found to contain pyridone glucosides (acalyphin, epiacalyphin, noracalyphin, epinoracalyphin, acalyphin amide, epiacalyphin amide cycloside, ar-acalyphidone and seco-acalyphin) [ 7 ].
Methanol extract of A. indica dried leaves were found to contain fatty acids (pentadecanoicacid, 14-methyl-, methyl ester, n-hexadecanoic acid, 10-octadecanoic acid, methyl ester, heptadecanoic acid, 16-methyl-methyl ester, hexadecanoic acid, 14-methyl-methyl ester, nonadecanoic acid, 18-oxo, methyl ester, 14-methylhexadecanoic acid, picolinyl ester, 9-octadecenoic acid [Z], 2-hydroxy-1-(hydroxymethyl) ethyl ester) and non fatty acids (13-hexyloxacyclotridec 10-en-2-one, imidazole, 4-fluoro-5-hydroxyazomethyl-, 2-propenoic acid, 3-[5-acetyl-2,2-dimethlcyclopenthyl],methyl ester, [1a(E),5a]-, 1-oxaspiro [2,5] ocatane, 5,5-dimethyl-4-(3-methyl-1,3-butadienyl)-, 2-formyl-5,7,dimethyl-1,2,3,4-tetrahydropyrimido(3,4-a) indole) [ 8 ].
Essential oil of A. indica dried leaves were found to contain alcohol ((Z)-3-hexen-1-ol, (E)-3,7-dimethyl-2,6-octadien-1-ol)) alkanes (eicosane, heneicosane, docosane) , fatty aldehydes (tridecanal, tetradecanal, palmitaldehyde) fatty acids (9,12,15-octadecatrienoic acid) terpene (10-(acetylmethyl)-(+)-3-carene, phytol isomer) sesquiterpenoids (neophytadiene) [ 9 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally used as a laxative, for which purpose the plant is boiled and the extract is consumed [ 10 ].
Biological and pharmacological activities supported by experimental data
Antioxidant activity
Chloroform extract of A. indica dried aerial exhibited 2,2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) radical scavenging activity with 50% inhibition concentration (IC50) value of 6.31 mg/mL compared to trolox (IC50 = 1.32 mg/mL) using ABTS assay [ 11 ].
Hexane extract of A. indica dried aerial exhibited ABTS radical scavenging activity with 50% inhibition concentration (IC50) value of 6.13 mg/mL compared to trolox (IC50 = 1.32 mg/mL) using ABTS assay [ 11 ].
Methanol extract of A. indica dried aerial exhibited ABTS radical scavenging activity with 50% inhibition concentration (IC50) value of 6.37 mg/mL compared to trolox (IC50 = 1.32 mg/mL) using ABTS assay [ 11 ].
Antimicrobial activity
Methanol extract of A. indica dried leaves (0.2 mg/mL) showed antifungal activity against Candida albicans with inhibition zone (IZ) of 25 mm diameter comparable to clotrimazole (0.01 mg/mL) (IZ = 20 mm) using disc diffusion method [ 12 ].
Methanol extract of A. indica dried stem (0.2 mg/mL) showed antifungal activity against C. albicans with inhibition zone (IZ) of 20 mm diameter comparable to clotrimazole (0.01 mg/mL) (IZ = 20 mm) using disc diffusion method [ 12 ].
Chloroform extract of A. indica dried leaves (0.2 mg/mL) showed antifungal activity against Aspergillus niger with inhibition zone (IZ) of 22 mm diameter comparable to clotrimazole (0.01 mg/mL) (IZ = 22 mm) using disc diffusion method [ 12 ].
Methanol extract of A. indica dried stem (0.2 mg/mL) showed antifungal activity against A. niger with inhibition zone (IZ) of 18 mm diameter comparable to clotrimazole (0.01 mg/mL) (IZ = 22 mm) using disc diffusion method [ 12 ].
Hexane extract of A. indica dried leaves (0.2 mg/mL) showed antibacterial activity against Escherichia coli with inhibition zone (IZ) of 25 mm diameter comparable to clotrimazole (0.01 mg/mL) (IZ = 22 mm) using disc diffusion method [ 12 ].
Chloroform extract of A. indica dried stem (0.2 mg/mL) showed antibacterial activity against E. coli with inhibition zone (IZ) of 22 mm diameter comparable to clotrimazole (0.01 mg/mL) (IZ = 22 mm) using disc diffusion method [ 12 ].
Methanol extract of A. indica dried leaves (5 mg/disc) showed antibacterial activity against Staphylococcus aureus with inhibition zone (IZ) of 15.8 mm diameter comparable to streptomycin (10 µg/disc) (IZ = 14 mm) using disc diffusion method [ 13 ].
Methanol extract of A. indica dried leaves (5 mg/disc) showed antibacterial activity against Bacillus cereus with inhibition zone (IZ) of 14 mm diameter comparable to streptomycin (10 µg/disc) (IZ = 12 mm) using disc diffusion method [ 13 ].
Methanol extract of A. indica dried leaves (5 mg/disc) showed antibacterial activity against Strepcoccus faecalis with inhibition zone (IZ) of 16.5 mm diameter comparable to streptomycin (10 µg/disc) (IZ = 13 mm) using disc diffusion method [ 13 ].
Anthelmintic activity
Ethanol extract of A. indica dried leaves extract (25, 50 and 100 mg/mL) showed significant (p < 0.05) anthelmintic activity in dose dependent manner towards Pheretima posthuma (use as model for anthelmintic screening activity due to its anatomical and physiological resemblance with intestinal roundworm parasites of human being). The ethanol extract (100 mg/mL) demonstrated paralysis time (P: 10 min) and death time (D: 29 min) of worms compared to positive control, piperazine citrate (P: 8 min and D: 20 min) [ 14 ].
Cytotoxicity activity
Methanol extract of A. indica dried aerial parts showed cytotoxic activity on NCI-H187-small lung cancer cell lines with IC50 values of 25 µg/mL compared to ellipticine (IC50 = 0.88 µg/mL) and doxorubicin (IC50 = 0.88 µg/mL) using Resazurin Microplate Assay (REMA) [ 11 ].
Hepatoprotective activity
Methanol extract of A. indica dried aerial parts (300 mg/kg) was administered orally to Wistar albino rats of either sex (175 – 225 g) in a single daily dose for three days. Thioacetamide as hepatoxicity inducer was administered orally on the second day 30 min after the administration of the extract. After 48 hr of thioacetamide administration, the extract showed hepatoprotective activity by decreasing the glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), alkaline phosphatase (ALKP) (GOT: 140.17 ± 7.67 IU/L; GPT: 84.31 ± 28.70 IU/L; ALKP: 427.48 ± 73.38 IU/L) compared to silymarin (GOT: 152.67 ± 7.61 IU/L; GPT: 77.71 ± 27.56 IU/L; ALKP: 385.92 ± 91.27 IU/L) [ 15 ].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
14-days toxicity study
Methanol extract of A. indica dried aerial parts (300 and 2000 mg/kg) administered orally to Wistar albino rats of either sex (175 – 225 g) for 14 days showed no mortality with lethal dose at 50% (LD50) > 2000 mg/kg [ 15 ].
Oral single dose acute toxicity on female Sprague Dawley rats (aged between 7 and 12 weeks old) using aqueous extract of A. Indica aerial showed no toxic effect on the parameters observed, including behavior, body weight, food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality but several histological lesions were found . No-observed-adverse -effect level (NOAEL) is not more than 2,000 mg/kg body weight [ 16 ]
Others (Adverse reactions, contraindications, side effects, warning, precautions)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The plant List. [Internet] Acalypha indica L.; [cited on 1st March 2018]. Available from: http://www.theplantlist.org/tpl1.1/record/kew-713?ref=tpl1
- Quattrocchi UFLS. CRC world dictionary of medicinal and poisonous plants: common names, scientific names, eponyms, synonyms, and etymology. Vol. III E-L. United States: CRC Press. 2012; p.36-37.
- Goh Kong Ling. Malaysian Herbs Chinese Edition. 2000;p.186.
- Government of India Ministry of Health & Family Welfare Department of Ayush New Delhi. The Ayurvedic Pharmacopoeia of India. Part I, Volume VI, First Edition. India: Department of Ayush New Delhi. 20118:63-65.
- Takle VV, Savad RV, Kandalkar AM, Akarte AM, Patel AM. Pharmacognostic and phytochemical investigations of aerial parts of Acalypha indica Linn. Pharmacognosy Journal. 2011;3(21):33-35.
- Nahrstedt A, Hungeling M, Petereit F. Flavonoids from Acalypha indica. Fitoterapia. 2006; 77(6): 484-486.
- Hungeling M, Lechtenberg M, Fronczek FR, Nahrstedt A. Cyanogenic and non-cyanogenic pyridone glucosides from Acalypha indica (Euphorbiaceae). Phytochemistry. 2009; 70: 270-277.
- Ravi S, Shanmugam B, Subbaiah GV, Prasad SH, Reddy KS. Identification of food preservative, stress relief compounds by GC-MS and HR-LC/1-TOF/MS; evaluation of antioxidant activity of A. indica leaves methanolic extract (in vitro) and polyphenolic fraction (in vivo). Journal Food Science Technology. 2017;54(6):1585-1596.
- Rahman MS, Hossain R, Saikot FK, Rahman SM, Saha SK, Hong J, Kim KH. Insights into the in vitro germicidal activities of Acalypha indica. Analytical Science & Technology. 2017;30(1):26-31.
- Burkill IH. A dictionary of the economic product of the Malay Peninsula, Vol.1. London; Published on behalf of the governments of the Straits settlements and Federated Malay states by the Crown agents for the colonies. 1935; p. 166-167
- Sanseera D, Niwatananun W, Liawruangrath B, Liawruangrath S, Baramee A. Antioxidant and anticancer activities from aerial parts of Acalypha indica L. Chiang Mai University Journal of Natural Sciences. 2012; 11(2): 157-168.
- Solomon RDJ, Kallidas S, Vimalan J. Isolation, identification and study of antimicrobial property of a bioactive compound in an Indian medicinal plant Acalypha indica (Indian-nettle). World Journal of Microbiology and Biotechnology. 2015; 21: 1231-1236.
- Govindarajan M, Jabanesan A, Reetha D, Amsath R, Pushpanathan T, Samidurai K. Antibacterial activity of Acalypha indica L. European Review for Medical and Pharmacological Sciences. 2008; 12:299-302.
- Ranju G, Niranjan S, Kumar PS, Kumar PV, Kumar PS. In vitro anthelmintic activity of Acalypha indica leaves extracts. International Journal of Research in Ayuverda & Pharmacy. 2011; 2(1):247-249.
- Kumar SVS, Kumar CV, Vardhan AV. Hepatoprotective activity of Acalypha indica Linn against thioacetamide induced toxicity. International Journal of Pharmacy and Pharmaceutical Sciencec. 2013;5(4):356-359.
- Elda Nurafnie IR, Nor Azlina Z, Umi Rubiah SZ, Janice CSW, Tavinraj R, Nurmaziah MS, Teh BP. Acute oral toxicity study of selected Malaysian medicinal herbs (Acalypha indica aerial) on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2020. Report no.: NON-GLP/2020/03/01.







