Kancing baju Aerial
Bidens pilosa L
Asteraceae







Figure 1 : Bidens pilosa. (a) Mature annual herb; (b) flowering plant; (c) whole plant consisting of flowers, leaves, stem and root; (d) leaves; (e) stem; (f) fruits achenes; (g) aerial powder 0.355 mm size. (Photos courtesy of UPM, 2019).
DEFINITION
Kancing baju aerial part consists of the powder of dried aerial system of Bidens pilosa L. (Asteraceae).
SYNONYM
Coreopsis leucantah L., Kerneria pilosa L., Bidens alausensis Kunth [1].
VERNACULAR NAMES
Beggar sticks, black fellows, blackjack, broom stick (English); kancing baju, rumput juala (Malay); gui zhen cao (Chinese) [1, 2, 3].
CHARACTER
| Colour | Peanut colour |
| Odour | Earthy smell |
| Taste | Earthy |
IDENTIFICATION
Plant morphology
B. pilosa is an annual herb up to 0.1 – 1.5 m; it is 4-angled branched stem or sometimes hardly branched, pale green or sometimes reddish. Leaves pinnately 3-5 lobed, 15-20 cm long; segments ovate to ovate-lanceolate, terminal segment 5-10 cm long, 2.5-5 cm wide, lateral segments 2–5 cm long, 1–2 cm wide and asymmetric, often lobed or bilobed; petiole up to 70 mm long. Capitulum terminal and solitary or few together; peduncle up to 16 cm long; outer phyllaries 7–10, 3–4 mm long, inner phyllaries 5–8, yellow-green to pale brown, 3–4.5 mm long with yellow scarious margins, glabrous but for the apex; receptacle club-shaped in fruit; pale light brown, striate, 3–5 mm long. Florets ray florets white or cream, 4–8 rays (when fully developed) 7–15 × 3–4.5 mm, tube 0.5–0.8 mm long; disc florets yellow or yellow to orange, ± 4 mm long, furry at base. Fruits achenes black, 4–6-ribbed, linear-tetragonal, 4–12 mm long, strigose or verrucose; aristae 2–4 mm long [2].
Microscopy
The powdered aerial part consists of basic plant tissues such as parenchyma cells; abaxial epidermal cells (below surface) with sinuous anticlinal walls and anomocytic stomata cells; simple and multicellular trichomes and solitary calcium oxalate crystals. Other characteristics observed are the presence of pollen grains, fiber, and fragments of scalariform, spiral and pitted vessels.
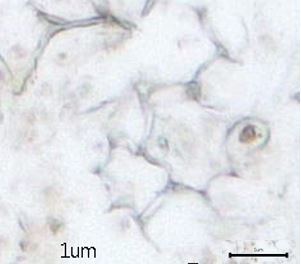
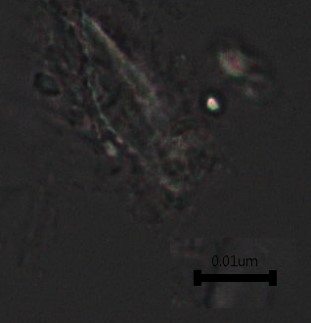
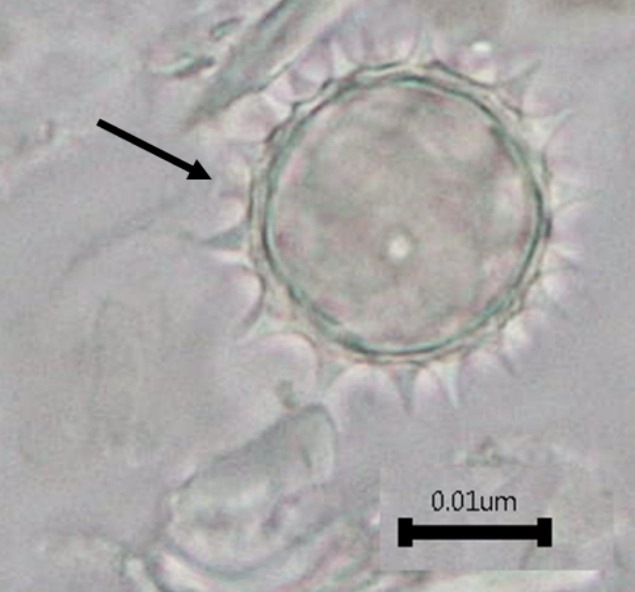

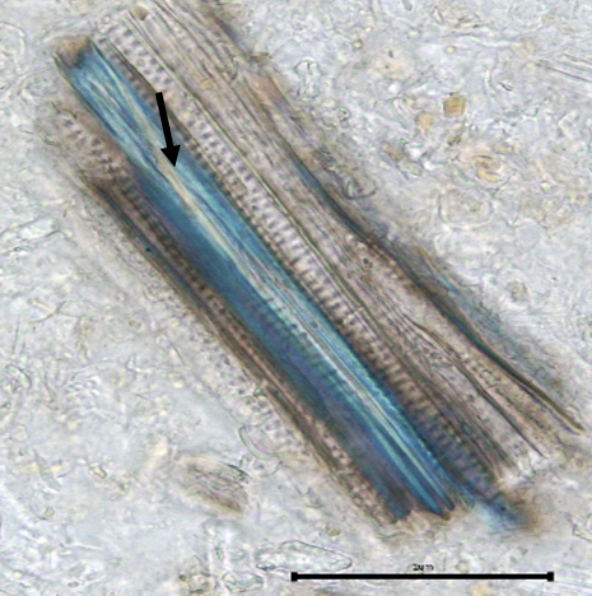
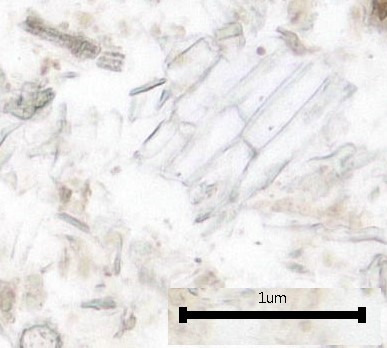
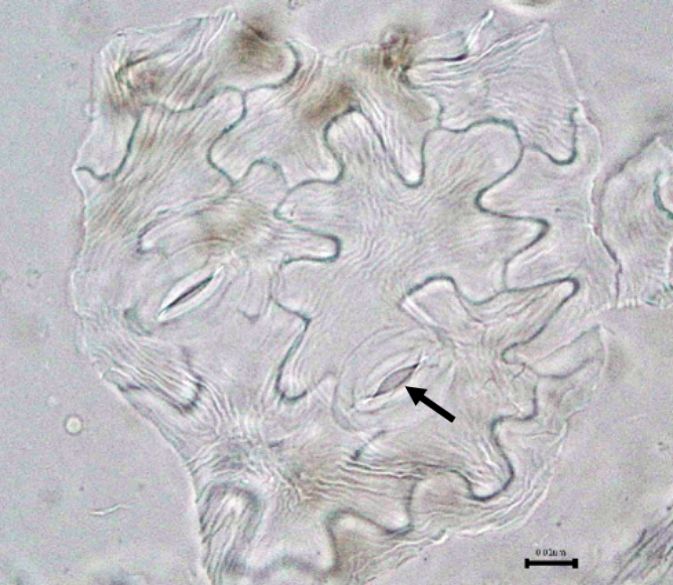
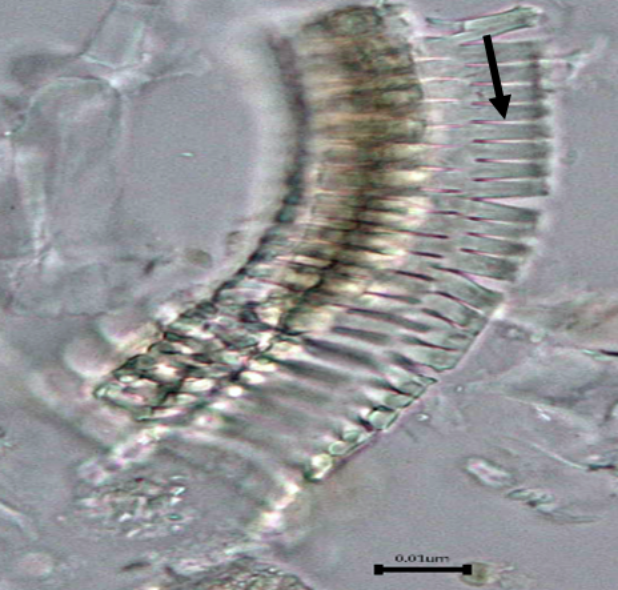
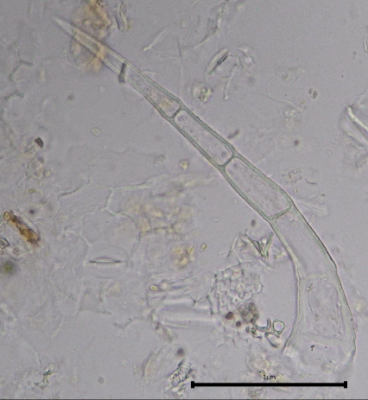
Figure 2: Microscopic characters of B. pilosa aerial powder of 0.355 mm size. (a) parenchyma cells (magnification 20×); (b) collenchyma cells (magnification 20×); (c) calcium oxalate crystals: solitary (prism) (arrow) (magnification 40×); (d) abaxial epidermal cells (lower surface) with sinuous anticlinal walled and anomocytic stomata cells (arrow) (magnification 40×); (e) pollen grains (black arrow) (magnification 40×); (f) vessels fragments: scalariform (black arrow) (magnification 40×); (g) vessel fragments: spiral (red arrow), annular (yellow arrow), scalariform (black arrow) (magnification 40×); (h) simple, uniseriate trichome; (i) Presence of fibre in the middle of vascular tissues (black arrow) (magnification 20×). [Scale bars: a, b, and h = 1 μm; c, d, e, f, and g = 0.01 μm].
Chemical Test
Observed colour of solution after treatment for the presence of:
| Test for the presence of alkaloid | orange red precipitate |
| Test for the presence of flavonoid | deep pink colour |
| Test for the presence of saponin | presence of froth |
Thin Layer Chromatography (TLC)
| Test Solutions |
Weigh about 5.0 g of B. pilosa dried aerial powder of 0.355 mm size in a 250-mL conical flask. Add 50 mL methanol and sonicate the solution for 30 minutes at room temperature. Filter the mixture with filter paper into a conical flask. Evaporate the filtrate to dryness. Use the filtrate as a test solution. |
| Standard solution |
Dissolve 3,5-dicaffeoylquinic acid [CAS no.: 2450-53-5] in methanol to give a standard concentration of 1.0 mg/mL. |
| Stationary Phase |
HPTLC glass plate silica 60 F254 10 cm x 10 cm, Merck. |
| Mobile phase |
Ethyl acetate: formic acid: water: toluene (10 : 1 : 1 : 0.5) (v/v/v/v) |
| Application |
(a) 3,5-dicaffeoylquinic acid standard (S); 6 μL, as a band. (b) Methanol extract of pilosa dried aerial part powder (L); 8 μL, as a band. |
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
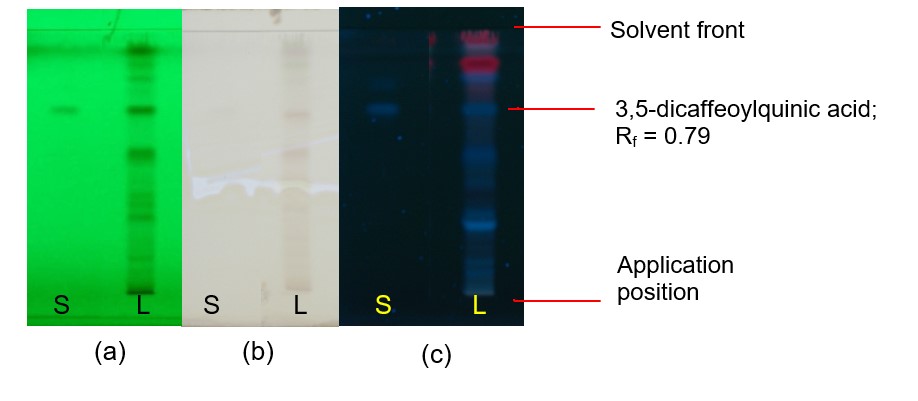
Figure 5: TLC chromatogram of 3,5-dicaffeoylquinic acid (S), methanol extract of Bidens pilosa dried aerial part powder (L) observed under (a) UV at 254 nm before derivatisation (b) visible light after derivatisation and (c) UV at 366 nm after derivatisation
High Performance liquid Chromatography (HPLC)
| Test Solutions | Weigh about 5.0 g of B. pilosa dried aerial part powder of 0.355 mm size in a 250-mL conical flask. Add 50 mL methanol and sonicate the solution for 30 minutes at room temperature. Filter the mixture with filter paper into a conical flask. Evaporate the filtrate to dryness. Reconstitute with methanol to be used as a test solution. Filter the solution through a 0.45 µm syringe filter and inject the filtrate into the HPLC column. Use the filtrate as a test solution. |
| Standard solution | Dissolve 3,5-dicaffeoylquinic acid [CAS no.: 2450-53-5] in methanol to produce a standard concentration of 1.0 mg/mL solution. |
| Chromatographic system | Detector: Photo Diode Array Detector (PDA) 330 nm Column: C18 (5.0 µm, 4.6 mm I.D. × 250 mm) Column oven temperature: 40°C Flow rate: 1.0 mL/min Injection volume: 10 µL |
| Mobile phase (gradient mode) | 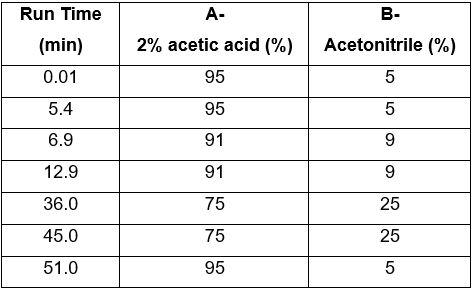 |
| System suitability requirements | Perform at least five replicate injections of (1.0 mg/mL). The requirements of the system suitability parameters are as follow: (1) Symmetry factor (As) for 3,5-dicaffeoylquinic acid standard is not more than 1.5. (2) Percentage of relative standard deviation (RSD) of the retention time (tr) for gallic acid standard is not more than 2.0%. |
| Acceptance criteria | (1) Retention time (tr) of 3,5-dicaffeoylquinic acid standard in the test solution is similar to the tr of the standard solution. (2)The ultraviolet (UV) spectrum of 3,5-dicaffeoylquinic acid standard in the test solution is similar to the UV spectrum of the standard solution (optional supportive data). |
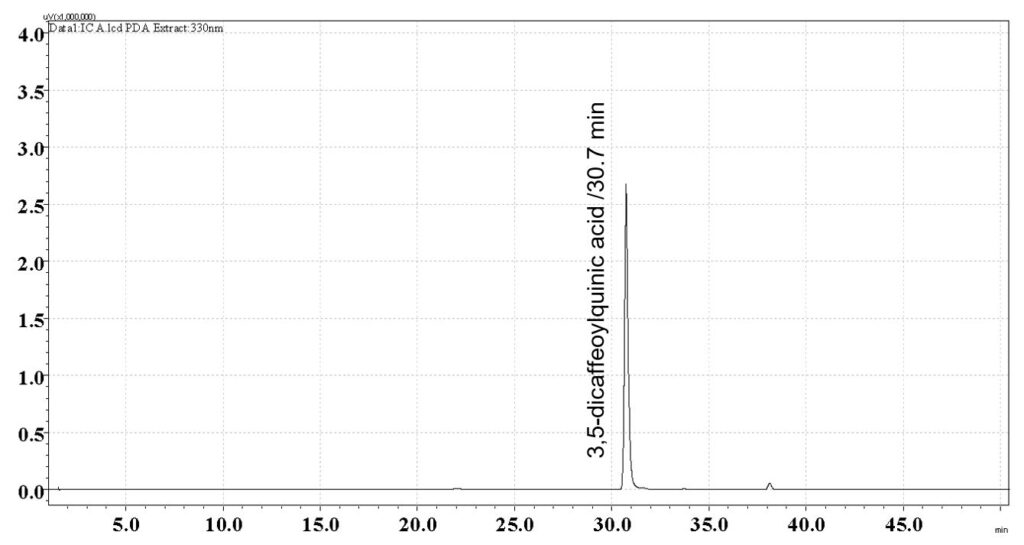
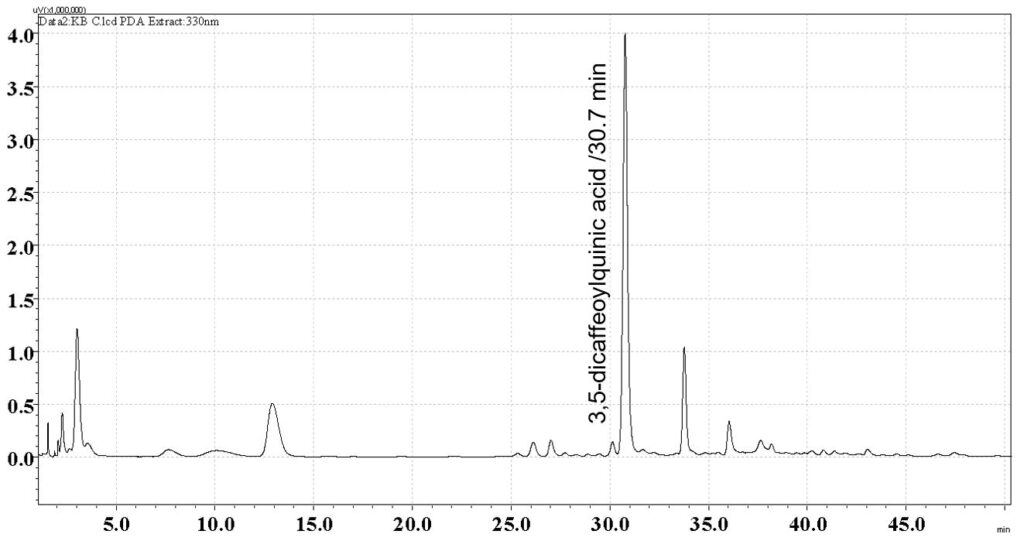
Figure 6: Whole HPLC chromatogram of (a) 3,5-dicaffeoylquinic acid standard solution (1 mg/mL) at tr = 30.7 min and (b) methanol extract of B. pilosa dried aerial part powder from the central showing peak corresponding to 3,5-dicaffeoylquinic acid standard solution at tr = 30.7 min.
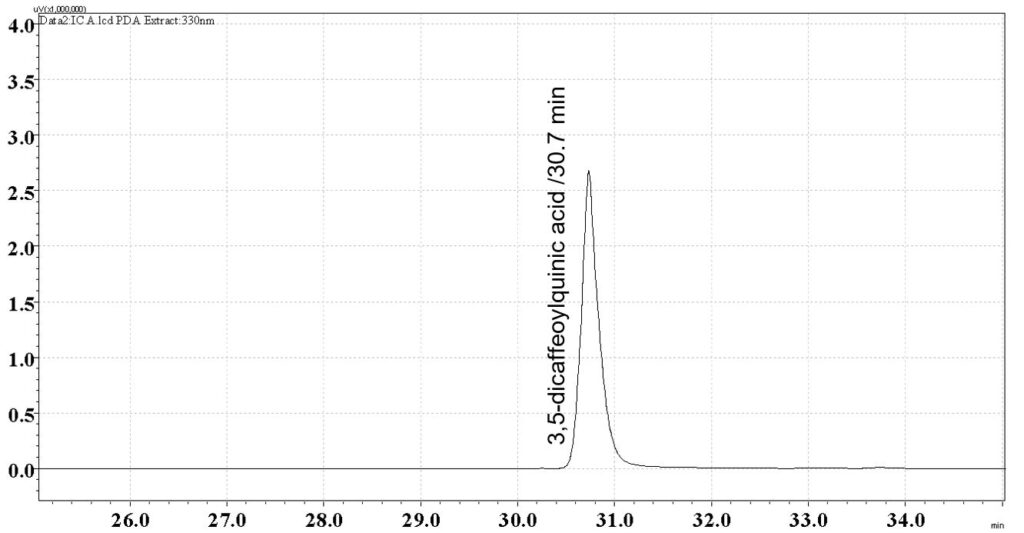
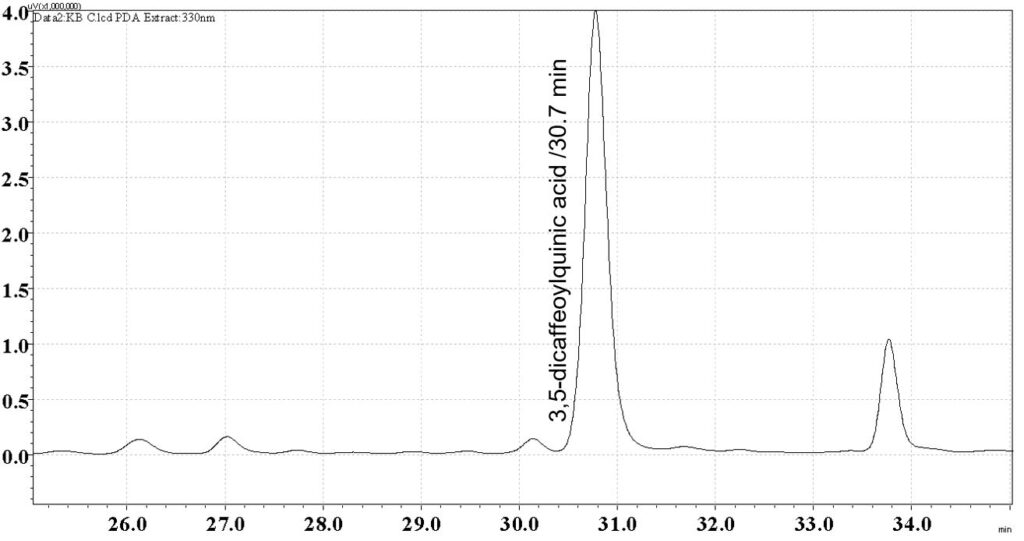
Figure 7: HPLC chromatogram highlighting the elution region of 3,5-dicaffeoylquinic acid in (a) 3,5-dicaffeoylquinic acid standard solution (1 mg/mL) and (b) methanol extract of B. pilosa dried aerial part powder showing peak corresponding to methanol extract of B. pilosa standard solution at tr = 30.7 min.
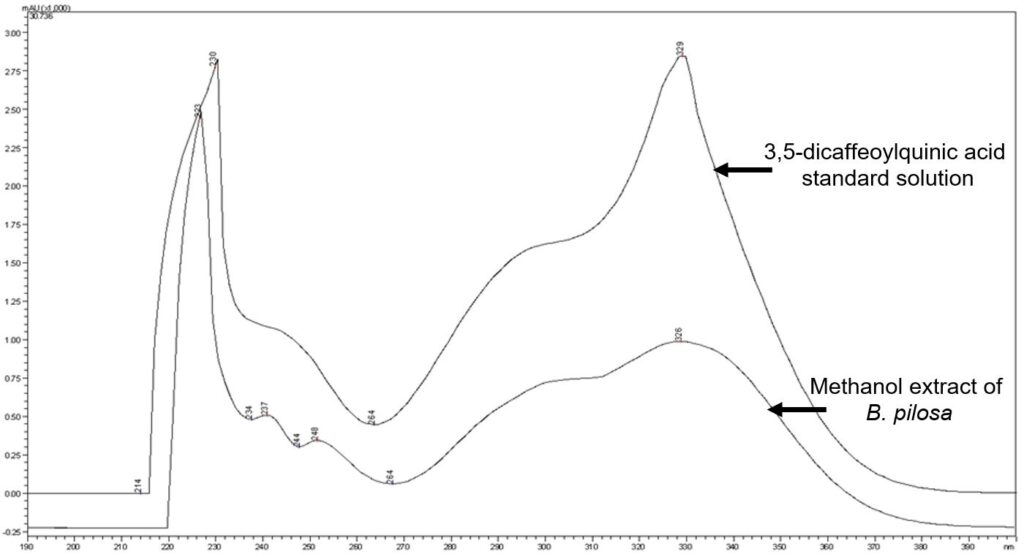
Figure 8: UV spectrum of 3,5-dicaffeoylquinic acid standard solution (1 mg/mL) and methanol extract of B. pilosa dried aerial part powder.
PURITY TEST
The purity tests except foreign matter test are based on B. pilosa dried aerial part powder of 0.355 mm particle size.
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 14% |
| Acid-insoluble ash | Not more than 3% |
| Water-soluble ash | Not more than 10% |
| Loss on Drying |
| Not more than 11% |
| Water-soluble extracts | |
| Hot method | Not less than 19% |
| Cold method | Not less than 19% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 10% |
| Cold method | Not less than 5% |
| Ash Contents | |
| Total ash | Not more than 14% |
| Acid-insoluble ash | Not more than 3% |
| Water-soluble ash | Not more than 10% |
SAFETY TEST
The safety tests are based on B. pilosa dried aerial part powder of 0.355 mm particle size.
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total aerobic microbial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Ethanol (95%) extract of B. pilosa aerial part has been reported to contain polyacetylenic (e.g., phenylheptatriyne (1-phenylhepta-1,3,5-triyne), 6-phenylhexa-1,3,5-triyn-1-ol, 6-phenylhexa-1,3,5-triyn-1-yl acetate, trideca-1,11-diene-3,5,7,9-tetrayne, trideca-2,12-diene-4,6,8,10-tetrayn-1-ol, trideca-2,12-diene-4,6,8,10-tetrayn-1-yl acetate, 6-phenylhex-1-ene-3,5-diyn-1-ol, tridec-1-ene-3,5,7,9,11-pentayne, 2-β-D-glucopyranosyloxy-1-hydroxy-5(E)-tridecene-7,9,11-triyne, 3-β-D-Glucopyranosyloxy-1-hydroxy-6(E)-tetradecene-8,10,12-triyne) [4].
Ethanol (95%) extract of B. pilosa aerial part contain polyacetylenic (e.g., 7-phenyl-hepta-4,6-diyn-1,2-diol, 7-phenyl-hepta-2,4,6-triyn-2-ol, 5-(2-phenylethynyl)-2-thiophene methanol, 5-(2-phenylethynyl)-2-β-glucosylmethyl-thiophene, (6E,12E)-3-oxo-tetradeca-6,12-dien-8,10-diyn-1-ol, (5E)-1,5-tridecadiene-7,9-diyn-3,4,12-triol) [5].
Dichloromethane extraction from aerial part of B. pilosa has isolated (R)-1,2-dihydroxytrideca-3,5,7,9,11-pentayne, 2-β-D-glycopyrasyloxy-1-hydroxytrideca 3,5,7,9,11-pentayne [6].
Ethyl acetate extract of B. pilosa var. radiata dried aerial parts has isolated flavonoid glucoside (e.g., (Z)-6-O-(3”,4″,6″-triacetyl-β-D-glucopyranosyl)-6,7,3’,4′-tetrahydroxyaurone, (Z)-6-O-(2”,4″,6″-triacetyl-β-D-glucopyranosyl)-6,7,3’,4′-tetrahydroxyaurone, (Z)-6-O-(4″,6″-diacetyl-β-D-glucopyranosyl)-6,7,3’,4′-tetrahydroxyaurone, okanin 4′-O-β -D-(3‘’,4″,6″-triacetyl)-glucopyranoside), acetylated okanin 4′-glucoside, iso-okanin 7-O-β-D-(2″,4″,6″-triacetyl)-glucopyranoside, luteolin, and 4-O-(2-O-acetyl-6-O-p-coumaroyl- β -D-glucopyranosyl)-p-coumaric acid, ethanoic acid (e.g., butanedioic acid) [7].
Petrol extract of B. pilosa var. radiata dried aerial parts has identified a polyacetylenes which is 1-phenyl-l,3,5-heptatriyne [7].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally, the leaves were used to treat cough and gum for toothache [8].
Biological and pharmacological activities supported by experimental data
Antimalarial activity
(R)-1,2-dihydroxytrideca-3,5,7,9,11-pentayne (0.8 mg/kg) isolated from dichloromethane extract of fresh aerial part of B. pilosa administered intravenously to ICR mice (24-26 g, 6 weeks old) infected with the Plasmodium berghei NK-65 strain with single daily doses for four consecutive days, significantly decreased average parasitaemia from 32.8% to 12.1% (p <0.05) compared to chloroquine (12 mg/kg) with a decrease in average parasitaemia from 32.8% to 1.1% using in vivo suppressive test [6].
Antibacterial activity
(R)-1,2-dihydroxytrideca-3,5,7,9,11-pentayne (0.25-1.28 µg/mL) isolated from dichloromethane extract of B. pilosa fresh aerial part showed highly inhibitory activities against several Gram-positive and Gram-negative pathogenic bacterial species including drug-resistant bacteria methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecalis (VREF) and Candida albicans. This compound was highly effective against several Gram-positive and Gram-negative bacteria including the drug-resistant bacteria Staphylococcus aureus N315 (MRSA) and Enterococcus faecalis NCTC12201 (VREF). (R)-1,2-dihydroxytrideca-3,5,7,9,11-pentayne had a similar MIC50 value to antibiotics amphotericin B (MIC50 > 8 µg/mL) in most of the bacteria tested [6].
Antitumour activity
Chloroform extract of B. pilosa dried aerial parts showed cytotoxicity activity on murine-derived Ehrlich ascites carcinoma (EAC) cell line with an IC50 of 97±7.2 and 83±5.2 µg/mL compared to doxorubicin IC50 = 0.6±0.1 µg/mL and IC50 = 0.4±0.1 µg/mL using neutral red uptake (NRU) assay and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay respectively [9].
Chloroform extract of B. pilosa dried aerial parts (150 mg/kg) administered intraperitoneally to isogenic BALB/c mice (male, 20±5 g) for 9 days significantly reduced (p <0.05) tumour body weight (6.09 ± 1.3 g), abdominal circumference (1.2 ± 0.4 cm), tumour volume (5.8 ± 2.7 mL), packed cell volume (2.4 ± 0.4 mL) and viable tumour cell count (8.6 ± 2.4 × 107 cells/mL) when compared to the Ehrlich ascites carcinoma (EAC) control group (8.1 ± 1.7 g, 2.7 ± 0.4 cm, 10.4 ± 2.1 mL, 3.4 ± 0.5 mL, 15.9 ± 3.4×107 cells/mL) [9].
Anti-inflammatory activity
Isolated ethyl acetate fraction of methylene chloride/methanol (1:1) extract of B. pilosa dried leaves (50, 100, and 200 mg/kg) was applied topically to carragenean-induced Wistar rats (100–140 g, 50−60 days). The extract (200 mg/kg) significantly (p<0.01) reduced paw edema volume by 58.51% at 3 hours treatment compared to indomethacin (10 mg/kg) 91.95% [10].
Isolated ethyl acetate fraction of methylene chloride/methanol (1:1) extract of B. pilosa dried leaves (50, 100, and 200 mg/kg b.w) was applied topically to dextran-induced Wistar rats (100–140 g, 50−60 days). The extract (200 mg/kg) significantly (p<0.05) decreased the paw edema volume by 39.26% at half hour compared to indomethacin (10 mg/kg) by 59.11% at the second hour [10].
Isolated ethyl acetate fraction of methylene chloride/methanol (1:1) extract of B. pilosa dried leaves (50, 100, and 200 mg/kg) was applied topically to histamine-induced Wistar rats (100–140 g, 50−60 days). The extract (200 mg/kg) significantly (p<0.05) decreased the paw edema volume by 32.18% at 1 hour compared to promethazine (1 mg/kg) which decreased paw edema volume by 41.48% [10].
Isolated ethyl acetate fraction of methylene chloride/methanol (1:1) extract of B. pilosa dried leaves (50, 100, and 200 mg/kg) was applied topically to Serotonin-induced Wistar rats (100–140 g, 50−60 days). The extract (100 mg/kg) significantly (p<0.05) decreased the paw edema volume by 56.11% compared to cyproheptadin (2 mg/kg) with (p <0.01) [10].
Antihyperglycemic activity
Mixture of 2-β-D-glucopyranosyloxy-1-hydroxy-5(E)-tride-cene-7,9,11-triyne and 3-β-D-glucopyranosyloxy-1-hydroxy-6(E)-tetradecene-8,10,12-triyne isolated from aqueous ethanolic extract of B. pilosa dried aerial parts ((3:2) (250 or 500 mg/kg)) administered orally by gavage to genetically altered obese diabetic mice (C5BL/Ks- db/db) (a model for type 2 diabetes) (male, 8 weeks old) for 0, 8, and 24 h significantly reduced blood glucose levels and food intake on the second day. Specifically, at a dose of 250 mg/kg, blood glucose levels decreased by 2% at 3 hours and 25% at 27 hours, with an average body weight reduction from 39.2 g to 38.8 g and a decrease in food intake to 3.2 g/mouse/day. At a higher dose of 500 mg/kg, blood glucose levels decreased by 13% at 3 hours and 41% at 27 hours, with an average body weight reduction from 40.0 g to 39.5 g and a decrease in food intake to 2.5 g/mouse/day. These effects were significant and comparable to the positive control, metformin (250 mg/kg), which showed a blood glucose reduction of 37% at 3 hours and 41% at 27 hours, with body weight decreasing from 39.8 g to 39.5 g and food intake to 4.2 g/mouse/day [11].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Information and data have not been established.
Others (Adverse reactions, contraindications, side effects, warning, precautions)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- Ridley HN. Malay Materia Medica. Vol. 5. Singapore: Singapore : Straits Medical Association; 1894.
- The Plant List. [Internet] Bidens pilosa L.; 2020 (cited on 11th July 2020]. Available from http://www.theplantlist.org/.
- Globinmed. [Internet] Bidens pilosa L.; 2020 (cited on 11th July 2020]. Available from https://www.globinmed.com.
- Bohlmann F, Burkhardt T, Zdero C. Naturally Occurring Acetylenes. Academic Press Inc, New York;1973.
- Wang R, Quan-Xiang W, Yan-Ping S. Polyacetylenes and flavonoids from aerial parts of Bidens pilosa. Planta Media. 2010; 76: 893-896.
- Tobinaga S, Sharma MK, Aalbersberg WGL, Watanabe K, Iguchi K, Narui K, Sadatsu M, Waki. Isolation and identification of a potent antimalarial and antibacterial polyacetylene from Bidens pilosa. Planta Med. 2009; 75:624–628.
- Wang J, Yang H, Lin ZW, Sun HD. Flavonoids from Bidens pilosa var. radiata. Phytochemistry.1997; 46:1275–1278.
- Burkill IH. A dictionary of the economic product of the Malay Peninsula, Vol.1. London; Published on behalf of the governments of the Straits settlements and Federated Malay states by the Crown agents for the colonies. 1935; p. 326
- Kviecinski MR, Felipe KB, Schoenfelder T. Study of the antitumor potential of Bidens pilosa (Asteraceae) used in Brazilian folk medicine. Journal of Ethnopharmacology. 2008;117(1); 69–75.
- Fotso AF, Longo, F, Djomeni, PD, Kouam, SF, Spiteller, M, Dongmo, AB, Savineau, JP. Analgesic and anti-inflammatory activities of the ethyl acetate fraction of Bidens pilosa (Asteraceae). Inflammopharmacology, 2014; 22(2):105-114.
- Ubillas RP, Mendez CD, Jolad SD. Antihyperglycemic acetylenic glucosides from Bidens pilosa. Planta Medica. 2000; 66(1):82–83.


