Hempedu bumi Herb
Andrographis paniculata (Burm.f.) Wall. ex Nees
Acanthaceae
DEFINITION
Hempedu bumi herb consists of the powder of dried aerial part of Andrographis paniculata (Burm.f.) Wall. ex Nees. (Acanthaceae) with occasionally flowers and pods are present [ 1 , 2 , 3 ].
SYNONYM
Justicia latebrosa Russ., J. paniculata Burm. f., J. stricta Lam. ex Steud [ 1 , 2 , 3 ].
VERNACULAR NAMES
Akar cerita, hempedu bumi, bidara (Malay), chuan xin lian (Chinese), nilavempu (Tamil), king of bitters (English) [ 3 , 4 , 5 ].
CHARACTER
The leaves are green to dark green in color, odourless and intensely bitter taste. The powder is green in colour with slight characteristic odour.
IDENTIFICATION
Plant Morphology
A herbaceous annual, erect, up to 1 m high. Stem quadrangular to acutely quadrangular with longitudinal furrows and numerous long divaricats branches. Leaves simple, opposite, lanceolate or ovate-lanceolate, tapering at both ends especially at the base of petiole, glabrous, 2-12 cm long, 1-3 cm wide, apex acuminate, margin entire, slightly undulate, upper leaves often bractiform, dark green above but much paler beneath; petiole short or nearly sessile. Numerous small flowers pale but blotched and spotted with brown and purple 10-30 mm long, distantly arranged singly or in pairs in a somewhat unilateral manner along the upper side of elongated, arching or slender branches, spreading axillary and terminal racemes or panicles, bract and bractlets small, ca. 1 mm long; pedicel short; calyx-lobed triangular-lanceolate 5-particle glandular pubescent, small, ca. 3 mm long, linear; corolla tube narrow, about 6-12 mm long; limb longer than the tube, nearly libiate to bilabiate; upper lip 2 lobate to oblong, white with a yellowish top; lower lip broadly cuneate to cuneate-decurrent, 3-lobed, white with violet markings or pale purple stripe; corolla same length as labellum; stamens 2, inserted in the throat of the corolla and far exerted; filaments are flattened and hairy in the upper part; anthers 2 chambers, 1 chamber base and one side of filament pubescent or basally bearded; superior ovary, 2-celled; style far exerted. Capsule/pod erect, linear-oblong, 1.0-2.3 cm long and 2-5 mm wide, flat or compressed, longitudinally furrowed or indentation in middle of broad faces, acute at both ends, thinly glandular-hairy, cracks into 2 when mature; numerous small seeds 6-12 per capsule, small, glabrous, yellowish brown to red, subquadrate, wrinkled.
Microscopy
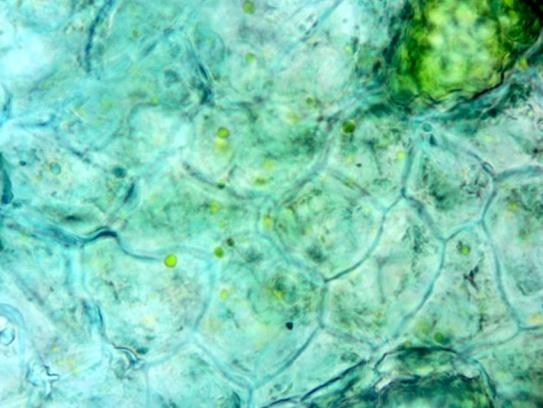
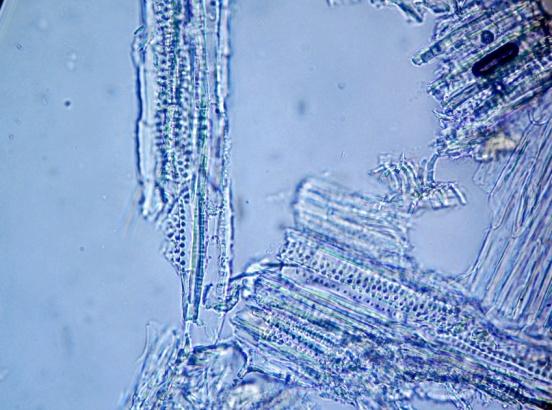
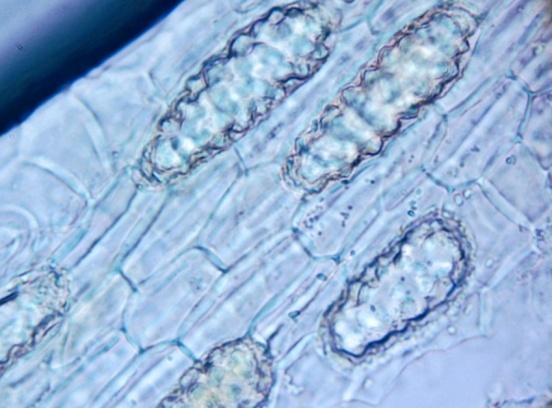
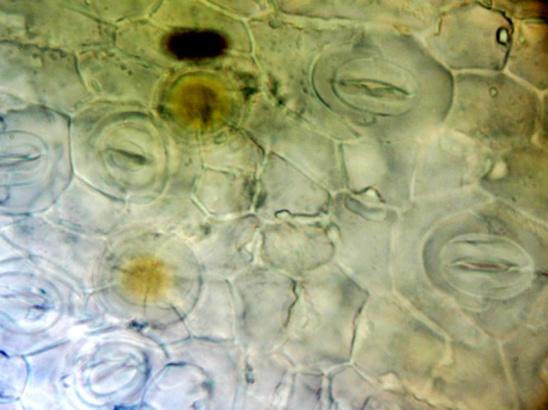
Shows abundant simple, uni- and 2-3 celled multicellular short and long trichomes; glandular trichomes with short and long multicellular stalk and multicellular head from leaf, stem and all parts of the flower; long acicular fibers from xylem region of stem, epidermal cells of the leaf and petiole in surface view embedded with diacytic stomata and cystolith; parenchymatous cells of the pith of stem embedded with acicular crystals of calcium oxalate and simple starch grains; fragments of spiral, pitted and scalariform vessels, polygonal thick-walled cells of the corolla tube, pollen grains and sclereidal layers from the stamen; fragments of cotyledon and endosperm, fibers and sclereids from the fruit wall
[ 1 ].
Colour Tests
Observed colour of solution after treatment with various reagents:
| H2SO4 (conc.) | Dark brown |
| KOH (5%) | Dark green |
| FeCl3 (5%) | Greenish brown |
Thin Layer Chromatography (TLC)
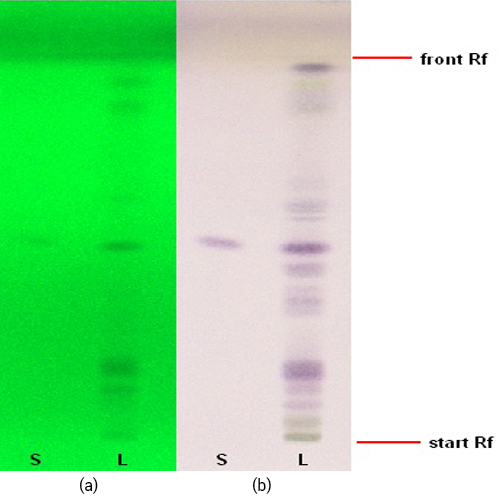
Figure 3 : TLC profiles of andrographolide (S) and ethanol extract of A. paniculata herb (L) observed under (a) UV at 254 nm and (b) visible light after spraying with anisaldehyde-sulphuric acid.
| Test Solutions | Weigh about 1.0 g of of A. paniculata dried herb powder in a 250 mL round bottom flask and add 50 mL of ethanol into the flask. Reflux the sample for 2 hr and allow to cool. Filter the mixture and use the filtrate as the test solution. |
| Standard solution | Dissolve 2.0 mg andrographolide in 20 mL methanol to give 100 µg/mL solution. |
| Stationary Phase | HPTLC silica gel 60 F254, 5 x 10 cm |
| Mobile phase | Toluene : Ethyl acetate : Formic acid, 5 : 3.5 : 1.5 (v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
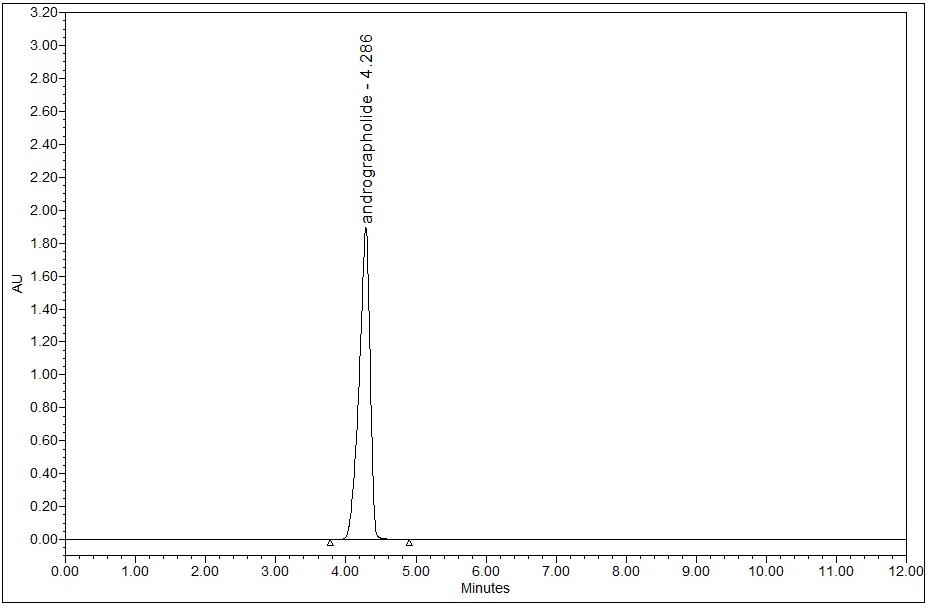
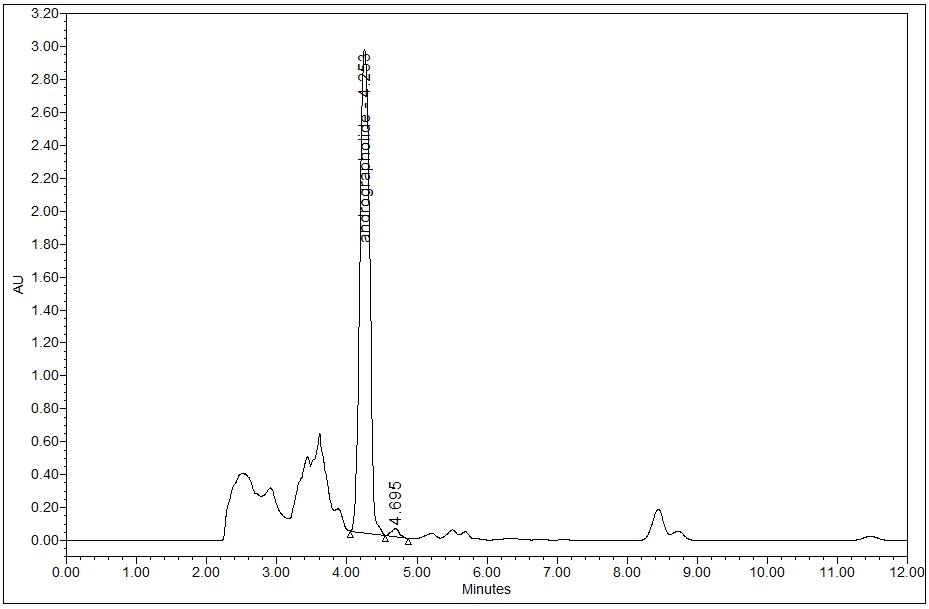
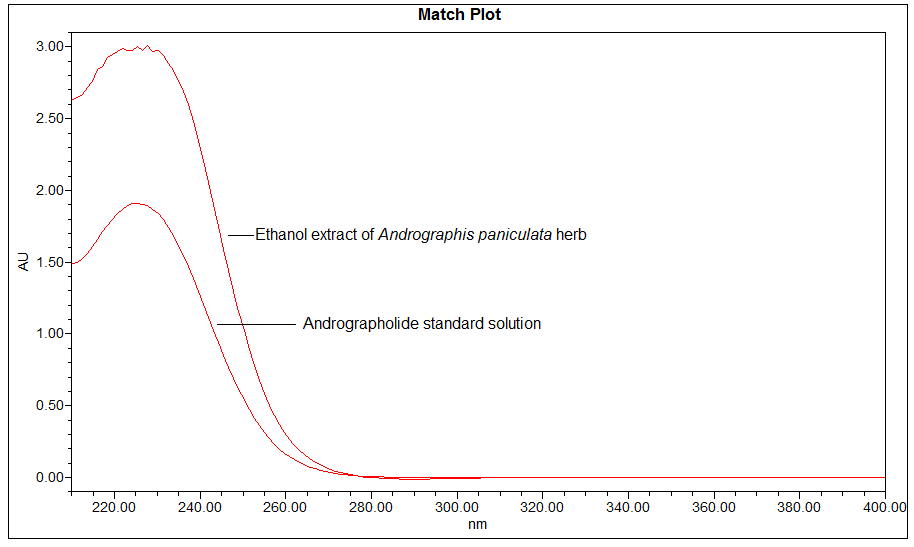
High Performance Liquid Chromatography (HPLC)
| Test solution | Extract about 1.0 g of A. paniculata dried herb powder with 10 mL ethanol in a 50 mL screw-capped conical flask. Sonicate the mixture for 30 min and allow to cool. Filter the mixture solution through a 0.45 µm syringe filter and inject the filtrate into the HPLC column. |
| Standard solution | Dissolve 5.0 mg of andrographolide standard in 10 mL of ethanol to produce 500 µg/mL solution. |
| Chromatographic system |
Detector: UV 223 nm Column: C18 (5 µm, 4.6 mm I.D x 250 mm) Column oven temperature: 25°C (Room temperature) Flow rate: 0.8 mL/min Injection volume: 10 µL |
| Mobile Phase (Isocratic mode) | 0.1% phosphoric acid in water: acetonitrile, 54:46 (v/v). Run time (min) : 15 |
| System suitability requirement |
Perform at least five replicate injections of andrographolide (500 µg/mL). The requirements of the system suitability parameters are as follow:
|
| Acceptance criteria |
|
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents Acid-insoluble ash | |
| Total ash | Not more than 11% |
| Acid-insoluble ash | Not more than 2% |
| Loss on Drying |
| Not more than 10% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 23% |
| Cold method | Not less than 14% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 20% |
| Cold method | Not less than 13% |
SAFETY TEST
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
The aqueous extract of A. paniculata aerial parts was found to contain andrographolide, neoadrographolide, 14-deoxy-11,12 didehydroandrographolide, 5,4′-dihydroxy-7-methoxyflavone-6-yl-β-D-glucopyranoside and 5,4′-dihydroxy-7-methoxyflavone-8-yl-β-D-glucopyranoside [ 6 , 7 ].
Methanol extracts contain diterpenoids (e.g. andrographolide, 8α-methoxyl-14-deoxy-17β-hydroxyandrographolide, dehydroandrographolide, 14-deoxy-11,12-didehydroandrographolide, 14-deoxy-15-isopropylidene-11,12-didehydroandrographolide, isoandrographolide, neoandrographolide, 19-O-β-D-glucopyranosyl-ent-labda-8(17),13-dien-15,16,19-triol, ent-14β-hydroxy-8(17),12-labdadien-16,15-olide-3β,19-oxide, deoxyandrographiside, andrographiside) [ 8 , 9 , 10 , 11 ].
Ethanol extracts had mainly diterpenoids (e.g. andrographolide, 12S,13S-hydroxyandrographolide, 12R,13R-hydroxyandrographolide, 14-deoxy-12-hydroxyandrographolide, 14-deoxyandrographolide, 3-oxo-14-deoxyandrographolide, 7S-hydroxy-14-deoxyandrographolide, 7R-hydroxy-14-deoxyandrographolide, 3,14-dideoxyandrographolide, 14-deoxy-11,12-didehydroandrographolide, 14-deoxy-11,12-didehydroandrographolide, isoandrographolide, (8S,12S)-isoandrographolide, (8S,12R)-isoandrographolide, (8R,12R)-isoandrographolide, (8R,12S)-isoandrographolide, neoandrographolide, bisandrographolide, 19-hydroxy-3-oxo-ent-labda-8(17),11,13-trien-16,15-olide, 3,18,19-trihydroxy-ent-labda-8(17),13-dien-16,15-olide, 3,19-dihydroxy-ent-labda-8(17),12-dien-16,15-olide, 19-[(β-D-lucopyranosyl)oxy]-19-oxo-ent-labda-8(17),13-dien-16,15-olide, 3,19-dihydroxy-15-methoxy-ent-labda-8(17),11,13-trien-16,15-olide, ent-labda-8(17),13-diene-15,16,19-triol, 3,15,19-trihydroxy-ent-labda-8(17),13-dien-16-oic acid, 3,19-dihydroxy-14,15,16-trinor-ent-labda-8(17),11-dien-13-oic acid, 13,14,15,16-tetranor-ent-labd-8(17)-ene-3,12,19-triol, 19-hydroxy-8(17),13-labdadien-15,16-olide, 19-norandrographolides A-C, andrographiside, 14-deoxyandrographiside, 14-deoxy-11,12-didehydroandrographiside) and flavonoids (e.g. 7-O-methylwogonin, 7-O-methyldihydrowogonin, 5-hydroxy-7,8,2′,5′-tetramethoxy-flavone, skullcapflavone-2′-methoxylether, 5-hydroxy-7,8,2′,3′-tetramethoxyflavone, 5,4′-dihydroxy-7,8,2′,3′-tetramethoxyflavone, dihydroskullcapflavone, 5,7,8-trimethoxydihydroflavone, 5,2′-dihydroxy-7,8-dimethoxyflvone, andrographidine C, 5,7,4′-trihydroxyflavone, 5,7,3′,4′-tetrahydroxyflavone) [ 12 , 13 , 14 , 15 , 16 , 17 , 18 ].
The acetone extract had 3,7,19-trihydroxy-8,11,13-ent-labdatrien-15,16-olide and 8α,17β-epoxy-3,19-dihydroxy-11,13-ent-labdatrien-15,16-olide while the ethyl acetate extract had andrographolactone [ 19 , 20 ].
The chloroform and dichloromethane extracts were found to contain andrographolide, dehydroandrographolide, 14-deoxyandrographolide, 14-deoxy-11,12-didehydroandrographolide and neoandrographolide, in addition to 14-deoxy-12-hydroxyandrographolide in the dichloromethane extract [ 21 , 22 , 23 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
A. paniculata acts as a bitter tonic in patients with diarrhoea or dysentery. The weed is chewed while the juice is swallowed and applied to the wound caused by snake-bites and stings of insects [ 4 ].
Biological and pharmacological activities supported by experimental data
Antimalarial activity
Andrographolide from the methanol fraction of A. paniculata aerial part (0.2-1.0 µM) inhibited the growth of Plasmodium falciparum with an IC50 value of 9.1 µM. [24] The compound (15 mg/kg/day) in combination with curcumin administered intraperitoneally to P. berghei ANKA-infected Balb/C mice for a duration of 15 days decreased parasitemia percentage at 29% [ 24 ].
Methanol extract of A. paniculata aerial part (1.8–500 μg/mL) inhibited the growth of P. falciparum with an IC50 value of 7.2 μg/mL [ 25 ]. The extract (7 mg/kg/day) in combination with Hedyotis corymbosa administered intraperitoneally to P. berghei ANKA-infected Balb/C mice for a duration of 13 days decreased parasitemia percentage at 32% [ 25 ].
Antithrombotic activity
14-deoxy-11,12-didehydroandrographolide from the aqueous extract of A. paniculata aerial part (1–100 μM) significantly (P<0.01) inhibited platelet aggregation induced by thrombin with an IC50 value ranged from 10 to 50 μM. [26] The compound (100 μM) also inhibited the phosphorylation of extracellular signal-regulated kinase 2 in thrombin-induced platelet aggregation [ 26 ].
Antidiabetic activity
Ethanol extract of A. paniculata aerial part (400 mg/kg) administered orally twice daily to streptozotocin-induced diabetic male Sprague Dawley rats for a duration of 14 days significantly decreased fasting serum glucose (P<0.001), liver and kidney thiobarbituric acid reactive substances values (P<0.0001) and significantly (P<0.05) increased liver superoxide dismutase and catalase enzymes [ 27 ].
Anticancer activity
Methanol, methanol-aqueous (1:1) and aqueous extracts of A. paniculata aerial part showed no cytotoxic effect on human HT-1080 fibrosarcoma cells with an EC50 value of 90 µg/mL for methanol extract, >100 µg/mL for methanol-aqueous extract and >100 µg/mL for aqueous extract [ 28 ].
Andrographolide from ethyl acetate extract of A. paniculata aerial part (up to 100 µg/ml) showed selective cytotoxic effect on human prostate adenocarcinoma PC-3 cells with an IC50 value of 5 µg/mL without affecting normal Chang liver cells with an IC50 value of > 50 µg/mL [ 29 ].
Antihypertensive activity
14-deoxy-11,12-dihydroandrographolide and 14-deoxyandrographolide from dichloromethane extract of A. paniculata aerial part (1 mg/mL) significantly (P<0.05) decreased the coronary perfusion pressure but no significant effect on cardiac contractility and heart rate in isolated Langendorff perfused rat hearts [ 30 ].
Clinical studies
A clinical trial to study the tolerance and adverse effect of A. paniculata in patients with diabetes mellitus type 2 was conducted. It is an open label clinical trial which involved 6 male and 14 female patients (35-70 years old). The patients took 2 capsules containing powdered of A. paniculata aerial part at the dosage of 600 mg/day followed by a gradual increase to a maximum dose of 1800 mg/day with breakfast for a duration of 12 weeks. Result showed no significant adverse effects and toxicity effects observed except for a significant reduction in glycosylated haemoglobin (P<0.01) and fasting serum insulin levels (P<0.003) [ 31 ].
A capsule of methanol extract of A. paniculata leaf (200 mg/day) when given to patients with uncomplicated upper respiratory tract infection significantly (p< ) reduced symptoms assessed which includes cough, expectoration, nasal discharge (running nose), headache, fever, sore throat, malaise/fatigue and sleep disturbance after 5 days [ 32 ].
SAFETY INFORMATION
Preclinical studies (toxicology study)
Acute toxicity
Oral single dose acute toxicity study using aqueous mixture of dried powder of A. paniculata aerial part on female Sprague Dawley rats (aged between 8 and 12 weeks old) showed no toxic effect on the parameters observed which includes behaviors, body weight, food and water intakes. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. No-observed-adverse-effect level (NOAEL) is more than 2,000 mg/kg body weight. [ 33 ].
Impairment of fertility
Standardized extract of A. paniculata (20-1000 mg/kg bw/day) administered orally to male rats for duration of 60 days showed no reproductive toxicity effect[34].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Adverse reactions
A. paniculata administered orally in high dose might result in gastric discomfort, vomiting and loss of appetite. These side effects appear to be due to the bitter taste of andrographolide [ 35 ].
Contraindications
A. paniculata should not be used during pregnancy or lactation. It is contraindicated in cases of known allergy to plants of the Acanthaceae family [ 31 ].
Precaution
Aqueous and alcohol extracts of A. paniculata aerial parts can cause herb-drug interactions through modulation of hepatic cytochrome P450 enzymes [ 36 , 37 ]. No related information available on drug and laboratory test interactions, or paediatric use; hence A. paniculata should not be given to children without medical supervision [ 3 ].
DOSAGE
For pyrexia: a decoction of 3 g of dried crude drug or 25 g fresh herb twice a day before meal [ 2 , 3 ].
For common cold: 1.5-3.0 g of powdered dried crude drug three times daily, after meals and at bedtime [ 2 , 3 ].
For diarrhea: a decoction from 3-9 g crude drug as a single dose as needed, or two tablets of 500 mg four times daily, after meals and at bedtime [ 3 ].
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- Quality Standards of Indian Medicinal Plants. Volume 8. New Delhi: Indian Council of Medicinal Research. 2010.
- Standard of Asean Herbal Medicine. Volume 1. Jakarta: ASEAN Countries. 1993.
- WHO. WHO monographs on selected medicinal plants. 2nd ed. Geneva: WHO. 2002
- Burkil IH. A dictionary of the economic products of the Malay Peninsula. Kuala Lumpur: Ministry of Agriculture Malaysia. 1935;p.156-157.
- Malaysian herbal monograph. Volume 1. Malaysian Monograph Committee. Kuala Lumpur. 1999;p.5.
- Ren X, Du G, Zhou B, Zong K, Ma B. Study on spectroscopy of two flavonyl glycosides from Andrographis paniculata. Frontiers of Chemistry in China. 2008;3(2):178-181.
- Thisoda P, Rangkadilok N, Pholphana N, Worasuttayangkurn L, Ruchirawat S, Satayavivad J. Inhibitory effect of Andrographis paniculata extract and its active diterpenoids on platelet aggregation. European Journal of Pharmacology. 2006;553:39-45.
- Qiong YZ, Li N, Dan C, Wen LD, Shu LP, Li SD. A new ent-labdane diterpenoid from Andrographis paniculata. Chinese Chemical Letters. 2010;21:1091-1093.
- Xiao CM, Zhan PG, Chang YW, Ji HY, Xiu LX, Yuan L, Ke XL. A new ent-labdane diterpenoid lactone from Andrographis paniculata. Chinese Chemical Letters. 2010;21(5):587-589.
- Reddya MK, Reddya MVB, Gunasekara D, Murthyb MM, Cauxc C, Bernard B. A flavone and an unusual 23-carbon terpenoid from Andrographis paniculata. Phytochemistry. 2003;62:1271-1275.
- Jantan I, Waterman PG. Ent-14β-hydroxy-8(17),12-labdadien-16,15-olide-3β,19-oxide: A diterpene from the aerial parts of Andrographis paniculata. Phytochemistry. 1994;37(5):1477-1479.
- Chen LX, Feng Q, Hong W, Qu GX, and Yao XS. Nine new ent-labdane diterpenoids from the aerial parts of Andrographis paniculata. Helvetica Chimica Acta. 2006;89:2654-2664.
- Chen L, Zhu H, Wang R, Zhou K, Jing Y, Qiu F. ent-Labdane diterpenoid lactone stereoisomers from Andrographis paniculata. Journal of Natural Products. 2008;71(5):852-855.
- Chen LX, Qu GX, Qiu F. Studies on diterpenoids from Andrographis paniculata. Zhongguo Zhong Yao Za Zhi. 2006;31(19):1594-1597.
- Poonam K, Tiwari UK, Shukla A, Gaur AK. Chemical constituents isolated from Andrographis paniculata. Indian Journal of Chemistery B. 2010;49B(3):356-359.
- Hu YM, Wang GC, Wang Y, Sung HHY, Williams ID, Ye WC. Diastereoisomeric ent-labdane diterpenoids from Andrographis paniculata. Helvetica Chimica Acta. 2012;95:120-126.
- Zhang XQ, Wang GC, Ye WC, Li Q, Zhou GX, Yao XS. New diterpenoids from Andrographis paniculata (Burm. f.) Nees. Journal of Integrative Plant Biology. 2006;48(9):1122-1125.
- Chen LX, Qu GX, Qiu F. Studies on flavonoids of Andrographis paniculata. Zhongguo Zhong Yao Za Zhi. 2006;31(5):391-395.
- Xiao CM, Bao JZ, Deng S, Huang SS, Ke XL, Jing MJ. A new ent-labdane eiterpenoid lactone from Andrographis paniculata. Chinese Chemical Letters. 2009;20(3):317-319.
- Wang GC, Wang Y, Williams ID, Ho HYS, Zhang XQ, Zhang DM, Jiang RW, Yao XS, Ye WC. Andrographolactone, a unique diterpene from Andrographis paniculata. Tetrahedron Letters. 2009;50:4824-4826.
- Parichatikanond W, Suthisisang C, Dhepakson P, Herunsalee A. Study of anti-inflammatory activities of the pure compounds from Andrographis paniculata (Burm.f.) Nees and their effects on gene expression. International Immunopharmacology. 2010;10:1361-1373.
- Sriramaneni RN, Omar AZ, Ibrahim SM, Amirin S and Mohd AZ. Vasorelaxant effect of diterpenoid lactones from Andrographis paniculata chloroform extract on rat aortic rings. Pharmacognosy Research. 2010;2(4):242-246.
- Awang K, Abdullah NH, Hadiand Yew SF. Cardiovascular activity of labdane diterpenes from Andrographis paniculata in isolated rat hearts. Journal of Biomedicine and Biotechnology. 2012;2012:876458.
- Mishra K, Dash AP, Dey N. Andrographolide: a novel antimalarial diterpene lactone compound from Andrographis paniculata and its interaction with curcumin and artesunate. Journal of Tropical Medicine. 2011;2011:579518.
- Mishra K, Dash AP, Swain BK, Dey N. Anti-malarial activities of Andrographis paniculata and Hedyotis corymbosa extracts and their combination with curcumin. Malaria Journal. 2009;8:26.
- Thisoda P, Rangkadilok N, Pholphana N, Worasuttayangkurn L, Ruchirawat S, Satayavivad J. Inhibitory effect of Andrographis paniculata extract and its active diterpenoids on platelet aggregation. European Journal of Pharmacology. 2006;553:39-45.
- Zhang XF , Tan BKH. Antihyperglycaemic and anti-oxidant properties of Andrographis paniculata in normal and diabetic rats. Clinical and Experimental Pharmacology and Physiology. 2000;27:358-363.
- Ueda JY, Tezuka Y, Banskota AH, Tran QL, Tran QK, Harimaya Y, Saiki I, Kadota S. Antiproliferative activity of Vietnamese medicinal plants. Biological & Pharmaceutical Bullettin. 2002;25(6):753-760.
- Kim TG, Hwi KK, Hung CS. Morphological and biochemical changes of andrographolide-induced cell death in human prostatic adenocarcinoma PC-3 cells. In vivo. 2005; 19: 551-558.
- Khalijah A, Nor Hayati A, Hadi AH, Fong YS. Cardiovascular activity of labdane diterpenes from Andrographis paniculata in isolated rat hearts. Journal of Biomedicine and Biotechnology. 2012;2012:876458.
- Renu A, Siti AS, Mafauzy M. Open label clinical trial to study adverse effects and tolerance to dry powder of the aerial part of Andrographis paniculata in patients type 2 with diabetes mellitus. Malaysian Journal of Medical Sciences, 2005;12(1):13-19.
- Saxena RC, Singh R, Kumar P, Yadav SC, Negi MP, Saxena VS, Joshua AJ, Vijayabalaji V, Goudar KS, Venkateshwarlu K, Amit A. A randomized double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmColdTM) in patients with uncomplicated upper respiratory tract infection. Phytomedicine. 2010;17:178–185.
- Teh BP, Hamzah NF, Rosli SNS, Yahaya MAF, Zakiah I, Murizal Z. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2012. Report No.: HMRC 11-045/01/AP/WP2/K.
- Shamsuzzoha M, Rahman MS, Ahmed MM, Islam AK. Antifertility effect in mice of medicinal plant family Acanthaceae. Lancet, 1978;2(8095):900.
- Chang HM, But PPH, eds. Pharmacology and applications of Chinese materia medica. 1 vol. Singapore: World Scientific; 1986:918–928.
- Jarukamjorn K, Don-in K, Makejaruskul G, Laha T, Daodee S, Pearaksa P, Sripanidkulchai BO. Impact of Andrographis paniculata crude extract on mouse hepatic cytochrome P450 enzymes. Journal of Ethnopharmacology. 2006;105:464-467.
- Pekthong D, Blanchard N, Abadie C, Bonet A, Heyd B, Mantion G, Berthelot A, Richert L, Martin H. Effects of Andrographis paniculata extract and andrographolide on hepatic cytochrome P450 mRNA expression and monooxygenase activities after in vivo administration to rats and in vitro in rat and human hepatocyte cultures. Chemico-Biological Interactions. 2009;179(2-3):247-255.