Melada pahit Fruit
Brucea javanica (L.) Merr.
Simaroubaceae
Definition
Melada pahit fruit consists of the powder of dried matured fruits of Brucea javanica (L.) Merr. (Simaroubaceae).
Synonym
Ailanthus gracilis Salisb., Brucea amarissima Desv. ex Gomes, Brucea glabrata Decne., Brucea gracilis (Salisb.) DC., Brucea sumatrana Roxb., Brucea sumatrana var. cambodiana Lecomte., Brucea amarissima (Lour.) Meyen ex Walp., Brucea sumatrensis Spreng., Gonus amarissimus Lour., Lussa amarissima (Lour.) Kuntze., Rhus fastuosa Salisb., Rhus javanica L., Tetradium amarissimum Poir.[ 1, 2 ].
Vernacular names
Java brucea, kosam, kosam seed, Macassar kernels (English); embalau padang, kusum, lada pahit, melada pahit, bidara pahit (Malay); ya dan zi (Chinese) [ 3 ].
Character
| Colour | Brown to dark brown |
| Odour | Characteristics |
| Taste | Very bitter |
Identification
Plant Morphology
Brucea javanica is shrub or small trees, monoecious or dioecious, up to 1–3 m high, with soft-haired twigs and leaves. Leaves compound-paripinnate, arranged spirally, 20–40 cm long, exstipulate; leaflets 3–15, opposite, petiolule 4–8 mm; blades ovate-oblong to ovate lanceolate, margin serrate, apex acuminate, base broadly cuneate or nearly rounded, both surfaces densely pubescent, especially underside and along veins. Inflorescence axillary, pubescent, raceme-like thyrses. Flowers unisexual, small, dark purple, 1.5–2 mm in diameter. Male flowers, petals sparsely puberulent or nearly glabrous; stamens 4; pistil reduces to a stigma. Female flowers, sepals and petals same as in males; stamens 4, much reduced; ovary with 4 carpels. Fruits consisting of 1–4 hardly fleshy drupelets, ovoid, 6–10 mm long, 4–7 mm in diameter, apex acuminate, base having a dented fruit stalk scar, shell hard and brittle, grey-black when ripe. Seeds ovoid, thinly membranous, 5–6 mm long, 3–5 mm in diameter, oily, blackish brown [ 4 , 5 , 6 , 7 ].
Microscopy
Powdered material consists of starch grains associated with oil globules, brachysclereid of pericarp, elongated fibres, parenchyma cells and solitary calcium oxalate crystals [ 5 ]
Figure 2 : Microscopic characters of Brucea javanica dried fruits powder of 0.355 mm size. (a) Starch grains, S and oil globules, OG (magnification 20x); (b) brachysclereid (magnification 40x); (c) fibre (magnification 20x); (d) parenchyma cells (magnification 20x); (e) fibre (magnification 10x); (f) solitary calcium oxalate crystals under polarizing filter (magnification 20x). [Scale bars: a, b, d, f = 50 µm; c, e = 100 µm]
Chemical Tests
Observation of solution after treatment with reagent
| Test for the presence of triterpenes | Red |
Thin Layer Chromatography (TLC)
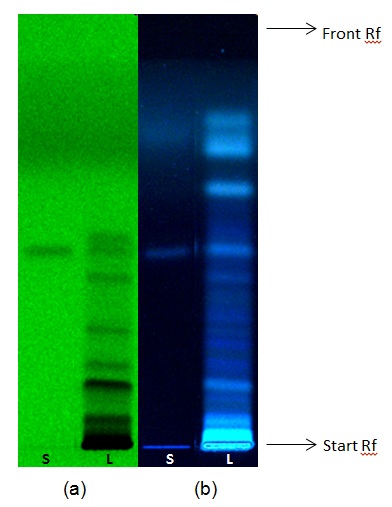
Figure 3 : TLC chromatogram of Bruceine A (S) at Rf: 0.47 and aqueous extract of Brucea javanica dried fruits powder (L) observed under (a) UV at 254 nm before derivatization and (b) UV at 366 nm after derivatization with anisaldehyde reagent.
| Test Solutions | Weigh about 1.5 g of B. javanica dried fruits powder in a 50 mL screw-capped sample bottle. Add 20 mL of aqueous and sonicate the mixture for 15 min at room temperature. Filter the mixture with filter paper (Whatman no. 1) and evaporate to dryness. Reconstitute with 4.0 mL methanol, filter the solution and use the filtrate as test solution. |
| Standard solution | Dissolve Bruceine A standard [CAS no.: 25514-31-2] in methanol to produce a standard concentration of 0.2 mg/mL. |
| Stationary Phase | HPTLC Glass Silica Gel 60 F254, 10 x 10 cm |
| Mobile phase | Chloroform : methanol (90:10) (v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
(Anisaldehyde was prepared using 4-methoxybenzaldehyde, 1.0 mL in 170 mL methanol and cautiously add 10 mL concentrated sulphuric acid and 20 mL acetic acid into the solution. Spray the solution onto the plate and heat the plate at 100°C for 5 min. Dry using air dryer. Observe plate at 366 nm and visible light.) |
High Performance Liquid Chromatography (HPLC)
| Test Solutions | Weigh about 1.5 g of B. javanica dried fruits powder in a 50 mL screw-capped sample bottle. Add 20 mL of aqueous and sonicate the mixture for 15 min at room temperature. Filter the mixture with filter paper (Whatman no. 1) and evaporate to dryness. Reconstitute with 4.0 mL methanol, filter the mixture solution through a 0.22 µm syringe filter and inject the filtrate into the HPLC column. | |||||||||||||||||||||
| Standard solution | Dissolve Bruceine A standard [CAS no.: 25514-31-2] in methanol to produce a standard concentration of 0.2 mg/mL. | |||||||||||||||||||||
| Chromatographic system |
Detector: UV 278 nmColumn: C18 (5.0 µm, 4.6 mm I.D x 150 mm) Oven temperature: 35°C Flow rate: 1.2 mL/min Injection volume: 10 µL |
|||||||||||||||||||||
| Mobile phase(gradient mode) |
|
|||||||||||||||||||||
| System suitability requirements |
Perform at least five replicate injections of the standard solutions (0.2 mg/mL). The requirements of the system suitability parameters are as follow:
|
|||||||||||||||||||||
| Acceptance criteria |
|
Figure 4 : Whole HPLC chromatogram of (a) Bruceine A standard solution (0.2 mg/mL) at tr = 19.846 min and (b) aqueous extract of Brucea javanica dried fruits powder showing peak corresponding to Brucein A standard solution at tr = 19.840 min.
(b)

(a)

Figure 5 : HPLC chromatogram highlighting the elution region of Brucein A in (a) Bruceine A standard solution (0.2 mg/mL) at tr = 19.846 min and (b) aqueous extract of Brucea javanica dried fruits powder at tr = 19.840 min.
(b)

(a)

Figure 6 : UV spectrum of Bruceine A standard solution (0.2 mg/mL) and aqueous extract of Brucea javanica dried fruits powder.

Purity Test
The purity tests, except foreign matter test, are based on B. javanica dried fruits powder of 0.355 mm particle size.
| Foreign Matter | |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 8% |
| Acid-insoluble ash | Not more than 1% |
| Water-soluble ash | Not less than 1% |
| Loss on Drying | |
| Not more than 10% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot Method | Not less than 17% |
| Cold Method | Not less than 12% |
| Ethanol-soluble extracts | |
| Hot Method | Not less than 24% |
| Cold Method | Not less than 19% |
Safety Test
The safety tests are based on B. javanica dried fruits powder of 0.355 mm particle size.
| Heavy Metals | |
| Arsenic | Not More than 5.0 mg/kg |
| Mercury | Not More than 0.5 mg/kg |
| Lead | Not More than 10.0 mg/kg |
| Cadmium | Not More than 0.3 mg/kg |
| Microbial limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
Chemical Constituents
Ethanol (30%) extract of B. javanica dried fruits was found to contain quassinoids (e.g. bruceine A, E, isobruceine B, bruceatin, brusatol, bruceantinoside A) [ 8 ].
Methanol (50%) extract of B. javanica dried fruits was found to contain quassinoids (e.g. bruceantinol, bruceine A, B, C, D, J, yadanziolide A, bruceantinol B, brusatol, bruceantin, dehydrobruceine A, B, dehydrobrusatol, bruceoside A and yadanzioside G) [ 11].
Ethanol (95%) extract of B. javanica dried fruits was found to contain quassinoids (e.g. brusatol, bruceene A, bruceine A, D, yadanzioside A-G, K, M, P, bruceoside A, B, bruceantinol, bruceantarin) [ 9 , 10].
Methanol extract of B. javanica dried fruits was found to contain pregnane glycoside (e.g. (20R)-O-(3)-β-D-glucopyranosyl-(1→2)-α-L-arabinopyranosyl-pregn-5-en-3β,20-diol), quassinoids (e.g. yadanziolide A-C, S, dehydrobrusatol, dehydrobruceantinol, brusatol, bruceine B, D, E), quassinoid glucosides (e.g. yadanziosides A-P, javanicosides B-F, bruceosides A-C, E and bruceantinoside A) [ 12 , 13 , 14 ,15 , 16 ].
Ethanol extract of B. javanica dried fruits was found to contain quassinoids (e.g bruceine B,C, brusatol, bruceantinol), lignans (e.g. erythro-syringylglycerol-β-O-4-sinapyl ether, wikstroemo, 1-(4-hydroxy-3-methoxyphenyl)-2-{2-methoxy-4-[1-(E)-propen-3-ol]-phenoxy}(10)-propane-3-diol (threo), 1-(4-hydroxy-3-methoxyphenyl)-2-{2-methoxy-4-[1-(E)-propen-3-ol]-phenoxy}-propane-3-diol (erythro), medioresinol, (+)-isolariciresinol, pinoresinol) [ 18 ].
Butanol extract of B. javanica dried fruits was found to contain quassinoids (e.g. Bruceine E-D) [20].
Ethyl acetate extract of B. javanica dried fruits was found to contain lignan (e.g guaiacylglyceroi-fl-O-6′-(2-methoxy)cinnamyl alcohol ether), quassinoids (e.g. brusatol, dehydrobrusatol, yadanziolide C), triterpenoid (e.g. blumenol A), coumarinolignan (e.g. cleomiscosin A), quassinoid glycoside (e.g. bruceoside B) [ 20 ].
Chloroform extract of B. javanica dried fruits was found to contain monoterpenoid glycoside and sesquiterpenes (e.g. brucojavans 1, 2, and 3), lignans (e.g. pinoresinol, cleomiscosin B, 7,8-epoxylignans, dihydrodehydrodiconiferyl alcohol, 70–hydroxylariciresinol, secoisolariciresinol, 4-methoxy-guaiacylglycerol, 7-carbonyl-guaiacylglycerol), phenolic acids (e.g. 4-hydroxy-3-methoxy-benzoic acid, and 3,4-dihydroxy-benzoic acid) [ 21 ].
Petroleum ether fraction of B. javanica dried fruits was found to contain esters (e.g. 2-ethyl-n-butyric acid ethyl ester, ethyl hexadecanoate, butanedioic acid, monomethyl ester, methyl 2-hydroxyhexadecanoate, pentanedioic acid, monomethyl ester, n-octadecanoic acid methyl ester, trans-2-hexenyl butyrate, (Z)-9-octadecenoic acid methyl ester, hydroxy-2-methylglutaric acid dimethyl ester, methyl cis,cis-9,12-octadecadienoate, methyl 8-hydroxyoctanoate, methyl cis-9,10-epoxyoctadecanoate, monomethyl pimelate, ethyl n-octadecanoate, dimethyl octanedioate, (Z)-9-octadecenoic acid ethyl ester, 9-oxo-nonanoic acid methyl ester, ethyl cis,cis-9,12-octadecadienoate, 4-hydroxy-benzeneacetic acid methyl ester, 2-[(2-nonylcyclopropyl)methyl]-cyclopropanebutyric acid, methyl ester, ethyl decanoate, methyl 7-hydroxystearate, 9-hydroxynonanoate, eicosanoic acid methyl ester, dimethyl onanedioate, methyl-(11E)-icosenoate, 4-hydroxy-benzeneacetic acid ethyl ester, eicosanoic acid ethyl ester, dodecanoic acid methyl ester, methyl 9,10-dihydroxyoctadecanoate, dodecanoic acid ethenyl ester, docosanoic acid methyl ester, methyl myristate, hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester, ethyl myristate, 8,11,14-docosatrienoic acid, methyl ester, methylpentadecanoate, tetracosanoic acid, methyl ester, phenylethyl cyclohexanecarboxylate, ethyl iso allocholate, n-hexadecanoic acid methyl ester, 1-O-hexadecanoyl-3-O-(9Z-octadecenoyl) glycerol, (Z)-7-hexadecenoic acid methyl ester, methyl 15-methylhexadecanoate, bis[(9E)-9-octadecenoyloxy]propyl(9E)-9-octadecenoate), acids (e.g. hexanoic acid, 3-methoxy-4-hydroxybenzoic acid, heptanoic acid, azelaic acid, benzenecarboxylic acid, pentadecanoic acid, octanoic acid, (Z)-9-octadecenoic acid, nonanoic acid, (Z,Z)-9,12-octadecadienoic acid, cinnamic acid, cis-9,10-epoxyoctadecanoic acid, 8-methoxy-8-oxooctanoic acid, erythro-9,10-dihydroxyoctadecanoic acid, 2-oxoadipic acid), alkenes (e.g. 1,4-cyclohexadiene, 1,3,12-nonadecatriene, cyclohexene, (17E)-17-pentatriacontene, (7E)-7-tetradecene), alcohols (e.g. 2-methyl-1-penten-3-ol, glycerin, cnidiol c, isooctanol, (2E)-2,6-dimethyl-2,7-octadiene-1,6-diol, 7-dimethyl-3-octanol, 2-cyclohexyl-hex-5-en-2-ol, 3,7-dimethyl-1,6-octadien-3-ol, 2-butyloctanol, cis-p-menth-2-n-1-ol, hexadecane-1,2-diol, phenylethyl alcohol, 2,6,10,15,19,23-hexamethyl-tetracosa-2,10,14,18,22-pentaene-6,7-diol, (3E)-2,6-dimethyl-3,7-octadiene-2,6-diol), ketones (e.g. acetophenone, 9-hydroxy-5-megastigmen-4-one, (3E)-3-nonen-2-one, 2-hydroxy-3-(1-propenyl)-1,4-naphthalenedione, n-nonaldehyde, 2-hydroxycyclopentadecanone, paroxypropiones), aldehydes (e.g. (2Z)-2-heptenal, vanillin, n-nonaldehyde, (2E)-2-tridecenal, (E)-2-nonenal, 2-amylnonen-2-al, p-hydroxybenzaldehyde, tridecanedial, (E,E)-2,4-decadienal, pentadecana), terpenes (e.g. copaene, α-caryophyllene, thujopsene, widdrol, δ-cadinene, platambin, caryophyllene, simiarenol, spathulenol, lupeol acetate, cedrol, deacetylpapyriferic acid, longiverbenone), pregnanones (e.g. pregn-4-ene-3,20-dione, 3-hydroxy-pregn-5-en-20-one), steroids (e.g. lanosterol, stigmasterol, (3β)-cholesta-4,6-dien-3-ol, 7-oxo-cholesterol, (3β)-ergost-5-en-3-ol, 3β-acetoxystigmasta-4,6,22-triene, (3β,5α,7β,8α,22E)-3′,7-dihydro-cycloprop [7,8]ergost-22-en-3-ol, 5α-stigmastane-3,6-dione, 22-stigmasten-3-one, (3β)-lanosta-8,24-dien-3-ol, stigmast-4-en-3-one, 9,19-cyclo-9β-lanostane-3β,25-diol, (22E)-stigmasta-4,6,22-trien-3-ol, 3-hydroxyergost-5-en-12-yl acetate) [ 27 ].
Medicinal Uses
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally, the poultice of its leaves is used to treat scurf, ringworm, boils and centipede bites. Meanwhile, the decoction of B. javanica whole plant or roots can be used to relieve colic, dysentery, fever and body ache [ 22 ].
Biological and pharmacological activities supported by experimental data
Antihypertensive activity
Aqueous fraction of alcohol (96%) extract of B. javanica dried fruits powder (0.0714 mg/kg) was administered orally to adrenaline-induced male Sprague Dawley rats (200 – 300 g) and blood pressure was measured regularly every 20 min for 80 min. The fraction significantly (p < 0.05) decreased the systolic blood pressure (180.75 ± 22.23 to 138.75 ± 17.73 mmHg) compared to bisoprolol (182.25 ± 25.62 to 176.25 ± 23.41 mmHg) [ 23 ].
Hexane fraction of alcohol (96%) extract of B. javanica dried fruits powder (0.0714 mg/kg) was administered orally to adrenaline-induced male Sprague Dawley rats (200 – 300 g) and blood pressure was measured regularly every 20 min for 80 min. The fraction significantly (p < 0.05) decreased the systolic blood pressure (160.50 ± 24.19 to 153.00 ± 14.28 mmHg) compared to bisoprolol (182.25 ± 25.62 to 176.25 ± 23.41 mmHg) [23].
Hypoglycemic effect
Ethyl acetate fraction from ethanol (95%) extract of B. javanica dried seeds powder (125 mg/kg) was administered orally to non-diabetic Sprague Dawley rats (200 – 250 g) fed with glucose after 30 min of treatment. The fraction significantly (p < 0.05) decreased the blood glucose levels after 90 min (5.60 mmol/L) compared to distilled water-control group (7.00 mmol/L) [25 ].
Butanol extract of B. javanica dried seeds powder (32, 64 and 128 mg/kg) was administered orally to male Mus musculus mice (25 – 30 g) and blood was withdrawn from tail vein immediately prior to the extract administration (0 hour) and every two hours after extract administration for eight hours. At eight hours, the extract showed significant (p < 0.05) reduction in blood glucose concentration (32 mg/kg: 39.14 ± 13.50%; 64 mg/kg: 37.02 ± 14.97%; 128 mg/kg: 32.31 ± 16.31%) compared to vehicle-control group (polyethylene glycol 3000) (18.33 ± 6.88%) [ 28 ].
Antidiabetic activity
Bruceine D isolated from butanol subfractions of methanol (80%) extract of B. javanica dried seeds powder (1.0 mg/kg) was administered intraperitoneally to male streptozotocin-induced diabetic Sprague Dawley rats (200 – 250 g) and blood glucose concentration was monitored for eight hours. At eight hours, the subfractions significantly (p < 0.05) decreased the blood glucose concentration (from 23.5 mmol/L to 3.0 mmol/L) comparable to glibenclamide (from 23.5 mmol/L to 6.0 mmol/L) [ 19 ].
Bruceine E isolated from butanol subfractions of methanol (80%) extract of B. javanica dried seeds powder (2.0 mg/kg) was administered intraperitoneally to male streptozotocin-induced diabetic Sprague Dawley rats (200 – 250 g) and blood glucose concentration was monitored for eight hours. At eight hours, the subfractions significantly (p < 0.05) decreased the blood glucose concentration (from 23.0 mmol/L to 5.5 mmol/L) comparable to glibenclamide (from 23.0 mmol/L to 6.0 mmol/L) [ 19].
Ethyl acetate fractions of ethanol (95%) extract of B. javanica seeds (25 and 50 mg/kg) was administered to streptozotocin-induced diabetic Sprague Dawley rats of both sexes (200 – 230 g, aged eight week old) once daily for 28 days. The fractions showed significant (p < 0.05) reduction at day-28 (25 mg/kg: 9.43 ± 1.12 mmol/L; 50 mg/kg: 7.87 ± 1.15 mmol/L) compared to day-0 (25 mg/kg: 13.43 ± 1.01 mmol/L; 50 mg/kg: 14.25 ± 0.93 mmol/L) [ 32 ].
Anti-inflammatory activity
Ethyl acetate fraction from ethanol (70%) extract of B. javanica seeds powder (5 and 25 mg/kg) was injected intraperitoneally to ICR male mice 30 min before induction of paw edema using carrageenan. After five hours of post-injection, the fraction significantly (p < 0.05) inhibited paw edema thickness (40% and 74%, respectively) compared to vehicle treated group (olive oil) (28%) [ 8 ].
Reduction in tumor size
Brucein D isolated from B. javanica fruits (1.5 mg/kg) was administered intraperitoneally (three times a week for duration of three weeks) to male nude mice (aged 4 – 6 weeks old) after induction of liver tumour using tumorigenic Hep3B cells. After 20 days, the tumor volume treated with Bruceine D significantly (p < 0.05) grew much slower (from 25 to 75 mm3) compared to solvent control (from 25 to 180 mm3) [ 27 ].
Petroleum ether extract of B. javanica seeds (49.5 mg/kg) was intravenously injected to Balb/c nude mice (aged 7 – 8 weeks old) after induction of leukemia using human leukemic monocyte lymphoma cell (U937) into the right flank of mice. After ten days, the tumor weight was significantly (p < 0.001) decreased (0.18 g) and the tumor volume also showed a smaller growth (from 100 to 200 mm3) compared to saline-treated control group (tumor weight = 1.58 g; tumor volume = from 100 to 1700 mm3) [ 30 ].
Oil of B. javanica dried seeds powder (1.5 g/kg) was administered subcutaneously (daily for seven days) to female H22-bearing mice (18 – 20 g) showed reduction in the average liver tumor weight (1.50 ± 0.39 g) and increased in the tumor inhibition ratio (38.27%) comparable to positive control, fluorouracil (5-Fu) (average tumor weight: 1.40 ± 0.62 g; 42.39% of inhibition ratio) [ 31 ].
Antioxidant activity
Ethyl acetate fraction from ethanol (95%) extract of B. javanica dried seeds powder (0.05 – 2 mg/mL) showed significant (p < 0.05) antioxidant activity with 2,2 diphenyl-1-picrylhydrazyl (DPPH) radical with inhibition of concentration at 50% (IC50) of 33.65 ± 3.04 µg/mL compared to butylated hydroxyanisole (BHA) (IC50 = 5.95 µg/mL) using DPPH radical scavenging activity assay [25 ].
Cytotoxicity activity
Ethyl acetate extract of B. javanica dried fruits powder inhibited the growth of cultured human cancer line i.e. human lung cancer cell line, A549 (IC50 = 0.02 µg/mL), human breast carcinoma cell line, MCF-7 (IC50 = 3.28 µg/mL), human hepatoma cell line, HepG2 (IC50 = 4.14 µg/mL), human lung cancer cell, NCI-H460 (IC50 = 5.79 µg/mL), human nasopharyngeal carcinoma cell line, CNE (IC50 = 0.48 µg/mL) and human epidermoid carcinoma cell line, KB-3-1 (IC50 = 9.66 µg/mL) compared to doxorubicin (A549: 0.16; MCF-7: 2.37; HepG2: 0.52; NCI-H460: 0.31; CNE: 0.37; KB-3-1: 0.17 µg/mL) using MTT assay [ 26 ].
Bruceine D isolated from B. javanica fruits (0.7 µM) showed cytotoxic activity on three hepatocellular carcinoma cell lines (HepG2, Hep3B and primary liver cancer). After 72 hr, bruceine D significantly (p < 0.05) increased the percentage of apoptotic cells (HepG2 = 19%, Hep3B = 16% and PLC = 16%) compared to solvent-control (HepG2: 6; Hep3B: 5 and PLC: 7%, respectively) using annexin V cell apoptosis assay [ 27 ].
Antimicrobial activity
Methanol extract of B. javanica dried fruits powder (10 mg/mL) showed anti-amoebic activity against Entamoeba histolytica with IC50 = 10.7 µg/mL compared to metronidazole (IC50 = 0.32 µg/mL) using broth microdilution assay [ 23 ].
Aqueous extract of B. javanica dried seeds powder (100 mg/mL) showed antifungal activity against Candida dubliniensis with diameter of inhibiton of 22.7 ± 1.0 mm, C. glabrata (15.6 ± 1.6 mm), C. lusitaniae (15.1 ± 0.8 mm)and C. parapsilosis (15.0 ± 0.9 mm) compared to positive control, chlorhexidine digluconate (range between 9.0 to 25.0 mm) using broth microdilution assay [ 28 ].
Clinical studies
Information and data have not been established.
Safety Information
Preclinical studies (Toxicology study)
24-hours toxicity study
Butanol extract of B. javanica dried seeds powder was administered orally at a single dose to male Mus musculus mice (25 – 30 g body weight). No toxicity effect were observed for a period of 24 hours (lethal dose at 50%, (LD50) > 438.43 mg/kg) [ 27 ].
9-weeks toxicity study
Butanol extract of B. javanica dried seeds powder (32 mg/kg) was administered orally to male Mus musculus mice (25 – 30 g body weight) once a day for duration of nine weeks. No significant changes in blood levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) was recorded. No toxicity effect and mortality was observed throughout the study [ 28 ].
14-weeks toxicity study
Oral single dose acute toxicity study on female Sprague Dawley rats (aged between 8 and 12 weeks old) using aqueous extract of B. Javanica fruits showed no toxic effect on the parameters observed, including behaviours, body weight, food and water intake. All rats were observed for 14 days prior to necropsy. One death was found after 8-hour administration of the extract (2,000 mg/kg body weight). Necropsy revealed no significant abnormality at doses 300 mg/kg body weight. Half lethal dose (LD50) is less than 2,000 mg/kg body weight [ 33].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
Dosage
Information and data have not been established.
Storage
Store below 30°C. Protect from light and moisture.
Reference
- The Plant List [Internet]. Brucea javanica (L.) Merr.; 2013 [cited on 27th May 2015]. Available from: http://www.theplantlist.org/tpl1.1/record/kew-2683970.
- WFO Plant List [Internet]. Brucea javanica (L.) Merr.; 2024 [ cited on 20th June 2024]. Available from: https://wfoplantlist.org/taxon/wfo-0000572705-2024-06?page=1
- Quattrocchi UFLS. CRC world dictionary of medicinal and poisonous plants: common names, scientific names, eponyms, synonyms, and etymology Volume 1 A-B. United States: CRC Press; 2012.
- PROSEA (Plant Resources of South-East Asia) Foundation Bogor, Indonesia. [Internet] Brucea javanica (L.) Merr.; 1999 [cited on 21st April 2015]. Available from: http://proseanet.org/prosea/e-prosea_detail.php?frt=&id=183.
- Flora of China. [Internet] Brucea javanica (Linnaeus) Merril, J. Arnold Arbor; 1928 [cited on 21st April 2015]. Available from: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200012489.
- World Health Organization. WHO Monographs on selected medicinal plants – Vol. 1. Geneva: World Health Organization. 1999; p.59-66.
- Mei HEH, Liping CUI. Pharmacopoeia of the people’s Republic of China. Vol. 1. Beijing: China Medical Science Press. 2010; p.78.
- Yang J, Li S, Xie C, Ye H, Tang H, Chen L, Peng A. Anti-inflammatory activity of ethyl acetate fraction of the seeds of Brucea javanica. Journal of Ethnopharmacology. 2013;147(2):442-446.
- Ye Q-M, Bai L-L, Hu S-Z, Tian H-Y, Ruan L-J, Tan Y-F, et al. Isolation, chemotaxonomic significance and cytotoxic effects of quassinoids from Brucea javanica. Fitoterapia. 2015;105:66-72.
- Feng X, Jiang H, Zhang Y, He W, Zhang L. Insecticidal activities of ethanol extracts from thirty Chinese medicinal plants against Spodoptera exigua (Lepidoptera: Noctuidae). Journal of Medicinal Plants Research. 2012;6(7):1263-1267.
- Bawm S, Matsuura H, Elkhateeb A, Nabeta K, Nonaka N, Oku Y, et al. In vitro antitrypanosomal activities of quassinoid compounds from the fruits of a medicinal plant, Brucea javanica. Veterinary parasitology. 2008;158(4):288-294.
- Liu Y-T, Wang F, Wang G-X, Han J, Wang Y, Wang Y-H. In vivo anthelmintic activity of crude extracts of Radix angelicae pubescentis, Fructus bruceae, Caulis spatholobi, Semen aesculi, and Semen pharbitidis against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus). Parasitology research. 2010;106(5):1233-1239.
- Sakaki T, Yoshimura S, Ishibashi M, Tsuyuki T, Takahashi T, Honda T, et al. Structures of new quassinoid glycosides, yadanziosides A, B, C, D, E, G, H, and new quassinoids, dehydrobrusatol and dehydrobruceantinol from Brucea javanica (L.) Merr. Bulletin of the Chemical Society of Japan. 1985;58(9):2680-2686.
- Tsuyuki T, Takahashi T, Honda T. Yandanziolide D, a new C19-quassinoid isolated from Brucea javanica (L.) Merr. Chemical and pharmaceutical bulletin. 1988;36(2):841-844.
- Mangunsong S, Dwiprahasto I. Spectroscopic analysis and cytotoxic activity of quas-sinoid isolated from the seeds of Brucea javanica on Hela cell. Indonesian Journal Of Pharmacy. 2011:137-143.
- Yoshimura S, Sakaki T, Ishibashi M, Tsuyuki T, Takahashi T, Honda T. Constituents of seeds of Brucea javanica. Structures of new bitter principles, yadanziolides A, B, C, yadanziosides F, I, J, and L. Bulletin of the Chemical Society of Japan. 1985;58(9):2673-2679.
- Kim IH, Takashima S, Hitotsuyanagi Y, Hasuda T, Takeya K. New Quassinoids, Javanicolides C and D and Javanicosides B− F, from Seeds of Brucea javanica. Journal of natural products. 2004;67(5):863-868.
- Dong S-H, Liu J, Ge Y-Z, Dong L, Xu C-H, Ding J, et al. Chemical constituents from Brucea javanica. Phytochemistry. 2013;85:175-184.
- Noor Shahida A, Wong TW, Choo CY. Hypoglycemic effect of quassinoids from Brucea javanica (L.) Merr (Simaroubaceae) seeds. Journal of Ethnopharmacology. 2009;124(3):586-591.
- Luyengi L, Suh N, Fong HH, Pezzuto JM, Kinghorn AD. A lignan and four terpenoids from Brucea javanica that induce differentiation with cultured HL-60 promyelocytic leukemia cells. Phytochemistry. 1996;43(2):409-412.
- Chen Q-J, Ouyang M-A, Tan Q-W, Zhang Z-K, Wu Z-J, Lin Q-Y. Constituents from the seeds of Brucea javanica with inhibitory activity of Tobacco mosaic virus. Journal of Asian natural products research. 2009;11(6):539-547.
- Burkill IH. A dictionary of the economic products of the Malay Peninsula. Vol. 1. London; Published on behalf of the governments of the Straits settlements and Federated Malay states by the Crown agents for the colonies. 1935;p.370-372.
- Roswiem AP, Kiranadi B, Bachtiar TSP, Ranasasmita R. Antihypertensive effect of Brucea javanica (L.) Merr. fruit extract. Makara Journal of Science. 2012;16(2):71-76.
- Wright CW, O’Neill MJ, Phillipson JD, Warhurst DC. Use of microdilution to assess in vitro antiamoebic activities of Brucea javanica fruits, Simarouba amara stem, and a number of quassinoids. Antimicrobial Agents and Chemotherapy. 1988;32(11):1725-1729.
- Ablat A, Mohamad J, Awang K, Shilpi JA, Arya A. Evaluation of antidiabetic and antioxidant properties of Brucea javanica seed. The Scientific World Journal. 2014;2014:1-8.
- Su Z, Huang H, Li J, Zhu Y, Huang R, Qiu SX. Chemical composition and cytotoxic activities of petroleum ether fruit extract of fruits of Brucea javanica (Simarubaceae). Tropical Journal of Pharmaceutical Research. 2013;12(5):735-742.
- Xiao Z, Ching Chow S, Han Li C, Chun Tang S, Tsui SK, Lin Z, Chen Y. Role of microRNA-95 in the anticancer activity of Brucein D in hepatocellular carcinoma. European Journal of Pharmacology. 2014;728:141-150.
- Shahida AN, Choo CY, Wong TW. Acute/subchronic oral toxicity of Brucea javanica seeds with hypoglycemic activity. Journal of Natural Remedies. 2011;11(1):60-68.
- Nordin M-A-F, Harun WHAW, Razak FA. Antifungal susceptibility and growth inhibitory response of oral Candida species to Brucea javanica Linn. extract. BMC Complementary and Alternative Medicine. 2013;13:342.
- Zhang H, Yang JY, Zhou F, Wang LH, Zhang W, Sha S, et al. Seed Oil of Brucea javanica Induces Apoptotic death of acute myeloid leukemia cells via both the death receptors and the mitochondrial-related pathways. Evidence-Based Complementary and Alternative Medicine. 2011;2011:14.
- Shi W-R, Liu Y, Wang Z-T, Huang Q-Y, Cai X-R, Wu S-R. Antitumor efficacy and mechanism in hepatoma H22-bearing mice of Brucea javanica oil. Evidence-Based Complementary and Alternative Medicine. 2015;2015:1-8.
- Ablat A, Halabi MF, Mohamad J, Hasnan MHH, Hazni H, Teh S, A.Shilpi J, Mohamed Z, Awang K. Antidiabetic effects of Brucea javanica seeds in type 2 diabetic rats. BMC Complementary and Alternative Medicine. 2017;17(94):1-14.
- Norzahirah A, Umi Rubiah SZ, Bazilah J, Nurul Syahira S, Dalnitha Lea N, Tavinraj R, Teh BP. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical research, Ministry of Health; 2019. Report no.: NON-GLP/2019/02/01.

