Scientific Name
Alpinia galanga (L.) Willd.
Synonyms
Alpinia alba (Retz.) Roscoe, Alpinia bifida Warb, Alpinia carnea Griff, Alpinia pyramidata Blume, Alpinia rheedei Wight, Alpinia viridiflora Griff, Amomum galanga (L.) Lour, Amomum medium Lour, Galanga major Garsault [Invalid], Galanga officinalis Salisb, Hellenia alba (Retz.) Willd, Heritiera alba Retz, Languas galanga (L.) Stuntz, Languas pyramidata (Blume) Merr, Languas vulgare J.Koenig Maranta, galanga L., Zingiber galanga (L.) Stokes , Zingiber medium Stokes, Zingiber sylvestre Gaertn. [1]
Vernacular Name
| Malaysia | Lengkuas, puar, lengkuas biasa, lengkuas benar, lawas, mengkanang [2], lankuas [3] |
| English | Galangal, spice ginger, languas [2], Java galangal [3], galanga, greater galangal [4] |
| China | Da gao liang jiang [2], san chiang, hong dou kou [5] |
| India | Kulinjan( Hindi); arattai, perattai (Tamil); sugandhmula (Sanskrit) [2] |
| Indonesia | Halawas (Bakat); langkuweh (Minang); lawas (Lampung) [2]; laos (Javanese), laja (Sundanese) [6]; lengkuas, langkuwas (Malay); aliku (Bugis) [5] |
| Thailand | Kha, khaa [4], kha yuak (Northern); katuk karohinea (Central) [6] |
| Philippines | Lankauas, langkauas [2] |
| Vietnam | Ri[eef]ng n[ees]p, s[ow]n n[aj]i, h[oof]ng d[aaj]u kh[aas]u [6], rieng, gieng [4] |
| Cambodia | Romdeng [2], rumdéng, pras [6] |
| Japan | Dai Koryokyo [3] |
| Myanmar | Pa da go ji, pa de gaw gyi [2] |
| Laos | Kha ta deng [2] |
| Netherlands | Grote galanga, galigaan, lengoewas [2] |
| France | Galanga de i’Indie, grand galanga [2] |
| Germany | Galgant, großer galgant, siam-ingwer, siamesische ingwerlilie [2] |
| Spain | Calanga, garengal [2] |
| Italy | Galangga maggiore [2] |
| Portugal | Gengibre do laos, gengibre tailandés [2]. |
Geographical Distributions
Alpinia galanga is a tropical plant and distribute to throughout Thailand and Southeast Asia. [7].
Botanical Description
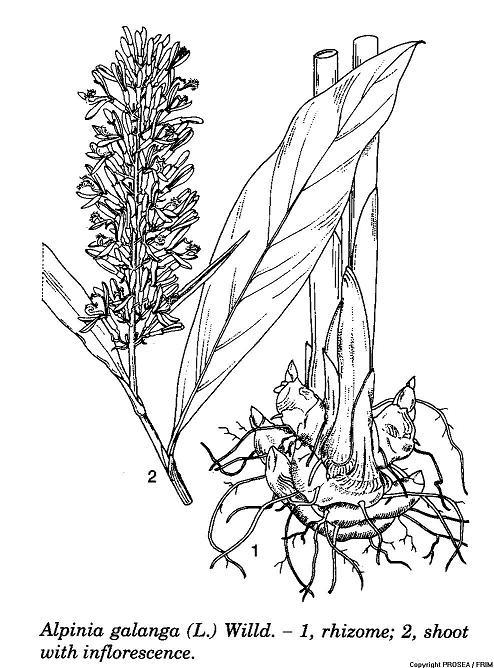
A. galanga is a perennial herb which belongs from Zingiberaceae family. It can grow up to 3.5 m tall and possesses a creeping rhizome [8].The plant grows from rhizome underneath the ground. The plant’s stem is soft, watery, smooth, non-woody and green in color [9].
Pseudostems formed by the rolled leaf sheaths and erect [10] [11]. The rhizome is extremely branches, 2.5-10.0 cm thick, 2.0-4.0 cm in diametre, hard, fibrous and subterete. The colour of rhizome is reddish brown externally and light orange internally [8] [10] [12].
The leaves are distichous, the bottom and top part is small, oblong-lanceolate shaped measure (20-)50(-60) x (4-)9(-15) cm, sheath obviously covered with short and soft hairs at the apex, the petiole is 1-1.5 cm long and hairy, base of the leaf is cuneate and short point at apex [8] [10]. The upper surface of the leaf is dark green and the underneath surface is lighter green, leaf margin is wavy [9]. The ligule present, measuring 1 cm long and truncate [10].
The inflorescence is terminal, many flowered, erect and behave in raceme measure 10-30 cm x 5-7 cm [8] [10] [11]. The bract ovate, up to 2 cm long with each of it subtending of 2-6 flowers in a cincinnus form [8] [10].
The flower is terminal with a large peduncle and bell shaped, measured 12 cm long [9], fragrant, tubular calyx with white in colour, corolla tube terete, both measured 1 cm long. The corolla has 3 oblong-lanceolate lobes measured 1.5 cm x 0.6 cm, the margin is fully ciliate, greenish white in color [8] [10]. Labellum spoon-shaped, 1.5-2.5 cm x 0.5-0.75 cm, white colour with purple vein, clawed, undulate crenate at the margin and the apex is recurved inwards. The staminode lateral; represent by 2 small pointed lobes at the base of labellum, 7-8 mm long, reddish in colour. Stamen one and erect, anther incurved; 2-2.5 cm long. The style is longer than the stamen, stigma obtriangular [10].
The fruits are globose to ellipsoidal capsules in shape measuring 1-1.5 cm in diametre [8] [10] [11]. The color of fruit is orange-red to wine red [10].
Cultivation



Usage
In Malaysia, A. galanga (lengkuas) are normally used as a spice for cooking, flavouring foods and ingredient for herbal preparation [10] [12]. Young shoot and flower of this plant usually used as vegetables [10]. Malay ethnics also use lengkuas as body lotion by boiling the leaves [13].
Soil Suitability and Climatic Requirement
A. galanga is very hardy and is adaptable to various soil types from the lowland to the upland areas with temperatures ranging from 25-35oC. However it is best suited to well-drained soil with high organic matter and the annual rainfall of 200–300 cm. The plants perform better in open areas with full sunlight. [12]
Field Preparation
Land Preparation
The planting area must be rotovated to improve the soil structure and eliminate the weeds. The first rotovation is also done to prepare the area for liming activity if the pH of the area is low (acid soil). The second ploughing must be carried out after application of the liming material to ensure the material is well incorporated into the soil to obtain the optimum effect. [12]
Production of Planting Materials
A. galanga can be propagated using its rhizomes. To ensure that it germinates, the matured rhizomes having 2 to 3 growing points and some roots are used. The mature rhizomes are bright red in colour with profusely active growing roots. The plant is very hardy and the fresh rhizomes can be directly planted to the field. [12]

Field Planting
The canopy diametre of a fully-grown A. galanga is about 1.5 m wide. Thus the recommended planting distance is about 1.0 m between rows and 1.0 m within plants in a row. This will give the population density of about 10,000 plants per ha. Planting should be done at the beginning of the planting seasons to minimise the transplanting shock. The rhizomes should be planted to about 4-6 cm deep into the planting hole measuring about 20 cm X 20 cm X 20 cm. Organic mulch such as dried leaves can be applied to conserve the soil moisture. [12]
Field Maintenance
Fertilisation
The fertilisers that are recommended for lengkuas are organic fertiliser (chicken dung), urea and compound fertiliser (NPK=12:12:17:2). Chicken dung should be given at the rate of 200 g per planting point 3-5 days before planting. Urea should be at 1 and 4 months after planting at the rate of 65 kg/ha. NPK compound fertiliser at the rate of 500 kg/ha should be given at 7 and 10 months after planting. [12]
Weed Control
Weeds should be controlled only during the early part of the crop growth (after 4 and 7 months) manually by using grass cutter or contact herbicides. The dense canopy of the crop will naturally control the weeds when it is full-grown. [12]
Water Management
A. galanga is very hardy. Thus no irrigation is required for field planting. However, to avoid transplanting stress, planting should be carried out at the beginning of the rainy seasons. [12]
Pest and Disease Control
Currently, there are no serious pest and disease problems on the field planting of A. galanga. [12]
Harvesting
Depending on the soil fertility, A. galanga reach its maturity at about 10-12 months after planting. At this growth stage, the plant starts to produce flowers. The rhizomes are harvested manually by using hoe. The harvested rhizomes are cut from the stems. The estimated yield is about 6 kg/clump (60 t/ha). [12]
Postharvest Handling
A. galanga harvested rhizomes are washed and dried at air temperature and sold as fresh rhizomes. The root hairs found on the rhizomes should be removed. The rhizomes are sliced into small pieces and dried for long-term storage and herbal preparations. [12]

Estimated Cost Of Production
The estimated cost of production per hectare of A. galanga is RM20,000. Based on 60 t/ha rhizome yield, the production cost is estimated to be RM 0.30/kg. The production cost was estimated based on the cost of current inputs during writing of this article. [12]
Chemical Constituent
A. galanga has been reported to contain kampheride, alpinin, galangin, methyl cinnamate, cincole, 1’-acetoxychavicol acetate, 1’-hydroxychavicol acetate, galantin-3-methyl ether, a-terpineol, 4-hydroxybenzaldehyde, trans-coniferyl diacetate, trans-p-courmaryl diacetate, α-bergamotene, β-bisabolene, borneol, borneol acetate, butanol acetate, campene, carveol I, carveol II, chavicol acetate, citronellol acetate, α-copaene, curcumene, ρ-cymene, ρ-cymenol, eugenol methyl ether, 1’-acetoxyeugenol acetate, trans-b-farnescene, α-humulene, limonene, myrcene, nerol acetate, pentadecane, linalool, propanol acetate, 2-methyl sabinene, santalene, β-sesquiphellandrene, γ-terpinene, terpinolene, tridecane, caryophyllene oxide, 1’hydroxycineol acetate, ρ-hydroxycinnamaldehyde, di-(ρ-hydroxy-cis-styryl)-methane, α-pinene, β-pinene, quercetin, kaempferol, quercetin-3-methyl ether, isorhamnetin and derivative of 4-allylphenol [14] [15] [16] [17] [18] [19] [20] [21] [22] [23].
Other constituents have been reported are methylcinnamate, cineole, camphor, pinene, 1’-acetoxychavicol acetate, 1’-acetoxyeugenol acetate, caryophyllenol I, caryophylleno II, isorhemnetic, kaemferide, galangin-3-methyl ether, eugenol, galangol, cadinene, tannin, phlobaphenes, starch, cedrol, 1,8-cineole, α-pinene, β-pinene, fatty acid methyl esters, trans-4-methoxycinnamyl alcohol, n-7-heptadecene, n-pentadecane, trans-3,4-dimethoxycinnamyl alcohol, trans-4-hydroxycinnamaldehyde, isoborneol, bornyl acetate, camphene, D3-carene, trans-β-cymene, citronellol, α-fenchene, α-fenchol, geranial, geraniol, geranyl acetate, -2-ρ-menthen-1-ol, cis-2-ρ-menthen-1-ol, cis-β-ocimene, α-phellandrene, β-phellandrene, sabinene, trans-sabinene hydrate, α-terpinene, terpinen-4-ol, neral, α-thujene, β-thujone, tricyclene, α-bergamotene, butyl acetate, chavicol, neryl acetate, citronellyl acetate, α-capaene, p-cymenol, ar-cucumene, eugenly acetate, trans-β-farnesene, methyleugenol, 2-methylpropyl acetate, pentadacane, eugenol, 1’-hydroxychavicol acetate, ρ-hydroxy-cinnamaldehyde, [di-(ρ-hydroxy-cis-styryl)]methane, (E)-8(17),12-labddiene-15,16-dial, (E)-8b(17)-epoxylabd-12-ene-15,16-dial, galanals A-B, galanolactone [24].
Plant Part Used
Rhizomes [23]
Traditional Use
In Malaysia, A. galanga are normally used as a spice for flavouring foods. It is also used as the ingredient in some herbal preparations. The rhizomes have antibacterial and digestive stimulant properties [13]. Thus its decoctions are normally used to treat flatulence, dyspepsia, vomiting and stomach bloating. The poultice of the rhizomes that were mixed with vinegar is used to treat eczema [25]. The leaves are used as ingredients in afterbirth herbal bath. The dried powdered rhizomes are taken with water to treat nausea due to motion sickness during air and sea travel [12].
The rhizome of A. galanga has been used to treat fever [23], pharyngopathy, hiccups, coughs, asthma, bronchitis, headache, inflammations, rheumatoid arthritis, colic [26] [27], ringworm [28], puerpera, gastralgia, boborygmus, purify blood, vomiting, diarrhoea, diabetes, tubercular glands, malaria and tinea, pityriasis versicolor [17] [27], insanity, menstrual pain [29], afterbirth, desquamation of the soles and hands [30]. It is also used for its antiseptic and antibacterial activities. Furthermore, the rhizome was described as a carminative, a stomachic and also a digestive stimulant. It can also be applied externally on carious teeth to relieve a toothache. Flavonoids from the rhizome were clinically proven to have antifungal activity against Trichophyton floccosum and other gram positive and gram negative bacteria [17]. Besides the multiple uses of the rhizome, the leaves of A. galanga can be boiled and used as a body lotion by the Malays.
Preclinical Data
Pharmacology
Antimicrobial activity
Antiviral
A compound (1’S’-1-acetoxychavicol acetate (ACA)) isolated from the rhizome extract of A. galanga through phytochemical screening had been found to have inhibitory activity against human immunodeficiency virus (HIV). This compound apparently inhibited the Rev transport at a low concentration by binding to chromosomal region maintenance 1 and accumulating full-length HIV-1 RNA in the nucleus, thus resulting in a block in HIV-1 replication in peripheral blood mononuclear cells. ACA in combination with didanosine acted synergistically to inhibit HIV-1 replication. [31]
Antifungal
Ethanol extract of A. galanga rhizome (2 mg (10 µL)) showed a pronounced antifungal activity against 9 fungi species which were Cryptococcus neoformans, Wangiellia dermatitidis, Alternaria alternate, Aspergillus fumigatus, Fusarium oxysporum, Microsporum gypseum, Pseudallescheria, Rhizopus sp.,and Trichophyton mentagrophytes, comparable to berberine when tested using disk diffusion assay. [32]
A bioactive compounds identified as [E}-8beta,17-epoxylabd-12-ene-15,16-dial was found to enhance the antifungal activity of quercetin and chalcone against Candida albicans. Its antifungal activity is attributed to a change of membrane permeability arising from membrane lipid alteration which caused the lysis of C. albicans protoplast. [33]
Chloroform extract of A. galanga showed prominent antifungal activity against Cryptococcus neoformans and Microsporum gypseum, but low inhibition towards C. albicans when tested using disc diffusion and hyphal extension-inhibition assays. [34]
Acetoxychavicol acetate compound from n-pentane/diethyl ether extract of A. galanga essential oil from dried rhizomes showed active antifungal activity against seven fungi (yeast and six dermatophytes) with MIC value for dermatophytes was within range 50- 250 µg/ml. [18]
Antibacterial
The crude acetone extract of of A. galanga dried rhizome and its isolated compound 1’-acetoxychavicol acetate showed antibacterial and antiplasmid activity against several antidrug resistant bacteria. The crude extract cured antibiotic resistant plasmids in Salmonella typhi, Escherichia coli and vancomycin resistant Enterococcus (VRE) with an efficiency of 92%, 82% and 8% respectively at 400 µg/ml SIC and the isolated compound showed antibiotic resistance inhibition for Enterococcus faecalis,, Salmonella typhi, Pseudomonas aeroginosa,, Escherichia coli and Bacillus cereus with curing efficiency of 66%, 75%, 70%, 32% and 6% respectively at SIC of 400–800 µg/ml. The inhibition was higher in crude extract which may be due to the synergistic effect of compounds in the extract. [35]
The valuable compound of 1′-acetoxychavicol acetate (ACA) from A. galanga as the Rev-export inhibitorinitiated the search for its robust analogs. The study on the structure-activity relationship of ACA and eleven ACA derivatives concluded that para substitution of the acetocyl and 1’-acetoxypropenyl groups at the benzene ring was essential and the linear ethyl and propyl chain carbonates were more active than branching chain carbonates. The formation of the quinone methide intermediate was essential for exerting the inhibitory activity of ACA. The study was able to sythesize four halogenated anologs which were more potent than ACA in particular the difluoroanalog 20d which showed four-fold potent activity. [36]
Hypoglycaemic activity
Methanol and aqueous extracts of A. galanga rhizome showed significant hypoglycaemic activity by lowering of blood glucose levels when administered orally (different doses to different group) to normal male New Zealand rabbits (1000-1500 g). The maximum activity was observed after 6 hours of treatment with 3 and 4 g/kg extract in dose-dependent manner where the glucose levels were decreased from 100 mg/dl (control) to 88.3 and 75.4 mg/dl, respectively, while no effects were seen in alloxan-diabetic rabbits (150 mg/kg i.v. of alloxan monohydrate). [37]
Antimelanogenesis activity
Ethanol extract of A. galanga dried rhizome (3.8-30 µg/mL) showed antimelanogenesis by suppressing tyrosinase activity and mRNA levels and UVA-mediated melanin production in human melanoma cells (G361). It was able to protect against UVA-induced cellular oxidant formation and depletion of CAT and GPx activities and GSH content in a dose-dependent manner. An antioxidant namely eugenol was detected to be present in the extract and its contribution to the inhibition of cellular oxidative stress and improving antioxidant defenses might be the mechanism of the extract protective effects on UVA-dependent melanogenesis. [38]
Gastroprotective activity
Two phenylpropaniods isolated from the 80 % aqueous acetone extract of A. galanga dried rhizome (1’S-1′-acetoxychavicol acetate and 1’S-1′-acetoxyeugenol acetate) exhibited marked inhibition of ethanol-induced gastric mucosal lesions (ED50 of 0.61 mg/kg and ca. 0.90 mg/kg, respectively) in male Sprague-Dawley rats (230-250 g). The effects were duplicated when tested against 0.6 M HCl and aspirin but not with indomethacid. The 1’-acetyl group was reported to be an essential component for their strong activity. It is also reported that endogenous prostaglandins and sulphydryl compound are involved in the protective effects of 1’S-1’-acetoxychavicol acetate. [39]
Antiallergic activity
Two isolated compounds from 80% aqueous acetone extract of A. galanga rhizome was reported to inhibit the release of beta-hexosaminidase, a marker of antigen-IgE-mediated degranulation in rat basophilic leukemia (RBL-2H3) cells. The compounds (1’S-1’-acetylchavicol acetate and 1’S-1’-acetyleugenol acetate) exhibited potent inhibitory activity (IC50 of 15 and 19 µM, respectively) against type I allergy. These compounds also inhibited ear passive cutaneous anaphylaxis in mice and the antigen-IgE-mediated TNF-alpha and IL-4 production, both of which participate in the late phase of type I allergic reactions in RBL-2H3 cells [40]. In addition, a development of a more potent and stable anologue of 1’S-1’-acetoxychavicol, 4-(methoxycarbonyloxyphenylmethyl) phenyl acetate was made. This compound also strongly inhibited the antigen-IgE-mediated TNF-alpha and IL-4 production. [41].
Antioxidant activity
1’S-1’acetoxychavicol acetate isolated from aqueous acetone extract of A. galanga rhizome showed Nitric Oxide (NO) production inhibition in lipopolysaccharide-activated mouse peritoneal macrophages (IC50 of 2.3 µM). The study determined the structure activity relationship where the para or ortho substitution of the acetoxyl and 1-acetoxypropenyl groups at the benzene ring was essential; the S configuration of the 1’-acetoxyl group was preferable; the presence of the 3-methoxyl group and the disappearance of the 2’-3’ double bond by hydorgenation reduced the activity; the substitution of acetyl groups with propionyl or methyl groups reduced the activity; and the lengthening of the carbon chain between the 1’- and 2’- positions reduced the activity. [42]
Aqueous acetone extract of A. galanga rhizomes showed antioxidant activity by inhibit the NO production in mouse peritoneal macrophages. Several compounds isolated from the extract were also successfully showed inhibitory effects on NO production which are galanganal (IC50 = 68 µM), galanganols B (88 µM) and C (33 µM), 1’S-1′-acetoxychavicol acetate (2.3 µM), 1’S-1′-acetoxyeugenol acetate (11 µM), trans-p-hydroxycinnamaldehyde (ca. 20 µM), trans-p-coumaryl alcohol (72 µM), and trans-p-coumaryl diacetate (19 µM) were found to show inhibitory activity. [43]
Antiarthritis activity
Acetone extract of A. galanga rhizome (6.25, 12.5, 25.0 µg/mL) showed significant inhibitory effect on the degradation of the extracellular matrix in cartilage human chondrocytes when cultured with interleukin-1β (25 ng/mL) for 3 days by inhibit up to 90 % sulphated glycosaminoglycans (s-GAGs) and up to 80 % of hyaluronun (HA) at concentration 25.0 µg/mL and 12.5 µg/mL respectively. An isolated compound from the acetone extract namely ρ-hydroxycinnamaldehyde was found to be able to reverse the interleukin-1β-inducing effect including by suppressing the release of s-GAGs and HA into the media, reduce the increased of catabolic genes expression levels (MMP-3 and MMP-13), and increase the reduced expression levels of the anabolic genes collagen, SOX9 and aggrecan core protein, thus make it a possible anti-arthritic and anti-inflammatory agents. [44]
Another study showed that the combination of A. galanga and Zingiber officinale extract (10 µg/mL) could inhibited chemokine expression in human synoviocytes and acted synergistically to suppress inflammation due to arthritis compared to control media. Synoviocytes cells activated with 1 ng/mL tumour necrosis factor alpha (TNF-α) suppressed the MCP-1 mRNA by 60 % and barely detect the IP-10 mRNA level in cell line and reduced the production of IP-10 protein in cell line A (48 pg/mL) and cell line B (10 pg/mL) compared to control (153 pg/mL and 400 pg/mL, respectively). [45]
Cytotoxic activity
Crude aqueous extract of A. galanga rhizome were tested for cytotoxixity, apoptasis and DNA damage against six different human cell lines including normal and p53-inactive fibroblasts, normal epithelial and tumour mammary cells and a lung adenocarcinoma cell line. Preliminary results did not show evidence of preferential cytotoxicity of tumour cells, however there was indication that p53-active cell lines may be more sensitive than the p53-inactive cells. Apoptosis was only observed at exposure to 300 µg/ml of the extract. At a concentration as little as 100 µg/ml, the extract generated signficant level of DNA single-strand break. The presence of 1’-acetoxychavicol and its deacetylaed derivatives was not responsible for the cytotoxicity induced by the complete aqueous extract. [46]
A compound 1’acetoxychavicol acetate (ACA) isolated from dicloromethane extract of A. galanga rhizome from Thailand exhibited cytotoxic activity by inhibit the growth of lung cancer cell line (COR L23) and breast cancer cell line (MCF7) with IC50 of 4.7 and 6.1 respectively after 48 hours exposure. [47]
A compound galangin isolated from A. galanga showed cytotoxic activity (IC50 of 30.15 mg/L) by inducing morphological changes including nuclear chromatin condensation and florescence strength of BEL-7402 cells. The activity of caspase-9, -6 and -3 value was peaked at 6h, 12h and 18h respectively. The cell apoptosis was appeared after 24 hours treated with 20-80 mg/L galangin, thus, showed that the apoptotic action of galangin on VEL-7402 was via the mitochondrial pathway. [48]
Antitumour activity
1’-acetoxychavicol acetate (ACA) isolated from A.galanga (100 or 200 ppm) dietary administered to male F344 rats for 5 weeks showed xanthine oxidase inhibition by significantly (P<0.01) inhibit azoxymethane (AOM)-induced colonic aberrant crypt foci (ACF) with 41 % and 37 % inhibition for 100 ppm and 200 ppm ACA feeding, respectively. The study suggested the inhibition was due to its cell proliferation suppression in the colonic mucosa and ACA as antitumourigenesis. [49]
Another study to test the chemopreventive efficacy of A. galanga was administered to male F344 rats for 4 weeks by the initiation feeding (before the tumour was induced) and post-initiation feeding (after the tumour was induced) of 500 ppm ACA which caused 71% and 93% inhibition in the incidence of colon carcinoma, respectively. [50]
Toxicity
No documentation.
Clinical Data
Clinical findings
A clinical trial on the efficacy of the combination of Zingiber officinaleand A. galanga on pain relieve in osteoarthritic patients was done in a randomized, double-blind, placebo-controlled, multicentre and parallel-group for 6 weeks study. It was found that the highly purified and standardized ginger extract had a noteworthy effect on reducing symptoms of osteoarthritis of the knee. This effect was moderate with good safety profile especially on the gastrointestinal adverse events. [51]
Precautions
No documentation.
Interaction & Depletion
No documentation.
Contraindications
No documentation.
Case Report
It was reported that a localized contact dermatitis with subsequent generalized erythema multiforme-liked eruption occurred in an individual following topical application of a herbal remedy. Patch tests showed the presence of an allergen in fresh and dried A. galanga. [52]
Dosage
No documentation.
Poisonous Management
No documentation.
Line drawing
References
- The Plant List. Ver 1.1. Alpinia galanga (L.) Wild. [homepage on the Internet]. c2013 [updated 2012 March 23; cited 2014 Oct 14]. Available from: http://www.theplantlist.org/tpl1.1/record/kew-296861
- Philipine Medicinal Plants. Lankauas. Alpinia galanga (L.) Wild. [updated 2013 Apr; cited 2014 Oct 14] Available from: http://www.stuartxchange.com/Lankauas.html
- Wiart C. Medicinal plants of China Korea, Japan: Bioresources for tomorrow’s drug and cosmetics. Boca Raton, Florida: CRC Press; 2012. p. 35.
- Staples G, Kristiansen MS. Ethnic library herbs: A guide to identification and cultivation in Hawai’i. Hawaii: University of Hawai’I Press. 1999. p. 12.
- Arief H. Tumbuhan Obat dan Khasiatnya 2. Jakarta: Penebar Swadaya. 2008. p. 95.
- Ibrahim H. Alpinia galanga (L.) Willd. In: van Valkenburg JLCH, Bunyapraphatsara N, editors. Plant Resources of South-East Asia No. 12(2): Medicinal and poisonous plants 2. Leiden, Netherlands: Backhuys Publisher, 2001; p. 58-59.
- Chairgulprasert V, Japakeya A, Samaae H. Phytoremediation of synthetic wastewater by adsorption of lead and zinc onto Alpinia galanga Willd. Songklanakarin J. Sci. Technol. 2013;35(2):277-233.
- Peter KV. Handbook of herbs and spices Volume 2. Cambridge: Woodhead Publishing Ltd; 2004. p. 74-76.
- Ismail Z, Ismail N, Lassa J. Malaysian herbal monograph Volume 1. Kuala Lumpur: Malaysian Monograph Committee; 1999. p. 49.
- De Guzman CC, Siemonsma JS. Plant Resources of South East Asia No. 13: Spices. Netherlands: Backhuys Publishers; 1999. p. 65-68.
- Thomas ANS. Tanaman obat tradisional 2. Yogyakarta: Penerbit Kanisius; 1992; p. 77–78.
- Kertas maklumat penanaman lengkuas. Kuala Lumpur: Department of Agriculture Peninsular Malaysia (unpublished); 2010.
- Herbal Medicine Research Centre, Institute for Medical Research. Compendium of medicinal plants used in Malaysia. Volume 1. Kuala Lumpur: HMRC IMR; 2002. p. 34.
- Labhaya RH. Bayanul adviya. Delhi: Goswami Pharmacy Press; 1997. p. 235.
- 18 Satyavati GV, Raina MK, Sharma M. Indian council medical research. Delhi: Cambridge Printing Works; 1976. p.46.
- Jansen AM, Scheffer JJC, Baerheim-Svendsen A. Abstract from Int Res Cong Nat Prod Coll Pharm. University of North Carolina. 1985 Jul; 45.
- Lily MP, Judith M. Medicinal plants of East and Southeast Asia: Attributed properties and uses. Cambridge: The MIT press; 1980. p. 436.
- Janssen AM, Scheffer JJC. Acetoxychavicol acetate, an antifungal component of Alpinia galanga. Planta Med. 1985;51 (6):507-511.
- Nori T, Sekiya T, Katoh M, et al. Inhibitors of xanthine oxidase from Alpinia galanga. Chem Pharm Bull. 1988;36:244-248.
- De Pooter HL, Omar MN, Ciiksaet BA, Schamp NM. The essential oil of greater galanga (Alpinia galanga). Phytochemistry. 1985;24:93-96.
- Mitsui S, Kobayashi S, Naghori H, Ogiso A. Constituents from seeds of Alpinia galanga Wild, and their anti-ulcer activities. Chem Pham Bull. 1976;24:2377.
- Barik BR, Kundu AB, Dey AK. Two phenolic constituents from Alpinia galanga rhizomes. Phytochemistry. 1987;26:2126-2127.
- Hasnah MS. Kumpulan sebatian semulajadi. Paper presented at Seminar tahunan ke-4 Jabatan Kimia Universiti Pertanian Malaysia; 1987 Feb; Selangor.
- Dr Daniel M.Medicinal plants: Chemistry and properties. New Hampshire: Science Publishers; 2006. p. 62-63.
- Musa Y, Azimah K, Zaharah H. Tumbuhan Ubatan Popular Malaysia. Selangor: MARDI; 2009.
- Burkill IH. A Dictionary of the economic products of the Malay Peninsula Vol. 1 & 2. Kuala Lumpur: Ministry of Agriculture Malaysia; 1966. p. 1327-1332.
- Noro T, Miyase T, Kuroyanagi M, Ueno A, Fukushima S. Monoamine Oxidase Inhibitor from the Rhizomes of Kaempferia galanga L. Chem Pharm Bull. 1983;31(8):2708-2711.
- Sastriamidjojo AS. Obot Asli Indonesia. Djakarta: Penerbit Dian Rakyat, 1948; p. 238.
- Samad Ahmad A. Warisan Perubatan Melayu. Kuala Lumpur: Dewan Bahasa dan Pustaka; 1988. p. 126.
- Gimlette JD, Thomson HW. A Dictionary of Malayan Medicine. London: Oxford Univ. Press, 1939; p. 145-146.
- Ye Y, Li B. 1’S-1′-acetoxychavicol acetate isolated from Alpinia galanga inhibits human immunodeficiency virus type 1 replication by blocking Rev transport. J Gen Virol. 2006;87(Pt 7):2047-2053.
- Ficker CE, Smith ML, Susiarti S, Leaman DJ, Irawati C, Arnason JT. Inhibition of human pathogenic fungi by members of Zingiberaceae used by the Kenyah (Indonesian Borneo). J Ethnopharmacol. 2003;85(2-3):289-293.
- Haraguchi H, Kuwata Y, Inada K, et al. Antifungal activity from Alpinia galanga and the competition for incorporation of unsaturated fatty acids in cell growth. Planta Med. 1996;62(4):308-313.
- Phongpaichit S, Subhadhirasakul S, Wattanapiromsakul C. Antifungal activities of extracts from Thai medicinal plants against opportunistic fungal pathogens associated with AIDS patients. Mycoses. 2005;48(5):333-338.
- Latha C, Shriram VD, Jahagirdar SS, Dhakephalkar PK, Rojatkar SR. Antiplasmid activity of 1′-acetoxychavicol acetate from Alpinia galanga against multi-drug resistant bacteria. J Ethnopharmacol. 2009;123(3):522-525.
- Tamura S, Shiomi A, Kimura T, Murakami N. Halogenated analogs of 1′-acetoxychavicol acetate, Rev-export inhibitor from Alpinia galanga, designed from mechanism of action. Bioorg Med Chem Lett. 2010;20(7):2082-2085.
- Akhtar MS, Khan MA, Malik MT. Hypoglycaemic activity of Alpinia galanga rhizome and its extracts in rabbits. Fitoterapia. 2002;73(7-8):623-628.
- Panich U, Kongtaphan K, Onkoksoong T. Modulation of antioxidant defense by Alpinia galanga and Curcuma aromatica extracts correlates with their inhibition of UVA-induced melanogenesis. Cell Biol Toxicol. 2010;26(2):103-116.
- Matsuda H, Pongpiriyadacha Y, Morikawa T, Ochi M, Yoshikawa M. Gastroprotective effects of phenylpropanoids from the rhizomes of Alpinia galanga in rats: structural requirements and mode of action. Eur J Pharmacol. 2003;471(1):59-67.
- Matsuda H, Morikawa T, Managi H, Yoshikawa M. Antiallergic principles from Alpinia galanga: Structural requirements of phenylpropanoids for inhibition of degranulation and release of TNF-alpha and IL-4 in RBL-2H3 cells. Bioorg Med Chem Lett. 2003;13(19):3197-3202.
- Yasuhara T, Manse Y, Morimoto T, et al. Acetoxybenzhydrols as highly active and stable analogues of 1’S-1′-acetoxychavicol, a potent antiallergic principal from Alpinia galanga. Bioorg Med Chem Lett. 2009;19(11):2944-2946.
- Matsuda H, Ando S, Morikawa T, Kataoka S, Yoshikawa M. Structure-activity relationships of 1’S-1′-acetoxychavicol acetate for inhibitory effect on NO production in lipopolysaccharide-activated mouse peritoneal macrophages. Bioorg Med Chem Lett. 2005;15(7):1949-1953.
- Morikawa T, Ando S, Matsuda H, Kataoka S, Muraoka O, Yoshikawa M. Inhibitors of nitric oxide production from the rhizomes ofAlpinia galanga: structures of new 8-9′ linked neolignans and sesquineolignan. Chem Pharm Bull (Tokyo). 2005;53(6):625-630.
- Phitak T, Choocheep K, Pothacharoen P, Pompimon W, Premanode B, Kongtawelert P. The effects of p-hydroxycinnamaldehyde from Alpinia galanga extracts on human chondrocytes. Phytochemistry. 2009;70(2):237-243.
- Phan PV, Sohrabi A, Polotsky A, Hungerford DS, Lindmark L, Frondoza CG. Ginger extract components suppress induction of chemokine expression in human synoviocytes. J Altern Complement Med. 2005;11(1):149-154.
- Muangnoi P, Lu M, Lee J, et al. Cytotoxicity, apoptosis and DNA damage induced by Alpinia galanga rhizome extract. Planta Med. 2007;73(8):748-754.
- Lee CC, Houghton P. Cytotoxicity of plants from Malaysia and Thailand used traditionally to treat cancer. J Ethnopharmacol. 2005;100(3):237-243.
- Luo H, Ma C, Wang YJ, Chen J, Liu JQ, Zhang HT. Study on apoptosis of BEL-7402 cells induced by galangin. Zhong Yao Cai. 2008;31(8):1204-1207.
- Tanaka T, Kawabata K, Kakumoto M, et al. A xanthine oxidase inhibitor 1’acetoxychavicol acetate inhibits azoxymethane-induce colonic aberrant crypt foci in rats. Carcinogenesis. 1997;18(5):1113-1118.
- Tanaka T, Kawabata K, Kakumoto M, et al. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by a xanthine oxidase inhibitor 1’acetoxychavicol acetate. Jpn J Cancer Res. 1997;88(9):821-830.
- Altman RD, Marcussen KC. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001;44(11):2531-2538.
- Hong SJ, Chang CH. Erythema multiforme-like generalized allergic contact dermatitis caused by Alpinia galanga. Contact Dermat. 2006;54(2):118-120


