Durian belanda Leaves
Annona muricata L.
Annonaceae
DEFINITION
Durian belanda leaves consist of the powder of dried leaves of Annona muricata (Annonaceae).
SYNONYM
Annona bonplandiana Kunth, Annona cearaensis Barb.Rodr., Annona macrocarpa Werckle, Annona muricata var. borinquensis Morales, Annona muricata f. mirabilis R.E.Fr., Guanabanus muricatus M. Gomez [ 1 ].
VERNACULAR NAMES
Boat durian, prickly custard apple, soursop, sweetsop (English); durian belanda, durian bengala, durian benggala, durian europa, durian makah, durian makkah, nagka belanda, seri kaya belanda (Malay); ci guo fan li zhi (Chinese); mulcita, mullu citta, mulluccitta, mutcita, mutcitamaram, pulippala (Tamil) [ 2 , 3 ].
CHARACTER
| Colour | Greenish brown |
| Odour | Offensive smell [ 4 ] |
| Taste | Slightly bitter |
IDENTIFICATION
Plant Morphology
Annona muricata is a shrub or small tree, 3.0–10.0 m in height, low-branching and bushy but slender because of its upturned limbs. Leaves have an offensive smell, normally evergreen, are alternate, smooth, glossy and is dark green on the surface, lighter beneath; oblong, elliptic or narrow obovate, pointed at both ends and is 6.25–20 cm long and 2.5–6.25 cm wide. Flowers may appear anywhere on the trunk, branches or twigs and is borne singly, short stalked, 4–5 cm long, plump, in a triangle-conical shape, have 6 petals, with 3 yellow green slightly spreading fleshy petals as the outer layer and 3 pale yellow close-set petals as the inner petals. Fruit is compound and covered with a reticulated, leathery-appearing but tender, inedible, bitter skin from which protrude few or many stubby, or more elongated and curved, soft, pliable spines, more or less oval or heart-shaped, some times irregular, lopsided or curved, due to improper carper development or insect injury, size ranges from 10–30 cm long and up to 15 cm in width, weight may be up to 4.5–6.8 kg, the tips break off easily when the fruit is fully ripe, skin is dark-green in the immature fruit, becoming slightly yellowish-green before the mature fruit is soft to the touch. Seed is a single oval, smooth, hard, black seed, 1.25–2.0 cm long [ 4 , 5 ].
Microscopy
Powdered material consists of epidermal cells, in surface view, composed of polygonal cells with wavy to sinuous anticlinal walls and abundant of tetracytic and anomocytic type of stomata; fragments of spiral, pitted and scalariform vessels; fragment of parenchyma cells; fragment of lignified fibre found isolated; numerous solitary crystals and druses crystal found isolated, under light polarizing filter.

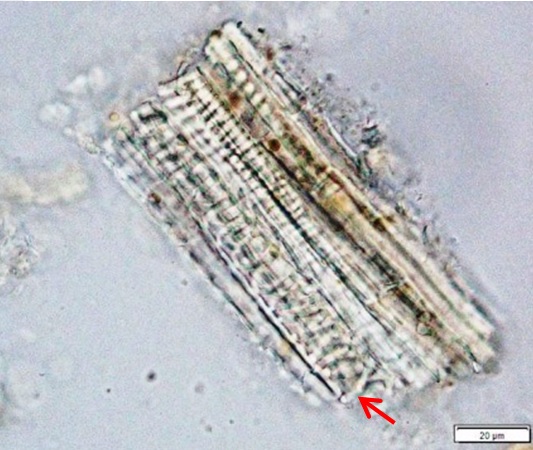

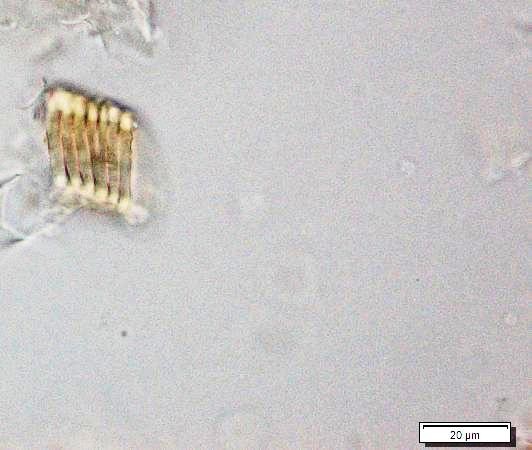


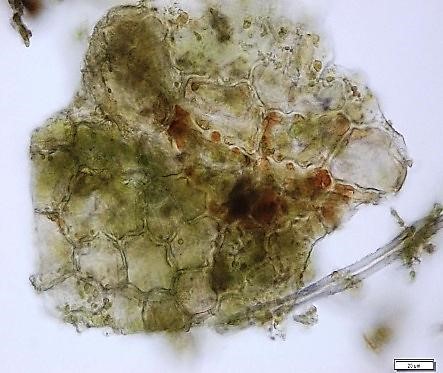
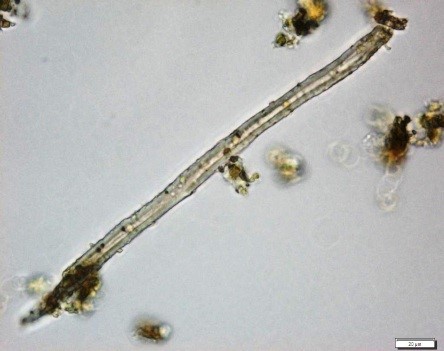
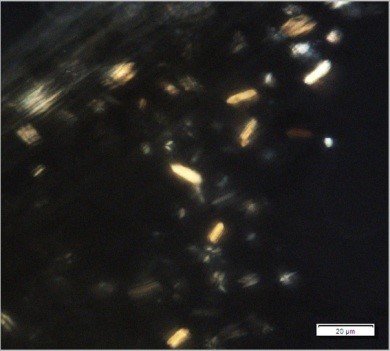

Figure 2 : Microscopic characters of Annona muricata leaves powder of 0.106 mm size. (a) Tetracytic stomata (b) tetracytic (red arrow) and anomocytic stomata (black arrow) (magnification 20x & 40x); (c) & (d) spiral vessel (magnification 20x); (e) pitted vessel and brachysclereids (arrow) (magnification 20x); (f) scalariform vessel; (g) spiral vessel (magnification 40x & 20x); (h) parencyma cells (magnification 40x); (i) adaxial epidermis (magnification 40x); (j) fibre (magnification 40x); (k) solitary crystal, under light polarizing filter (magnification 40x); (l) druses crystal, under light polarizing filter (arrow) (magnification 20x). [Scale bars: a = 50 µm; b–l = 20 µm
Chemical Tests
Observation of solution after treatment with various reagents:
| Test for the presence of steroids | Bluish green |
| Test for the presence of phenolic | Green |
Thin Layer Chromatography (TLC)
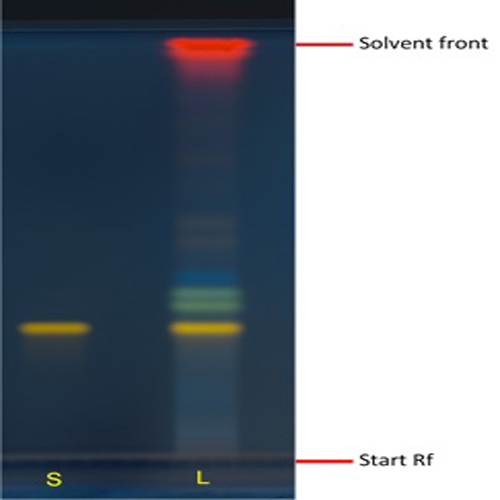
Figure 3 : TLC chromatogram of rutin (S), methanol extract of Annona muricata dried leaves (L) observed under UV at 366 nm after derivatisation.
| Test Solutions | Weigh about 1.0 g of A. muricata dried leaves powder of 0.335 mm size in 50 mL of conical flask. Add 10 mL of methanol. Sonicate the mixture for 15 min. The mixture was then centrifuged and supernatant was collected. |
| Standard solution | Dissolve rutin standard [CAS no. 153-18-4] in methanol to produce a standard concentration of 1.0 mg/mL. Vortex standard for 10 sec and sonicate 15 min at room temperature before use. |
| Stationary Phase | HPTLC Silica gel 60 F254 10 x 10 cm |
| Mobile phase | Ethyl Acetate : water : formic acid : acetic acid; (40 : 8 : 2.5 : 5.5) (v/v/v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
UV at 366 nm after derivatisation with NP-PEG reagent. Derivatizing reagent preparation: Derivatized with natural product reagent and polyethylene glycol 4000 reagent. Heat for 5 min at 100°C. Natural Product Reagent (NP) Dissolve 1.0 g of 2-aminoethyldiphenylborinate in 100 mL of methanol. Polyethylene glycol 4000 (PEG) Dissolve 5.0 g of polyethylene glycol 4000 in 100 mL ethanol. |
High Performance Liquid Chromatography (HPLC)
| Test solution | Weigh about 1.0 g of A. muricata dried leaves powder of 0.335 mm size in 50 mL of conical flask. Add 10 mL of methanol. Sonicate the mixture for 15 min. The mixture was then centrifuged and supernatant was collected. | |||||||||||||||||||||
| Standard solution | Dissolve rutin standard [CAS no. : 522-12-3] in methanol to produce a standard concentration of 0.25 mg/mL. Vortex standard for 10 sec and sonicate for 15 min at room temperature before use. | |||||||||||||||||||||
| Chromatographic system |
Detector: 350 nm Column: C18 (150 X 4.6 mm, 3.5 µm) (Zorbax SB-C18 if necessary) Column oven temperature: 25˚C Flow rate: 1 mL/min Injection volume: 10 µL |
|||||||||||||||||||||
| Mobile Phase (gradient mode) |
|
|||||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the standard solutions (0.25 mg/mL). The requirements of the system suitability parameters are as follow:
|
|||||||||||||||||||||
| Acceptance criteria |
|
(a)
(b)
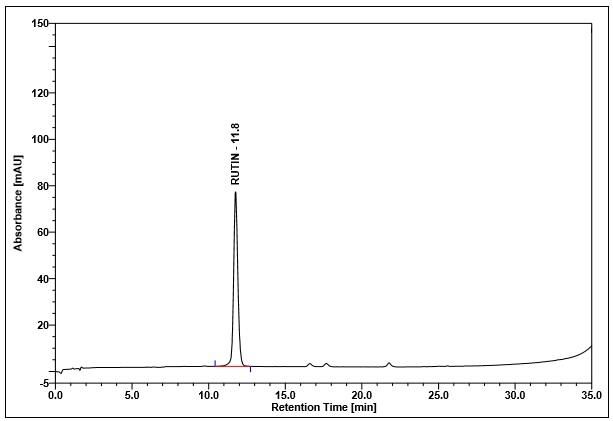
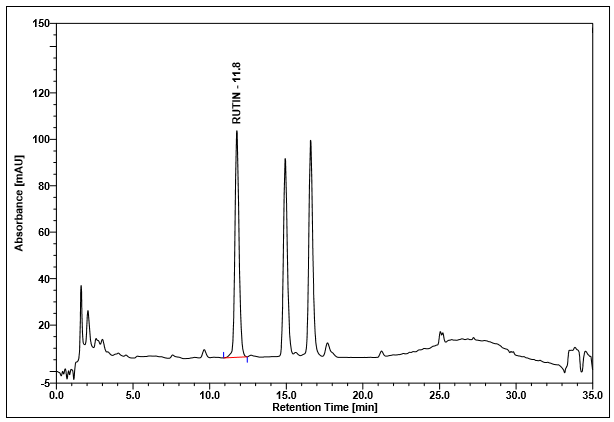
Figure 4 : Whole HPLC chromatogram of (a) rutin standard solution (0.25 mg/mL) at tr= 11.8 min and (b) methanol extract of Annona muricata dried leaves powder showing peak corresponding to rutin standard standard solution at tr= 11.8 min.
(a)
(b)
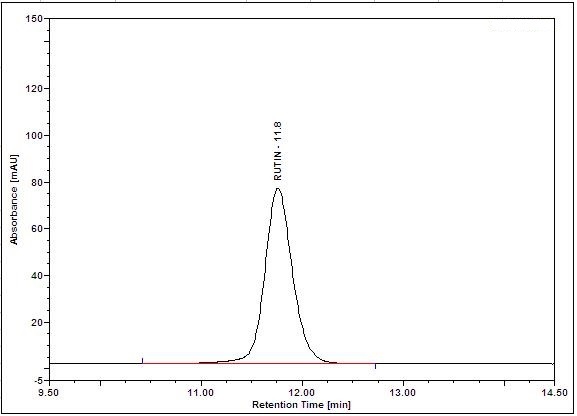
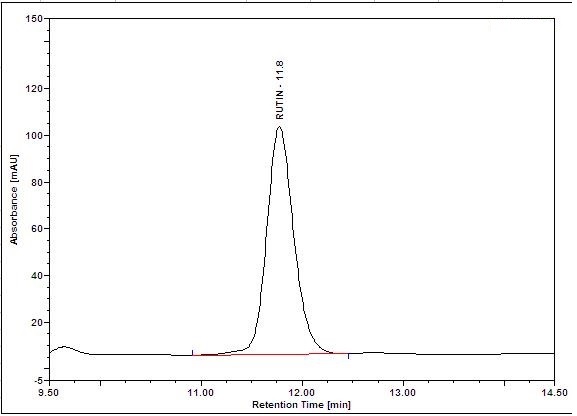
Figure 5 : HPLC chromatogram highlighting the elution region of (a) rutin standard standard solution (0.25 mg/mL) at tr= 11.8 min and (b) methanol extract of Annona muricata dried leaves powder showing peak corresponding to rutin standard standard solution at tr= 11.8 min.
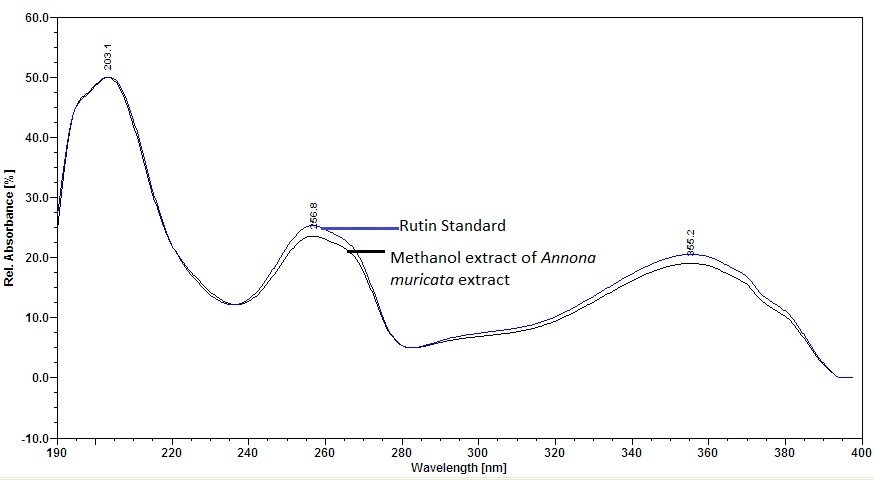
Figure 6 : UV spectrum of rutin standard solution (0.25 mg/mL) and methanol extract of Annona muricata dried leaves powder.
PURITY TESTS
The purity tests, except foreign matter test are based on A. muricata dried leaves powder of 0.355 mm particle size.
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 10% |
| Acid-insoluble ash | Not more than 1% |
| Water soluble ash | Not less than 1% |
| Loss on Drying |
| Not more than 9% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 24% |
| Cold method | Not less than 17% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 17% |
| Cold method | Not less than 12% |
SAFETY TESTS
The safety tests are based on A. muricata dried leaves powder of 0.355 mm particle size.
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Ethanol extract of A. muricata dried leaves was found to contain flavonoids (e.g rutin, kaempferol, quercetin and quercetin-3-glucoside) [ 6 ].
Ethanol (95%) extract of A. muricata dried leaves was found to contain acetogenins (e.g annonacin, annopentocin A, annopentocin B, annopentocin C, cis-annomuricin-D-ones, trans-annomuricin-D-ones, bullatacin, squamocin, squamostatin-A and squamostatin-D) [ 7 , 8 , 9 ].
Ethanol (75%) extract of A. muricata dried leaves was found to contain flavonol triglycosides (e.g quercetin 3-O-α-rhamnosyl-(1”” → 6”)-β-sophoroside) and phenolics (e.g argentinine, catechin, chlorogenic acid, epicatechin, gallic acid, kaempferol, kaempferol-3-O-rutinoside, quercetin, quercetin-3-O-rutinoside, quercetin-3-O-neohispredoside, quercetin-3-O-robinoside, quercetin-3-O-glucoside) [ 10 ].
Ethanol extract of A. muricata dried leaves was found to contain acyclic diterpene (e.g phytol) and fatty alcohol (e.g 2-pentadecanol, oleyl alcohol) and fatty acids (e.g n-hexadecanoic acid, hexadecanoic acid, ethyl ester, 9,12-octadecadienoic acid, ethyl ester) [ 11 ].
Methanol extract of A. muricata leaves was found to contain acetogenins (e.g annonacin and squamocin) [ 12 ].
Methanol (70%) extract of A. muricata dried leaves was found to contain acyclic diterpene (e.g phytol) and fatty acids (e.g n-hexadecanoic acid, hexadecanoic acid, methyl ester, octadecanoic acid, cis-10-heptadecenoic acid, tetradecanoic acid, (Z,Z,Z)-9,12,15-octadecatrienoic acid) [ 13 ].
Ethyl acetate extract of A. muricata dried leaves was found to contain sesquiterpenoids (e.g caryophyllene and α-copaene) [ 14 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Leaves of A. muricata is traditionally used as a poultice, or use an infusion externally for skin complaints in children, and for coughs and joint pain. The sap of young leaves is applied to pus from acne to make them burst [ 15 ].
Biological and pharmacological activities supported by experimental data
Antioxidant activity
Methanol extract of A. muricata dried leaves exhibited 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity with 50% inhibition concentration (IC50) value of 4.36 µg/mL compared to ascorbic acid (IC50 = 9.22 µg/mL) using DPPH assay [ 16 ].
Antimicrobial activity
Aqueous extract of A. muricata dried leaves (10 µg/mL) showed antibacterial activity against Enterococcus faecalis with inhibition zone (IZ) of 13.9 mm diameter comparable with sodium hypochlorite solution (1%) (IZ = 13.9 mm) using disc diffusion method [ 17 ].
Cytotoxic activity
Ethanol soluble fraction of A. muricata dried leaves water extract showed cytotoxicity towards human colorectal cancer cell lines DLD-1 (derived from Dukes’ type C) with IC50 value of 20.59 µg/mL compared to 5-fluorouracil (IC50 value = 2800 µg/mL) using 3-(4,5)-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [ 18 ].
Ethanol soluble fraction of A. muricata dried leaves water extract (containing 0.36% acetogenin) showed cytotoxicity towards human colorectal cancer cell lines COLO 205 (derived from Dukes’ type D) with IC50 value of 654.9 µg/mL compared to 5-fluorouracil (IC50 value = 1200 µg/mL) using MTT assay [ 18 ].
Muricoreacin isolated from ethanol (95%) extract of A. muricata dried leaves showed cytotoxicity towards human prostate adenocarcinoma (PC-3) with 50% effective dosage (ED50) value 0.025 µg/mL compared to adriamycin (ED50 = 0.11 µg/mL) using MTT assay [ 27 ].
Antidiabetic activity
Aqueous extract of A. muricata dried leaves (100 and 200 mg/kg) administered orally to streptozotocin-induced diabetic male Albinos Wistar rats (150 – 250 g) for duration of 28 days decreased the blood glucose level (100 mg/kg; from 344.60 ± 9.78 mg/dL to 80.75 ± 5.68 mg/dL) and (200 mg/kg; from 338.80 ± 12.47 mg/dL to 141.25 ± 10.25 mg/dL) compared to insulin (10 UI/kg; from 308.25 ± 3.57 to 81.00 ± 9.00 mg/dL) [ 19 ].
Anti-inflammatory activity
Aquoeus extract of A. muricata dried young leaves (500 mg/kg) was administered orally to male Swiss albino mice (25 – 30 g) one hour before induction of paw edema into the sub-plantar surface of the right hind paw using carrageenan. At five hour of post-injection, the extract significantly (p < 0.001) decreased paw edema volume (103.3 ± 0.95 mL) compared to ibuprofen (50 mg/kg; 119.72 ± 1.58 mL) [ 20 ].
Aquoeus extract of A. muricata dried young leaves (5 mg/ear) applied topically to the right ear of male Swiss albino mice (25 – 30 g) after application of tetradecanoylphorbol acetate (TPA) as phlogistic agent. At four hour of post-application, the extract inhibited edema (78.09 ± 3.61%) compared to ibuprofen (0.05 mg/ear; 79.60 ± 0.22%) [ 20 ].
Ethanol (70%) extract of A. muricata dried leaves (300 mg/kg) administered orally to carrageenan-induced paw oedema of Sprague Dawley rats of either sex (170 – 250 g) inhibited oedema (79.3%) compared to indomethacin (10 mg/kg; 84.4%) [ 21 ].
Ethanol (80%) extract of A. muricata dried leaves (300 mg/kg) administered orally to Complete Freund’s adjuvant-induced arthritis paw edema of Sprague Dawley rats of either sex (170 – 250 g) decreased the arthritic paw edema volume (0.16 ± 0.03 mL) compared to indomethacin (10 mg/kg; 0.20 ± 0.03 mL) [ 22 ].
Ethanol (80%) extract of A. muricata dried leaves (300 mg/kg) administered orally to Complete Freund’s adjuvant-induced arthritis paw edema of Sprague Dawley rats of either sex (170 – 250 g) decreased interleukin-1 beta (IL-1β) level in local arthritic tissue with value of 648.6 ± 35.11 pg/mg protein compared to indomethacin (664.6 ± 87.67 pg/mg protein) [ 22 ].
Ethanol (80%) extract of A. muricata dried leaves (300 mg/kg) administered orally to Complete Freund’s adjuvant induced arthritis paw edema of Sprague Dawley rats of either sex (170 – 250 g) decreased tumour necrosis factor alpha (TNF-α) level in local arthritic tissue with value of 242.9 ± 51.4 pico/mg protein compared to indomethacin (278.9 ± 11.25 pico/mg protein) [ 22 ].
Anti-nociceptive activity
Ethanol (70%) extract of A. muricata dried leaves (300 mg/kg) was administered to adult Balb/c albino mice of either sex (20 – 30 g) 30 min before induction of acetic acid via intraperitoneal injection. After 60 min of post-injection, the extract inhibited writhings (70.2%) compared to indomethacin (10 mg/kg; 63.7%) [ 21 ].
Antiproliferative activity
Ethanol (96%) extract of A. muricata dried leaves (300 mg/kg) administered orally to 7,12-dimethylbenz[a]anthracene (DMBA) induced of female Sprague Dawley rats (80 – 100 g) reduced the mean mAgNOR proliferative index with value of (~1.85) compared to tamoxifen (0.18 mg/kg;~1.75) [ 23 ].
Gastroprotective activity
Ethyl acetate extract of A.muricata dried leaves (400 mg/kg) was administered orally to Sprague Dawley rats of both sexes (180 – 250 g) one hour before induction of gastric ulcer using absolute ethanol. The extract lower the gastric acidity with pH value of 6 compared to omeprazole (20 mg/kg; pH value of 6.2) and ulcer lesion control group (pH value of 4.2) [ 14 ].
Laxative activity
Ethanol extract of A.muricata dried leaves (400 mg/kg) administered orally to loperamide-induced constipation of Wistar rats of either sex (180 – 250 g)) increased faeces output with value of 4.22 ± 0.0421 g compared to sodium picosulfate (5 mg/kg;5.45 ± 0.0208 g) [ 25 ].
Clinical studies
A randomized double-blind placebo-controlled trial involving thirty patients (male and female) with colorectal cancer was conducted to study the effects in human colorectal cancer cell line ex vivo by investigating the level of caspase 9 and caspase 8 enhancement activity in blood serum patient. The treatment group was given 300 mg of ethanol soluble fraction of A. muricata dried leaves water (ESFAM) capsule per day after breakfast for eight weeks. Peripheral blood sample was drawn at the end of the study period and the blood samples were centrifuged to obtain serum for ex vivo study. Ex vivo study was performed by treating DLD-1 colorectal cell lines with the serum of patients from treatment group and placebo group. The serum of treatment group significantly (p < 0.05) enhanced the expression of caspase-9 with value of 0.68 unit compared to placebo group (0.54 unit). Expression of caspase-8 tended to decrease in both group [ 18 ].
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
7-days toxicity study
Aqueous extract of A. muricata dried leaves (100, 1000, 2000 and 5000 mg/kg) administered orally to Swiss albino mice (either sex, 20 – 25 g) for seven days showed no mortality with LD50 > 5000 mg/kg [ 24 ].
14-days toxicity study
Ethyl acetate extract of A. muricata dried leaves (1000 and 2000 mg/kg) administered orally to female Sprague Dawley rats (180 – 250 g) for 14 days showed no mortality with lethal dose at 50% (LD50) > 2000 mg/kg [ 14 ].
Aqueous extract of A. muricata dried leaves (2000 and 5000 mg/kg) administered orally to Albino Wistar rats (either sex, 150 – 250 g) for 14 days showed no mortality with LD50 > 5000 mg/kg [ 19 ].
Oral single dose acute toxicity study on female Sprague Dawley rats (aged 8 and 12 weeks old) using aqueous extract of A. muricata leaves showed no toxic effect on the parameters observed, including behaviours, body weight, food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. Half lethal dose (LD50) is not more than 2,000 mg/kg body weight [ 28 ].
4-weeks toxicity study
Aqueous extract of A. muricata dried leaves (200, 400 and 800 mg/kg) administered daily by gavage to Albino Wistar rats (either sex, 150 – 250 g) for four weeks showed no mortality with LD50 > 800 mg/kg [ 19 ].
Reproductive toxicity
Aqueous extract A. muricata dried leaves (45 mg/kg) administered orally daily throughout the gestational period of female Wistar rats (200 – 300 g). There was significant (p < 0.05) reduction in maternal weight during second trimester pregnancy, litter birth weight, litter length, placental weight and placental/birth weight ratio with the value of (315.02 ± 3.22 g; 4.19 ± 0.01 g; 4.75 ± 0.01 cm; 0.38 ± 0.01 g; 0.090 ± 0.001) compared to pregnant control group (327.85 ± 4.64 g; 4.60 ± 0.07 g; 4.84 ± 0.02 cm; 0.57 ± 0.01 g; 0.125 ± 0.002) [ 26 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The plant List. [Internet] Annona muricata L.; Version 1.1; [cited on 1st March 2018]. Available from: http://www.theplantlist.org/tpl1.1/record/kew-2640944
- Quattrocchi UFLS. CRC world dictionary of medicinal and poisonous plants: common names, scientific names, eponyms, synonyms, and etymology. Vol. III E-L. United States: CRC Press. 2012; p.310.
- Flora of China. [Internet] Annona muricata L. [cited on 1st March 2018]. Available from: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200008507
- Singapore National Park Flora & Founa Web. [Internet] Annona muricata [cited on 1st March 2018]. Available from: https://florafaunaweb.nparks.gov.sg/Special-Pages/plant-detail.aspx?id=3258
- JF Morton. Soursop In: Fruits of warm climates. Miami, Florida: Julia F. Morton. 1987; p.75-80.
- C Yang, SR Gundala, R Mukkavilli, S Vangala, MD Reid, R Aneja. Synergistic interactions among flavonoids and acetogenins in Graviola (Annona muricata) leaves confer protection against prostate cancer. Carcinogenesis. 2015; 36(6): 656-665.
- K Mulia, E Krisanti, T Maulana, D. Selective polarity-guided extraction and purification of acetogenin in Annona muricata L. leaves. International Journal of Technology. 2015; 7: 1221-1227.
- L Zeng, F Wu, NH Oberlies, JL McLaughin. Five New Monotetrahydrofuran Ring Acetogenins from the Leaves of Annona muricata. Journal Natural Products. 1996;59:1035-1042.
- K Mulia, SW Winarcahyo, E Krisanti, D Kurniasuci. Practical isolation of bullatacin from Annona muricata leaves extract using an open column chromatography technique. Advanced Materials Research. 2013; 789:545-550.
- M Nawwar, N Ayoub, S Hussein, A Hashim, R El-Shawarawy, K Wende, M Harms, U Lindequist. A flavonol triglycoside and investigation of the antioxidant and cell stimulating activities of Annona muricata Linn. Archives of Pharmacal Research. 2012; 35(5): 761-767.
- Y Gavamukulya, F Abou-Elella, F Wamunyokoli, HA El-Shemy. GC-MS Analysis of Bioactive Phytochemicals Present in Ethanolic Extracts of Leaves of Annona muricata: A Further Evidence for Its Medicinal Diversity. Pharmacognosy Journal. 2015; 7(5): 300-304.
- IVMD Moraes, PRV Ribeiroa, FL Schmidt, KM Canutoa, GJ Zocoloa, ESD Britoa, R. Luoc, KM Richard, K Tran, RE Smith. UPLC–QTOF–MS and NMR analyses of graviola (Annona muricata) leaves. Brazilian Journal of Pharmacognosy. 2016; 26: 174-179.
- K Shibula, S Velavan. Determination of Phytocomponents in Methanolic Extract of Annona muricata Leaf Using GC-MS Technique. International Journal of Pharmacognosy and Phytochemical Research. 2015; 7(6): 1251-1255.
- SZ Moghadamtousi, E Rouhollahi, H Karimian, M Fadaeinasab, MA Abdulla, HA Kadir. Gastroprotective activity of Annona muricata leaves against ethanol-induced gastric injury in rats via Hsp70/Bax involvement. Drug Design, Development and Therapy. 2014;4:2099-2111.
- Burkill IH. A dictionary of the economic product of the Malay Peninsula, Vol.1. London; Published on behalf of the governments of the Straits settlements and Federated Malay states by the Crown agents for the colonies. 1935; p. 166-167.
- KM Ezealisiji and B Tamuno-Eli. Comparative evaluation of the phenolic and antioxidant properties of the leaves, root, stem bark, and root bark of Annona muricata (Annonaceae). Journal of Pharmacognosy and Phytochemistry. 2017; 6(2): 274-278.
- J Mathew, R George, R Theruvil, TC Padavil, L Tomy, A Kurian. Antibacterial Activity of Leaf Extract of Annona muricata and Simarouba glauca on Enterococcus faecalis. Journal of Contemporary Dental Practice. 2016;17(8):650-653.
- L Indrawati, P Ascobat, B Bela, M Abdullah, IS Surono, S Pramono. Antiproliferative activity and caspase enhancement properties of Annona muricata leaves extract against colorectal cancer cells. Medical Journal of Indonesia. 2016;25(3):136-142.
- NT Florence, MS Benoit, K Jonas, T Alexandra, DDP Desire, K Pierre, D Theophile. Antidiabetic and antioxidant effects of Annona muricata (Annonaceae), aqueous extract on streptozotocin-induced diabetic rats. Journal of Ethnopharmacology. 2014; 151: 784–790.
- AM Quilez, SM Paz, RD Puerta, MAF Arche, MDG Gimenez. Validation of Ethnopharmacological Use as Anti-inflammatory of a Decoction from Annona muricata Leaves. African Journal Complimentary Alternative Medicine. 2015;12(4):14-20.
- AH Roslida. CE Tay, A Zuraini, PF Chan. Anti-inflammatory and Anti-nociceptive activities of the ethanolic extract of Annona muricata leaf. Journal of Natural Remedies. 2010; 10(2): 97-104.
- CP Foong, AH Roslida. Evaluation of anti-inflammatory activities of ethanolic extract of Annona muricata leaves. Brazilian Journal of Pharmacognosy. 2012;22(6):1301-1307.
- E Sulistyoningrum, EPN Rachmani, HN Baroroh, L Rujito. Annona muricata Leaves Extract Reduce Proliferative Indexes and Improve Histological Changes in Rat’s Breast Cancer. Journal of Applied Pharmaceutical Science. 2017;7(1):149-155.
- FKN Arthur, E Woode, EO Terlabi, C Larbie. Evaluation of acute and sub chronic toxicity of Annona muricata (Linn.) aqueous extract in animals. European Journal of Experimental Biology. 2011; 1(4):115-124.
- J Somasundaram. Evaluation of laxative effect of ethanol extract of leaves of Annona muricata L. International Journal of Innovative Drug Discovery. 2013; 3(1):28-32.
- E Obianoju, O Pamela, I Eghosa, U Princewill, AU Bond. Effects of aqueous leaf of Annona muricata on pregnancy and pregnancy outcome. Journal of Advances in Medical and Pharmaceutical Sciences. 2017;12(1):1-6.
- GS Kim, L Zeng, F Alali, LL Roger, F Wu, S Sastrodihardjo, JD McLaughlin. Muricoreacin and murihexocin C, mono tetrahydrafuran acetogenins, from the leaves of Annona muricata. Phytochemistry. 1998;49(2):565-571.
- Teh BP, Jumriani AA, Noorashikin AH, Lalitha Suganthi S. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2019. Report no.: NON-GLP/2019/08/01.









