Scientific Name
Amaranthus spinosus L.
Synonyms
Galliaria spitosa (L.) Nieuwl., Amaranthus spinosus var. viridicaulis Hassk., Amaranthus spinosus var. rubricaulis Hassk., Amaranthus spinosus var. pygmaeus Hassk., Amaranthus spinosus var. purpurascens Moq., Amaranthus spinosus f. inermis Lauterb. & K.Schum., Amaranthus spinosus var. indehiscens Thell., Amaranthus spinosus var. circumscissus Thell., Amaranthus spinosus var. basiscissus Thell. [1]
Vernacular Name
| Malaysia | Bayam duri, bayam hutan [2] |
| English | Spiny amaranth, prickly amaranth, spiny pigweed [2] |
| China | Chih hsien, ci xian, le xian cai [3] |
| India | Acchadro, bahuvirya, cauleyi, didau, erramullugoranta, hati khutura, ikkiri, janum arak, kandakamarisha, kante ki cholai, konta koda, lure, meghanada, mullen keera, mulluithota koora, mulugire soppu, nalladoggali, neu, paniccakkirai, rajgira, sphurjathu, tandula, valiya mullen cheera, varataru, yerra mullu-gorinta [3] |
| Brunei | Bayam berduri (Malay) [2] |
| Indonesia | Bayam duri (General); bayem eri (Javanese); senggang cucuk (Sundanese) [2] |
| Thailand | Mang-lang-du (Karen-Mae Hong Son); pa-tue (Khmer); phak hom nam (Peninsular) [2] |
| Laos | Hôm hna:m [2] |
| Philippines | Urai (Tagalog); harum (Bisaya); kalunai (Iloko) [2]; alayon, ayantoto, aynatoto, baoan, bayambang, gitin-giting, hakum, harum kolitis, kaunton, kilitis, kolitis, kuantong, kulitis, oort, orai, oray, tadtad, taikuda, tilitis, urai, uri, uray [3] |
| Cambodia | Phti: bânla: (Pursat) [2] |
| Vietnam | Rau d[eef]n gai (General); gi[eef]ngai (Ha Nam Ninh) [2] |
| Nepal | Ban lunde, bangaidhap, banlude, lunde [3] |
| Japan | Hari-biyu (spiny Amaranthus) |
| Nigeria | Ebe-egban, efo [3] |
| South Africa | Doringmisbredie, hanekam, misbredie, rooiduiwel [3] |
| France | Epinard malabre [2] |
| Mexico | Bala chi, guie lachi, quiltonil de burro, quiltonil de pájaro [3] |
| Hawaii | Pakai kuku [3]. |
Geographical Distributions
Amaranthus spinosus occurs in all tropical and subtropical regions, including the whole of Southeast Asia, often gregariously and as a weed. It is sometimes found in temperate zones as well. It has been suggested that spiny amaranth originates from lowland tropical South and Central America, and that it was introduced in other warmer parts of the world from about 1700 AD onwards. Nowadays, it is rarely cultivated. [2]
Botanical Description
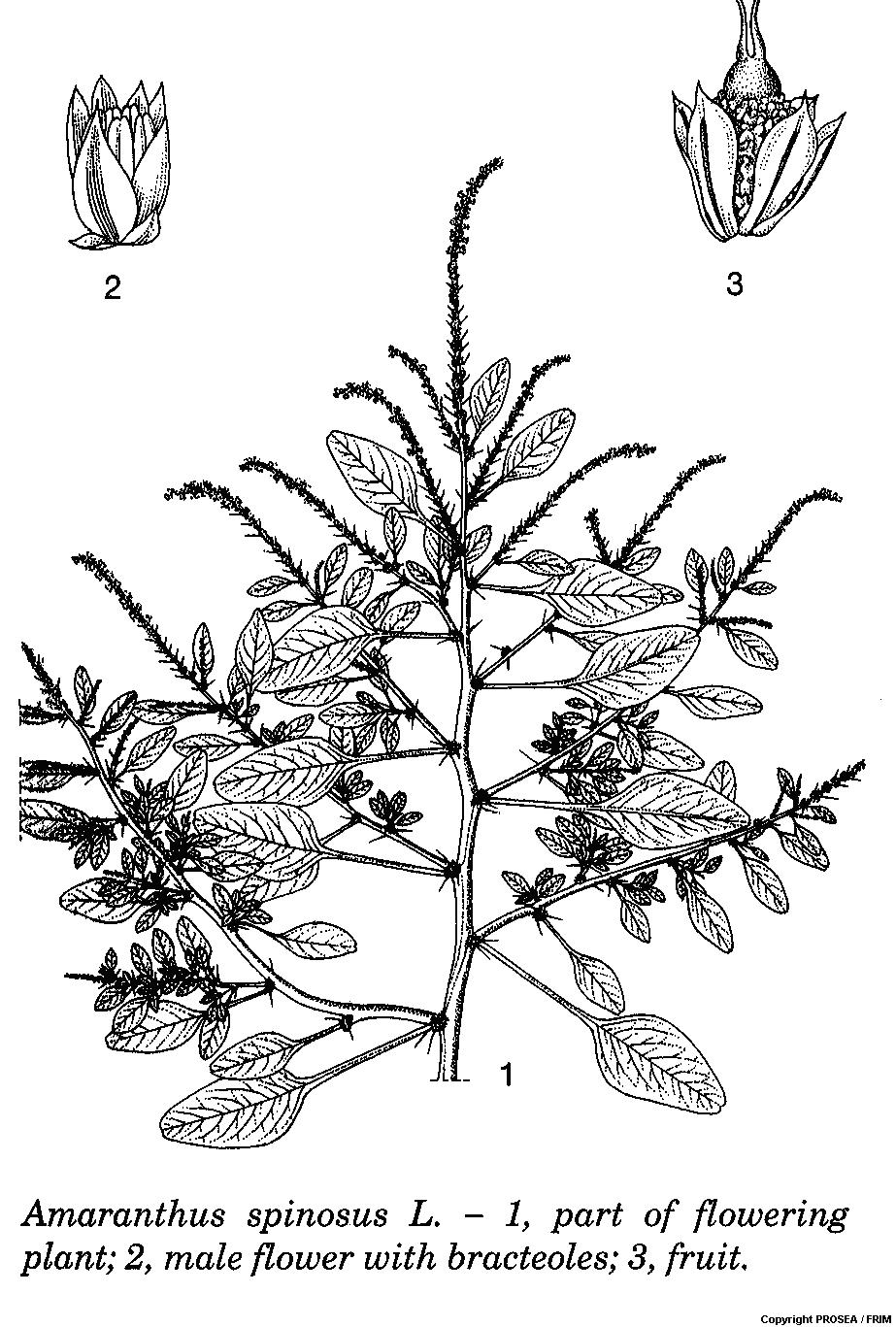
A. spinosus is a member of the family Amaranthaceae. It is an annual, erect monoecious herb up to 100(-130) cm tall and it is much branched. The stem is terete or obtusely angular, smooth or slightly hairy, and green or variably suffused with purple. [2]
The leaves are arranged alternate, simple and entire, ovate-lance-shaped to rhomboid, measuring 3.5-11 cm x 1-4.5 cm, acute and slightly decurrent at base, obtuse, rounded or slightly retuse and often short mucronate at apex, hairless or slightly hairy on veins when young. The petiole is rather long, approximately as long as leaf blade. Stipules are absent. [2]
The inflorescence consists of dense clusters. The lower ones are axillary while the higher ones are often collected in an axillary and terminal spike which is often branched in its lower part. The axillary clusters are usually armed with (1-)2(-3) very sharp spines up to 2 cm long. The flowers are solitary in the axil of a bract, subtended by 2 bracteoles, with scarious bracts and bracteoles, mucronate from a broad base, shorter or as long as the perianth and unisexual. The male flowers are usually arranged in a terminal spike above the base of the inflorescence and it is green. There are 5 tepals or often 3 in male flowers, free, subequal, ovate-oblong to oblong-spatulate, measure up to 2.5 mm long, very convex, membranous, with transparent margins and green or purple median band. There are 5 stamens and they are about as long as tepals. The ovary is superior, oblong, 1-celled, with 2-3 styles and ultimately recurved. [2]
The fruit is an oblong utricle with persisting styles, circumscissile a little below the middle or indehiscent and 1-seeded. The seed is about 1 mm in diametre, shiny black or brownish-black with thin margin. Seedling is with epigeal germination. Cotyledons are leafy and hairless while apex is rounded to slightly acute. The hypocotyl is up to 12 mm long while epicotyl is absent. [2]
Cultivation
A. spinosus is adapted to a wide range of climatic and edaphic factors. It grows best in the sun or in light shade; light intensity of less than 30% completely suppresses flowering. Flowering is the earliest and most abundant in areas with day length of 11-12 hours. It is nitrophilous and prefers soils with high organic matter content, but is also able to grow on sandy soils. Optimal growth is obtained on soils with moderate moisture content, but spiny amaranth is capable of growing on wet soils as well. It is drought-resistant and can even grow under arid conditions. A. spinosus is a very noxious weed in many parts of the world. It is, for instance, troublesome in upland rice, sugar cane and carrot in Indonesia, in maize in the Philippines, in groundnut and soya bean in Taiwan, and in tomato and field pea in India. In Southeast Asia, it is very common in roadsides, waste places, railway yards, cropped lands and gardens up to 1400 m altitude. [2]
Chemical Constituent
A. spinosus has been reported to contain 7-p-coumaroyl apigenin 4-O-β-D-glucopyranoside, coumaroyl flavone glycoside (e.g. spinoside, xylofuranosyl uracil, β-D-ribofuranosyl adenine, β-sitosterol glucoside, hydroxycinnamates, quercetin and kaempferol glycosides), betalains, betaxanthin, betacyanin; amaranthine and isoamaranthine, gomphrenin, betanin, β-sitosterol, stigmasterol, linoleic acid, 0.15% rutin and β-carotene. [4][5]
The carbohydrate content is 1.16 g/100 g leaves, energy 27 kcal, moisture 91 g, protein 4 g, fat 0.6 g, fibre 2.48 g, ash 2.76 g. [6]
The mineral content has been reported such as iron (38.4 mg/100 g dry weight), calcium (968.7 mg/100 g dry weight), magnesium (912.4 mg/100 g dry weight), phosphorus (816.3 mg/100 g dry weight), manganese (6.8 mg/100 g dry weight), copper (1.2 mg/100 g dry weight), zinc (6.8 mg/100 g dry weight). [7]
Plant Part Used
Root, leaf, tender shoot. [8][9][10]
Traditional Use
In Thai traditional medicine, A. spinosus is used to treat diarrhea. [11]
The leaves of amaranthus spinosus was reported to be used to treat tuberculosis [8]. In Ladakh, the leaves are made into tea and taken twice daily for 7 to 8 days to promote kidney function [9].
The tender shoot of Amaranthus spinosus is used to promote the secretion of milk among nursing mothers. [10]
The roots of the plant are used in India to treat indigestion. [10]
Preclinical Data
Pharmacology
Antiprotozoal activity
The dichloromethane extract of A. spinosus (2mg/mL) was moderately inhibitory to Blastocystis hominis, a common human protozoan. The reference anti-protozoan agent, metronidazole (40 µg/mL) killed 97% of the protozoan and inhibited all protozoan samples at concentrations of 1.25-20 µg/mL. [11]
Anti-inflammatory activity
Methanol extract of A. spinosus extract (25-100 mg/kg) showed anti-inflammatory activity by significantly inhibited carrageenan-induced rat paw edema and produced significant inhibition of acetic acid-induced increase in vascular permeability indicating that the extract has anti-inflammatory activity. In the cotton pellet granuloma test, rats were treated orally with the extract for 4 consecutive days after the subcutaneous implantation of a sterile pellet. The highest dose of the extract (100 mg/kg) was able to significantly reduce the post-implantation weight of cotton pellets compared to controls indicating its effectiveness against acute inflammation. The extract (25-100 mg/kg) elicited a significant reduction in castor oil-induced diarrhea. [12]
Severe gastric erosion was seen in rats given the extract (50 and 100 mg/kg) repeatedly for 4 days, which may reflect its ability to inhibit prostaglandin synthesis. This was not seen in the controls or with a lower dose of the extract [25 mg/kg]. The extract (25-100 mg/kg) also delayed castor oil-induced diarrhea in rats, which was postulated to reflect its prostaglandin synthesis inhibitory activity. [12]
A. spinosus extract also exhibited a highly specific prostaglandin synthesis inhibitory activity in vitro in an anti-inflammatory model test system, indicating its anti-inflammatory properties. [13]
Antioxidant activity
The antioxidant capacity of A. spinosus was studied in roadside plants which were postulated to be continuously exposed to the high levels of nitrogen oxides and sulphur dioxide from automobile emissions. A. spinosus was shown to possess a very good free radical scavenging system for combating air pollution through analysis of the enzymes superoxide dismutase, catalase, ascorbate peroxidase, glutathione reductase and phenolic peroxidase activities. [14]
Amaranthaceae plants contain betalain pigments which showed strong antioxidant activities by the DPPH assay. Their EC50 values range from 3.4 to 8.4 µM. The antioxidant activity of A. spinosus extract may be due to its betalain content. [15]
Antimalarial activity
The aqueous extract of A. spinosus bark obtained from mature stems was screened for antimalarial properties in mice inoculated with erythrocytes parasitized with Plasmodium berghei berghei. The bark extract showed a dose-dependent antimalarial activity in a 4-day suppressive antimalarial assay using chloroquine as the reference antimalarial drug. ED50 valuesfor the antimalarial activities of the extract and chloroquine were 789.4 and 14.6 mg/kg, respectively. [16]
Analgesic activity
Methanolic extract of A. spinosus leaves (25-100 mg/kg) produced a dose-dependent decrease in acetic acid-induced writhing with the highest dose producing an effect (56.2% inhibition of writhing) which was comparable to that of 5 mg/kg indomethacin (58.4% inhibition of writhing). These doses of the extract also reduced the licking time at the late phase (20 minutes post formalin), not the early phase of the formalin-induced paw licking assay in mice. These results indicate that A. spinosus extract has analgesic activity. Positive results in the late phase of the formalin test indicate that the extract inhibited pain which was associated with inflammation. [12]
Immunomodulatory activity
The water extract of A. spinosus leaves showed immuno-modulatory effects by significantly stimulating splenocyte proliferation in primary splenocytes from female BALB/c mice. The extract stimulated isolated B lymphocytes, not T lymphocytes, in a dose response manner. The water extract (1250 µg/mL) elicited a much higher proliferation rate in bulk splenocytes than in isolated purified B and T cells, suggesting some sort of interaction between these cells. Thus, the immuno-stimulating effects of the water extract may lead to B lymphocyte activation which will subsequently, through secondary signaling, lead to T lymphocyte proliferation. A novel immuno-stimulatory protein (GF1) with a molecular weight of 313 kDa was obtained after sequential purification of the water extract. GF1, which was assumed to be a glycoprotein and was heat labile, had an immuno-stimulatory activity which was 309 times higher than that of the water extract [17]. The phenolic and flavanoid contents of the plant showed significant cytokine secretion that might demonstrate immuno-modulatory activities via Th1/Th2 cytokines [18].
Antinociceptive activity
Amaranthus Spinosus has antinociceptive properties based on previous studies that shows its inhibitory effects on stimuli caused by chemical-induced nociceptive. Due to this, the plant acts on the peripheral and central system as an analgesic. [19]
Hepatoprotective activity
The phytochemical constituents that are extracted from the plants showed inhibition of lipid peroxidation that contributes to antioxidant and hepatoprotective activities [20]. In addition, at increasing concentration used to treat rats with CCl4 toxicity, it can be seen improvement on the biochemical parameters. This indicates that Amaranthus spinosus possessed enzyme restoring ability due to its free scavenging radical activity [21].
Toxicity
The aqueous extract of the bark of A. spinosus has a relatively low toxicity LD50 value of 1450 mg/kg. [16]
A. spinosus was reportedly the culprit in cases of spontaneous poisoning of cattle in Brazil during a severe drought. Clinical signs appeared after 30 days in 11 out of 35 adult cows and 8 out of 20 yearling calves which were introduced into a 15 ha maize plantation heavily infested with A. spinosus. However, only one calf died within 3-7 days. The clinical signs were depression, anorexia, marked weight loss, foul-smelling diarrhea occasionally tinged with blood, and subcutaneous oedema. Subacute cases showed distended abdomens, the animals were reluctant to stand and walked with difficulty. Sloughing of the hooves occurred in some animals. The main post-mortem findings in 5 animals were moderately pale and swollen kidneys, perirenal oedema and varying degrees of oedema in several tissues and cavities. In some cases petechiae and suffusions were associated with the subcutaneous oedema. The mucosa of the digestive system showed necrotic glossitis, oesophagitis and pharyngitis, abomasal hemorrhages and button-like ulcerations in the large intestine. The contents of ileum, colon and rectum were blood stained. Hemorrhagic diathesis was apparent by the presence of intra-abdominal hematomas. Histologically, there was marked tubular nephrosis associated with epithelial regeneration and hyaline intra-tubular casts. The mucosal lesions consisted of large necrotic areas in the epithelium which extended into the lamina propria and were associated with inflammatory reaction with massive infiltrations of mastocytes. The omasal mucosa had selective necrosis of the basal layer cells. Renal failure was suggested as the primary lesion which triggered the other changes. [22]
Clinical Data
No documentation.
Poisonous Management
No documentation.
Line Drawing
References
- The Plant List. Ver1.1. Amaranthus spinosus L. [homepage on the Internet]. c2013 [updated 2012 Apr 18; cited 2016 May 26]. Available from: http://www.theplantlist.org/tpl1.1/record/kew-2633107
- Lemmens RHMJ, Bunyapraphatsara N. Amaranthus spinosus L. In: de Padua LS, Bunyapraphatsara N, Lemmens RHMJ, editors. Plant Resources of South-East Asia No. 12(1): Medicinal and poisonous plants 1. Leiden, Netherlands: Backhuys Publishers, 1999; p. 110-113.
- Quattrocchi U. CRC world dictionary of medicinal and poisonous plants: Common names, scientific names, eponyms, synonyms, and etymology. Volume I A-B. Boca Raton, Florida: CRC Press, 2012; p.231-232.
- Azhar-ul-Haq, Malik A, Khan AU,Shah MR, Muhammad P. Spinoside, new coumaroyl flavone glycoside from Amaranthus spinosus. Arch Pharm Res. 2004;27(12):1216-1219.
- Suryavanshi VL, Sathe PA, Baing MM, Singh GR, Lakshmi SN. Determination of rutin in Amaranthus spinosus Linn. whole plant powder by HPTLC. Chromatographia. 2007;2(2):134-152.
- Odhava B, Beekrumb S, Akulaa U, Baijnath H. Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal, South Africa. J Food Comp Anal. 2007;20:430-435.
- Barminas T, Charles M, Emmanuel D. Mineral composition of non-conventional leafy vegetables. Plant Food Hum Nutr. 1998;53(1):29-36.
- Tabuti JR, Kukunda CB, Waako PJ. Medicinal plants used by traditional medicine practitioners in the treatment of tuberculosis and related ailments in Uganda. J Ethnopharmacol. 2010;127(1):130-136.
- Ballabh B, Chaurasia OP, Ahmed Z, Singh SB. Traditional medicinal plants of cold desert Ladakh-used against kidney and urinary disorders. J Ethnopharmacol. 2008; 2(118):331-339.
- Buragohain J. Folk medicinal plants used in gynecological disorders in Tinsukia district, Assam, India. Fitoterapia. 2008;5(79):388-392.
- Sawangjaroen N, Sawangjaroen K. The effects of extracts from anti-diarrheic Thai medicinal plants on the in vitro growth of the intestinal protozoa parasite: Blastocystis hominis. J Ethnopharmacol. 2005;98(2005):67-72.
- Olajide OA, Ogunleye BR, Erinle TO. Antiinflammatory properties of Amaranthus spinosus leaf extract. Pharm Biol. 2004;42(7):521-525
- Ibewuike J, Ogundaini AO, Bohlin L, Ogungbamila FO. Anti-Inflammatory activity of selected Nigerian medicinal plants. Nig J Nat Prod Med. 1997;1:10-14
- Mandal M, Mukherji S. A study on the activities of a few free radicals scavenging enzymes present in five roadside plants. J Environ Biol. 2001;22(4):301-305.
- Cai Y, Sun M, Corke H. Antioxidant activity of betalains from plants of the amaranthaceae. J Agric Food Chem. 2003; 51(8):2288-2294
- Hilou A, Nacoulmaa OG, Guiguemdeb TR. In vivo antimalarial activities of extracts from Amaranthus spinosus L. and Boerhaavia erecta L. in mice. J Ethnopharmacol. 2006;103(2):236-240.
- Lin B, Chiang B, Lin J. Amaranthus spinosus water extract directly stimulates proliferation of B lymphocytes in vitro. Int Immunopharmacol. 2005;5(4):711-722.
- Lin JY, Tang CY. Total phenolic contents in selected fruit and vegetable juices exhibit a positive correlation with interferon-γ, interleukin-5, and interleukin-2 secretions using primary mouse splenocytes. J Food Comp Anal. 2008;1(21):45-53.
- Zeashan H, Amresh G, Rao CV, Singh S. Antinociceptive activity of Amaranthus spinosus in experimental animals. J Ethnopharmacol. 2009;3(122):492-496.
- Zeashan H, Amresh G, Singh S, Rao CV. Hepatoprotective activity of Amaranthus spinosus in experimental animals. Food Chem Toxicol.2008;11(46):3417-3421.
- Zeashan H, Amresh G, Singh S, Rao CV. Hepatoprotective and antioxidant activity of Amaranthus spinosus against CCl4 induced toxicity. J Ethnopharmacol. 2009;2(125):364-366.
- Kawade RM, Ghiware NB, Sarje SK, Vadvalkar SM. A pharmacognostic and pharmacological review: Amaranthus spinosus. World J Pharm. 2013;2(6);2099-2110

