Pegaga Herb
Centella asiatica (L.)Urb.
Umbelliferae
DEFINITION
Pegaga herb consists of dried aerial part or whole plant of C. asiatica (L.) Urb.
SYNONYM
Centella coriacea Nannfd., Hydrocotyle asiatica L., Hydrocotyle lunata Lam., Trisanthus cochinchinensis Lour [ 1 , 2 , 3 , 4 ].
VERNACULAR NAMES
Pegaga (Malay), ji xui cao (Chinese), vallarai (Tamil), asiatic pennywort (English) [ 5 , 6 , 7 ].
CHARACTER
The whole plant powder is greyish green in colour with slightly bitter taste and characteristic odour.
IDENTIFICATION
Plant Morphology
A slender creeping herb with long-stalked leaf. The leaves are green, reniform, rounded apex and base deeply cordate stipulate with palmate netted veins and smooth texture; the petiole is relatively long, up to 20 cm. The flowers are in fascicled umbels with very small (about 4 mm) reddish petal and hermaphrodite; each flower bears 4-6 stamen and 2 styles. The fruit is small, mericarp in nature. The rootstock consists of rhizomes growing vertically down and stolon growing horizontally, interconnecting one plant to another [ 5 ].
Microscopy
The powder contains fragment of lamina consisting of upper epidermis with polygonal cells and underlying palisade cells that are rather small and closely packed. The lower epidermis has numerous paracytic stomata. Calcium oxalate prisms and macles are present. Fragments of parenchyma from the stolon and petiole are composed of longitudinal elongated cells. Fragments of bordered pitted vessel are also seen [ 5 , 6 , 7 , 8 ].
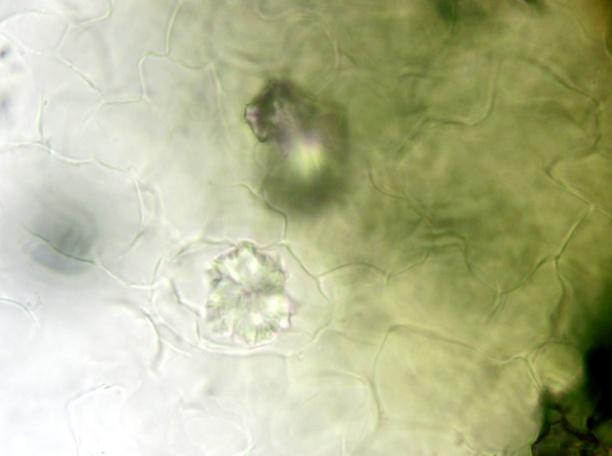
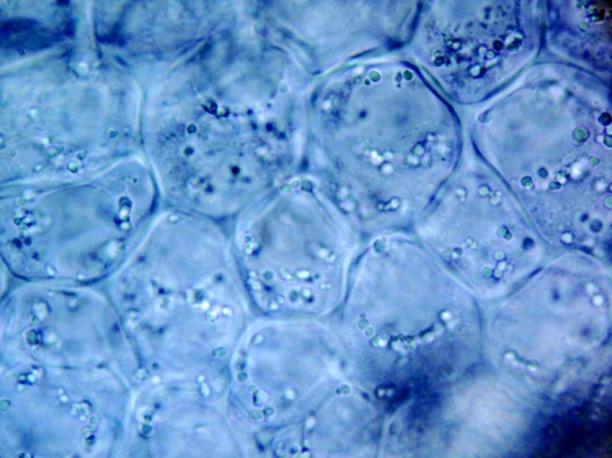
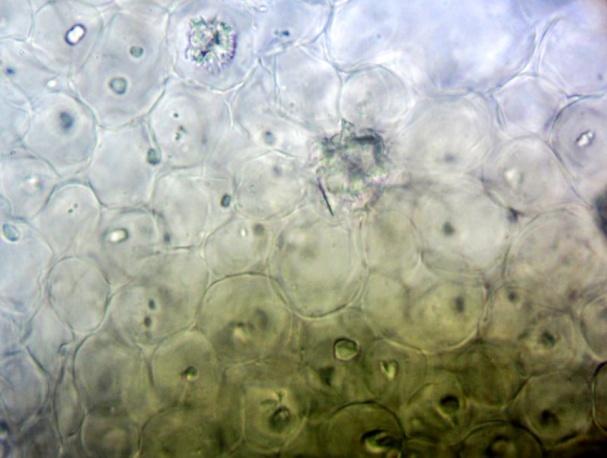

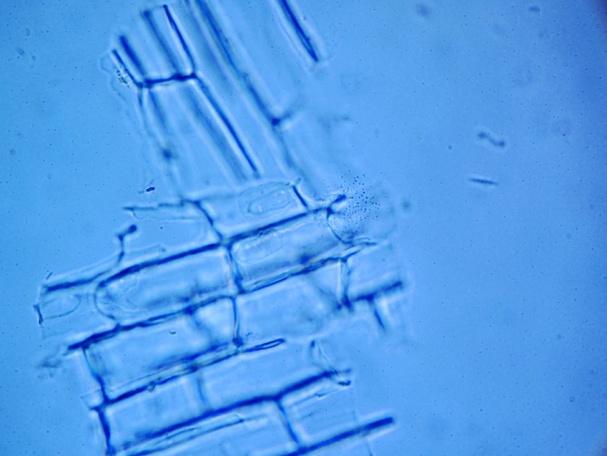
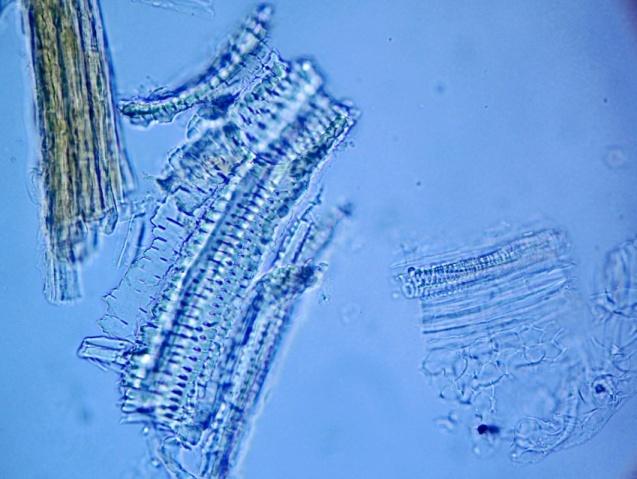
Figure 2 : Microscopic characters of C. asiatica herb powder. (a) Parenchyma cells with calcium oxalate macles (magnification 400X); (b) fragment of leaf epidermis with polyglonal cells (magnification 400X); (c) collenchyma cells with calcium oxalate macles (magnification 400X); (d) paracytic stomata (magnification 400X); (e) elongated parenchyma cells of the petiole (magnification 400X); (f) fragment of bordered pitted vessels.
Colour Tests
Observed colour of solution after treatment with various reagents:
| H2SO4 (conc.) | Dark brown |
| 5% NaOH | Light brown |
| 5% KOH | Light brown |
| 25% NH4OH | Yellow |
Thin Layer Chromatography (TLC)
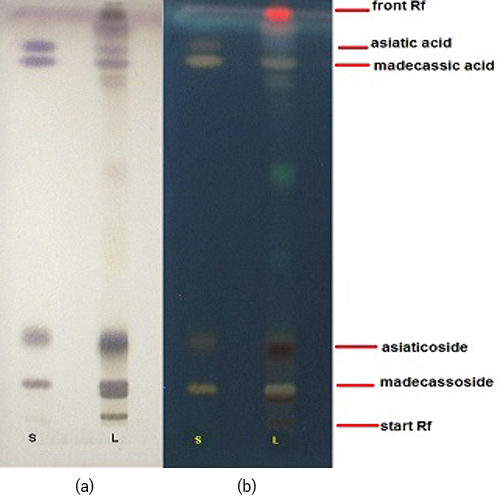
Figure 3 : TLC profiles of a standard mixture of asiaticoside, asiatic acid, madecassoside and madecassic acid standards (S) and ethanol extract of C. asiatica herb (L) after spraying with 10% sulphuric acid in ethanol and observed under (a) visible light and (b) UV at 366 nm
| Test Solutions | Weigh about 5.0 g of C. asiatica dried herb powder in a 50 mL round bottom flask and add 50 mL ethanol into the flask. Reflux the sample for 1 hr and allow to cool. Transfer 500 µL of the extract and add with 500 µL of ethanol. Filter the mixture and use the filtrate as the test solution. |
| Standard solution | Separately dissolve 2.0 mg of asiaticoside, asiatic acid, madecassoside and madecassic acid standards in 1 mL of ethanol to give 2000 µg/mL solutions. Mix the standards to give 500 µg/mL solution. |
| Stationary Phase | HPTLC Silica gel 60 F254, 10 x 10 cm |
| Mobile phase | Chloroform-methanol-formic acid, 5:1:4 (v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
Spray with 10% sulphuric acid in ethanol and view under
|
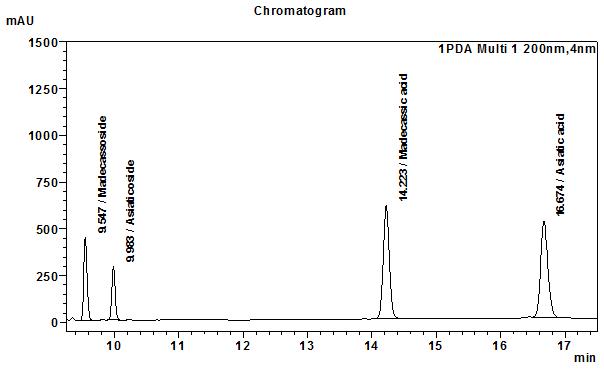
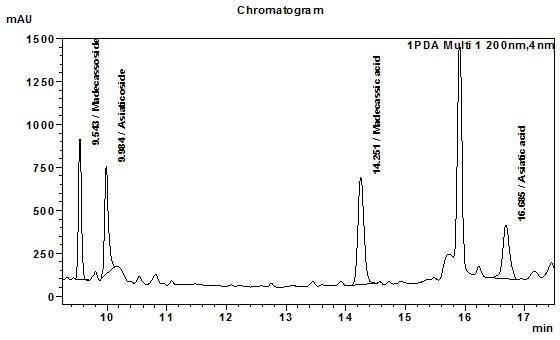
High Performance Liquid Chromatography (HPLC)
| Test solution | Extract about 5.0 g of dried powder C. asiatica dried herb powder with 80 mL of ethanol by reflux method at a temperature of 60°C for 30 min. Filter the mixture through a filter paper. Evaporate the filtrate to dryness using a rotary evaporator. Then, dissolve 20.0 mg of the dried extract in 2 mL of methanol. Sonicate the mixture for 15 min. Filter the solution through a 0.45 µm syringe filter and inject the filtrate into the HPLC column. | ||||||||||||
| Standard solution | Separately dissolve 2.0 mg of asiaticoside, asiatic acid, madecassoside and madecassic acid standards in 2 mL of methanol to give 1000 µg/mL stock solutions. Sonicate the solutions for 15 min. Mix the standard solutions to produce a 250 µg/mL solution. | ||||||||||||
| Chromatographic system |
Detector: UV 200 nm |
||||||||||||
| Mobile Phase (gradient mode) |
|
||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the standard mixture (250 µg/mL). The requirements of the system suitability parameters are as follow:
|
||||||||||||
| Acceptance criteria |
|
Table 1 : The Relative Retention Times (RRT) for the four characteristic peaks
| Standard | RRT |
| Madecassoside | 0.67 |
| Asiaticosside | 0.69 |
| Madecassic acid (as reference) | 1.00 |
| Assiatic acid | 1.17 |
Note: The RRTs provided only serve as a guidance.
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 14% |
| Acid-insoluble ash | Not more than 4% |
| Loss on Drying |
| Not more than 10% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 23% |
| Cold method | Not less than 21% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 17% |
| Cold method | Not less than 7% |
SAFETY TEST
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Water extract of C. asiatica has been found to contain centellosides D-E, pectin and acidic arabinogalactan [ 9 , 10 , 11 ].
The ethanol extract had triterpenes (e.g. 2α,3β,20,23-tetrahydroxyurs-28-oic acid, 2α,3β,23-trihydroxyurs-20-en-28-oic acid), triterpenoid glycosides (e.g. asiaticoside, asiaticosides A-F, madecassoside, scheffuroside B) and a saponin (e.g. 2α,3β,23-trihydroxyurs-20-en-28-oic acid-O-α-L-rhamnopyranosyl-(1→4)-O-b-D-glucopyranosyl -(1→6)-O-β-D-glucopyranosyl ester) [ 12 , 13 , 14 , 15 ].
The methanol extract had triterpenes and triterpenoids (e.g. ursolic acid lactone, ursolic acid, pomolic acid, 2α,3α-dihydroxyurs-12-en-28-oic acid, 3-epimaslinic acid, corosolic acid, asiaticoside, madecassoside, asiatic acid, 6β-hydroxyasiatic acid, 3-O-[a-L-arabinopyranosyl]-2α,3β,6β,23-a-tetrahydroxyurs-12-ene-28-oic acid), a phenolic (e.g. rosmarinic acid), a steroid (e.g. b-sitosterol 3-O-b-glucopyranoside) and others (e.g. 8-acetoxy-1,9-pentadecadiene-4,6-diyn-3-ol, centellin, asiaticin, centellicin) [ 16 , 17 , 18 , 19 ]. Whereas the methanol-water extract had phenolics (e.g. castilliferol, castillicetin, isochlorogenic acid) [ 20 ].
C. asiatica has also been reported to contain brahmic acid, 3β-6β-23-trihydroxyolean-12-en-28-oic acid, 3β-6β-23-trihydroxyurs-12-en-28-oic acid, α-terpinene, thymol methyl ether, sceffoleoside A, bayogenin, centellasaponins B-D, centellose, D-gulonic acid, meso-inositol, docosyl ferulates) [ 21 , 22 , 23 , 24 , 25 ]. In addition, its cell cultures had irbic acid, chlorogenic acid and triferulic acid [ 26 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
The C. asiatica plant is traditionally used for albinism, anemia, asthma, bronchitis, cellulite, cholera, constipation, dermatitis, diarrhea, dizziness, dysentery, dysmenorrhea, dysuria, epistaxis, epilepsy, haematemesis, hemorrhoids, hepatitis, hypertension, jaundice, leucorrhoea, nephritis, nervous disorders, neuralgia, measles, rheumatism, smallpox, syphilis, toothache, urethritis and varices. It is also used as an antipyretic, anti-inflammatory and a “brain tonic” [ 27 , 28 , 29 ]. Poultices of this plant have been used to treat contusions, closed fractures, sprains and furunculosis [ 29 ].
Biological and pharmacological activities supported by experimental data
Cytotoxic activity
Methanol (80%) extract and acetone fraction of methanol (80%) extract of C. asiatica whole plant inhibited proliferation of Ehrlich ascites tumour cells (concentration of 50% cell death 62 and 17 μg/mL, respectively) and Dalton’s lymphoma ascites tumour cells (75 and 22 μg/mL, respectively). The acetone fraction also suppressed multiplication of mouse lung fibroblast (L-929) cells at a concentration of 50% cell death of 8 μg/mL [ 30 ].
Methanol (80%) extract of C. asiatica whole plant exhibited concentration dependent cytotoxicity towards human breast cancer cell line MCF-7 by apoptosis with LD50 value of 66 mg. Asiatic acid (10 µM) was found to kill ~95% cells [ 31 ].
Anti-tumour effect
Oral administration of the methanol (80%) extract (1 mg/g body weight) and acetone fraction of methanol (80%) extract (0.04 mg/g body weight) of C. asiatica whole plant to the Dalton’s lymphoma ascites tumour-bearing Swiss Albino mice significantly (p < 0.001) retarded the development of solid tumour. The methanol extract (1 mg/g body weight) also significantly (p < 0.001) reduced the ascites tumour growth and increased the life span of Ehrlich ascites tumour-bearing mice. The treatments were effective when these were started simultaneously after and 10 days prior to the tumour transplantation [ 30 ].
Wound healing activity
Titrated extract of C. asiatica plant that constituted of asiatic acid, madecassic acid and asiaticoside (40 mg) injected to male Sprague-Dawley rats twice a week for 4 weeks had wound healing effect by stimulation of glycogen and glycosaminoglycan synthesis and fasten the new formation of connective tissue [ 32 ].
Antioxidant activity
The ethanol extracts of all plant parts (leaves, petioles and roots) of C. asiatica showed significant antioxidant activity (p<0.05) in the concentration range of 1000-3000 ppm [ 33 ].
Methanol extract of C. asiatica whole plant (50 mg/kg/day) administered orally to 2-month old lymphoma-bearing male Swiss mice for 14 days significantly increased the antioxidant enzymes of superoxide dismutase, catalase and glutathione peroxidase, as well as the glutathione and ascorbic acid antioxidants in the liver and kidney [ 34 ].
Anti-ulcer activity
The water extract of C. asiatica whole plant (0.10 and 0.25 g/kg) and asiaticoside (5 and 10 mg/kg) given orally for 7 days to male Sprague-Dawley rats with acetic acid induced gastric ulcers were found to decrease the ulcer sizes (p< 0.01). This was due to the reduction of myeloperoxidase activity (p< 0.01) and promotion of epithelial cell proliferation (p<0.01) by upregulated expression of basic fibroblast growth factor in the ulcer tissues [ 35 ]. The extract and asiaticoside also inhibited inducible nitric oxide synthase activity (p< 0.01) and protein expression at the ulcer tissues [ 36 ].
Radioprotection effect
The aqueous extract of C.asiatica whole plant (100 mg/kg body weight) given orally to 6-8 weeks old Swiss Albino mice irradiated with Co-60 gamma radiation was reported to significantly increase survival time [ 37 ].
Protective effect for neurodegenerative disorder
The aqueous extract of C.asiatica whole plant (300 mg/kg) given to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity in aged Sprague-Dawley rats for 21 days protected the brain against neurodegenerative disorders with reference to examination of the oxidative biomarker levels in corpus striatum and hippocampus homogenate
[ 38 ].
Clinical studies
Clinical studies have been conducted using leaf extracts of C. asiatica [ 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 ], however, none is available for the whole plant extract.
SAFETY INFORMATION
Preclinical studies (Toxicology study)
Acute toxicity
Oral single dose acute toxicity study of aqueous mixture of C. asiatica whole plant powder on female Sprague Dawley rats (aged between 8 and 12 weeks old) showed no toxic effects on the parameters observed, including behaviors, body weight, food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. No-observed-adverse-effect level (NOAEL) is more than 2,000 mg/kg body weight. [ 49 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Warning Asiaticoside (0.10%) has been found to cause development of papillomas in vivo [ 50 ]. Precaution Allergic contact dermatitis has been associated with several topical products containing C. asiatica extract [ 51 , 52 , 53 ].
DOSAGE
Oral dose : 0.33-0.68 g or oral infusion of similar amount three times daily [ 4 , 27 , 28 ].
Poultice : Apply to skin with wound, burns or inflammation after the fresh or dried whole plant is minced [ 54 ].
Juice : One handful of fresh whole plant made into fresh juice as tonic [ 54 ].
STORAGE
Store below 30˚C. Protect from light and moisture.
REFERENCES
- Australian plant name index (APNI). [Internet]. Centella asiatica (L.) Urb; [cited 28 November 2012]. Available from: http://www.anbg.gov.au/cgi-bin/wintab.
- African pharmacopoeia, 1st ed. Lagos, Organization of African Unity, Scientific, Technical & Research Commission.1985.
- Vietnam materia medica. Hanoi, Ministry of Health. 1972.
- Iwu MM. Handbook of African medicinal plants. Boca Raton, FL, CRC Press. 1993.
- Malaysian herbal monograph ed. MHM. committee. Vol. 2. Kuala Lumpur. 2003;p.23.
- Chinese-English manual of common-used in traditional Chinese medicine. China, Joint Publishing Co. & Guangdong Science & Technology. 1992;p.704.
- The Ayurvedic pharmacopoeia of India. Part I. Vol. IV.[Internet] India, Government of India, Ministry of Health and Family Welfare; 2004; p.69. [cited on 9th December 2012]. Available from http://www.ayurveda.hu/api/API-Vol-4.pdf
- Pharmacopoeia, British. “International edition.” HMSO Cambridge 1. 2009.
- Weng XX, Zhang J, Gao W, Cheng L, Shao Y, Kong DY. Two new pentacyclic triterpenoids from Centella asiatica. Helvetica Chimica Acta. 2012;95:255-260.
- Wang XS, Dong Q, Zuo JP, Fang JN. Structure and potential immunological activity of a pectin from Centella asiatica (L.) Urban. Carbohydrate Research. 2003;338:2393-2402.
- Wang XS, Zheng Y, Zuo JP, Fang JN. Structural features of an immunoactive acidic arabinogalactan from Centella asiatica. Carbohydrate Polymers. 2005;59:281-288.
- Yu QL, Duan HQ, Takaishi Y, Gao WY. A novel triterpene from Centella asiatica. Molecules. 2006;11:661-665.
- Yu QL, Duan HQ, Gao WY, Takaishi Y. A new triterpene and a saponin from Centella asiatica. Chinese Chemical Letters. 2007;18:62-64.
- Jiang ZY, Zhang XM, Zhou J, Chen JJ. New triterpenoid glycosides from Centella asiatica. Helvetica Chimica Acta. 2005;88:297-303.
- Sahu NP, Roy SK, Mahato SB. Spectroscopic determination of structures of triterpenoid trisaccharides from Centella asiatica. Phytochemistry. 1989;28(10):2852-2854.
- Thomas MT, Kurup R, Johnson AJ, Chandrika SP, Mathew PJ, Dan M, Baby S. Elite genotypes/chemotypes, with high contents of madecassoside and asiaticoside, from sixty accessions of Centella asiatica of South India and the Andaman Islands: for cultivation and utility in cosmetic and herbal drug applications. Industrial Crops Products. 2010;32:545-550.
- Yoshida M, Fuchigami M, Nagao T, Okabe H, Matsunaga K, Takata J, Karube Y, Tsuchihashi R, Kinjo J, Mihashi K, Fujioka T. Antiproliferative constituents from Umbelliferae plants VII. Active triterpenes and rosmarinic acid from Centella asiatica. Biological & Pharmaceutical Bulletin. 2005;28(1):173-175.
- Shukla YN, Srivastava R, Tripathi AK, Prajapati V. Characterization of an ursane triterpenoid from Centella asiatica with growth inhibitory activity against Spilarctia obliqua. Pharmaceutical Biology. 2000;38(4):262-267.
- Siddiqui BS, Aslam H, Ali ST, Khan S, Begum S. Chemical constituents of Centella asiatica. Journal Asian Natural Product Research. 2007;9(4):407-414.
- Subban R, Veerakumar A, Manimaran R, Hashim KM, Balachandran I. Two new flavonoids from Centella asiatica (Linn.). Journal of Natural Medicines. 2008;62:369-373.
- Singh B, Rastogi RP. Chemical examination of Centella asiatica Linn – III: constitution of brahmic acid. Phytochemistry. 1968;7(8):1385-1393.
- Singh B, Rastogi RP. A reinvestigation of the triterpenes of Centella asiatica. Phytochemistry. 1969;8(5):917-921.
- Asakawa Y, Matsuda R, Takemoto T. Mono- and sesquiterpenoids from Hydrocotyle and Centella species. Phytochemistry. 1982;21(10):2590-2592.
- Yu QL, Gao WY, Zhang YW, Teng J, Duan HQ. Studies on chemical constituents in herb of Centella asiatica. Zhongguo Zhong Yao Za Zhi. 2007;32(12):1182-1184.
- Matsuda H, Morikawa T, Ueda H, Yoshikawa M. Medicinal foodstuffs. XXVII. Saponin constituents of gotu kola (2): structures of new ursane- and oleanane-type triterpene oligoglycosides, centellasaponins B, C, and D, from Centella asiatica cultivated in Sri Lanka. Chemical & Pharmaceutical Bulletin(Tokyo). 2001;49(10):1368-1371.
- Antognoni F, Perellino NC, Crippa S, Toso RD, Danieli B, Minghetti A, Poli F, Pressi G. Irbic acid, a dicaffeoylquinic acid derivative from Centella asiatica cell cultures. Fitoterapia. 2011;82:950-954.
- The Indian pharmaceutical codex. vol. I. indigenous drugs. New Delhi, Council of Scientific & Industrial Research. 1953.
- British herbal pharmacopoeia, part 2. London, British Herbal Medicine Association. 1979.
- Medicinal plants in Viet Nam. Manila, World Health Organization, WHO Regional Publications, Western Pacific Series, No.3.1990.
- Babu TD, Kuttan G, Padikkala J. Cytotoxic and anti-tumour properties of certain taxa of Umbelliferae with special reference to Centella asiatica (L.) Urban. Journal Ethnopharmacology. 1995;48(1):53-57.
- Babykutty S, Padikkala J, Sathiadevan PP, Vijayakurup V, Azis TK, Srinivas P, Srivanas G. Apoptosis induction of Centella asiatica on human breast cancer cells. African Journal of Traditional Complementary and Alternative Medicines. 2008;6(1):9-16.
- Maquart FX, Chastang F, Simeon A, Birembaut P, Gillery P, Wegrowski Y. Triterpenes from Centella asiatica stimulate extracellular matrix accumulation in rat experimental wounds. European Journal Dermatology. 1999;9(4):289-296.
- Hamid AA, Shah ZM, Muse R, Mohamed S. Characterisation of antioxidative activities of various extracts of Centella asiatica (L) Urban. Food Chemistry. 2002;77:465-469.
- Jayashree G, Kurup Muraleedhara G, Sudarslal S, Jacob VB. Anti-oxidant activity of Centella asiatica on lymphoma-bearing mice. Fitoterapia. 2003;74(5):431-434.
- Cheng CL, Guo JS, Luk J, Koo MWL. The healing effects of Centella extract and asiaticoside on acetic acid induced gastric ulcers in rats. Life Sciences. 2004;74:2237-2249.
- Guo JS, Cheng CL, Koo MW. Inhibitory effects of Centella asiatica water extract and asiaticoside on inducible nitric oxide synthase during gastric ulcer healing in rats. Planta Medica. 2004;70(12):1150-1154.
- Sharma J, Sharma R. Radioprotection of Swiss albino mouse by Centella asiatica extract. Phytotheraphy Research. 2002;16(8):785-786.
- Haleagrahara N, Ponnusamy K. Neuroprotective effect of Centella asiatica extract (CAE) on experimentally induced parkinsonism in aged Sprague-Dawley rats. Journal of Toxicology Sciences. 2010;35(1):41-47.
- Cesarone MR, Belcaro G, Nicolaides AN, Geroulakos G, Bucci M, Dugall M, De Sanctis MT, Incandela L, Griffin M, Sabetai M. Increase in echogenicity of echolucent carotid plaques after treatment with total triterpenic fraction of Centella asiatica: a prospective, placebo-controlled, randomized trial. Angiology. 2001;52(Suppl 2):S19-25.
- Incandela L, Belcaro G, Nicolaides AN, Cesarone MR, De Sanctics MT, Corsi M, Bavera P, Ippolito E, Griffin M, Geroulakos G, Sabetai M, Ramaswami G, Veller M. Modification of the echogenicity of femoral plaques after treatment with total triterpenic fraction of Centella asiatica: a prospective, randomized, placebo-controlled trial. Angiology. 2001; 52:(Suppl 2):S69-73.
- Pointel JP, Boccalon H, Cloarec M, Ledevehat C,Joubert M. Titrated extract of Centella asiatica (TECA) in the treatment of venous insufficiency of the lower limbs. Angiology. 1987;38(1 Pt 1):46-50.
- Cesarone MR, Belcaro G, De Sanctis MT, Incandela L,Cacchio M, Bavera P, Ippolito E, Bucci M,Geroulakos G, Dugall M, Bucella S, Kelyweght S, Cacchio M. Effects of the total triterpenic fraction of Centella asiatica in venous hypertensive microangiopathy: a prospective, placebo-controlled, randomized trial. Angiology. 2001;52(Suppl2):S15-18.
- Cesarone MR, Belcaro G, Rulo A, Griffin M, Ricci A, Ippolito E, De Sanctics MT, Incadela L, Banvera P, Cacchio M, Bucci M. Microcirculatory effects of total triterpenic fraction of Centella asiatica in chronic venous hypertension: measurement by laser Doppler, TcPO2CO2 and leg volumetry. Angiology. 2001;52(Suppl 2):S45-48.
- Cesarone MR, Incandela L, De Sanctis MT, Belcaro G, Geroulakos G, Griffin M, Lennox A, Di Renzo AD, Cacchio M, Bucci M. Flight microangiopathy in medium to long distance flights: prevention of edema and microcirculation alterations with total triterpenic fraction of Centella asiatica. Angiology. 2001;52(Suppl2):S33-37.
- Cesarone MR, Incandela L, De Sanctis MT,Belcaro G, Bavera P, Bucci M, Ippolito E. Evaluation of treatment of diabetic microangiopathy with total triterpenic fraction of Centella asiatica: a clinical prospective randomized trial with a microcirculatory model. Angiology. 2001;52(Suppl 2):S49-54.
- Bosse JP, Papillon J, Frenette G, Belcaro G, Bavera P, Bucci M, Ippolitto E. Clinical study of new antikeloid agent. Annals of Plastic Surgery. 1979;3:13-21.
- Widgerow AD, Chait LA, Stals R, Stals PJ. New innovations in scar management. Aesthetic Plastic Surgery. 2000;24:227-234.
- Bradwejn J, Zhou Y, Koszycki D, Shlik J. A double-blind, placebo-controlled study on the effects of Gotu kola (Centella asiatica) on acoustic startle response in healthy subjects. Journal of Clinical of Psychopharmacology. 2000;20:680-684.
- Teh BP, Hamzah NF, Rosli SNS, Yahaya MAF, Zakiah I, Murizal Z. Acute oral toxicity study of selected Malaysian medicinal herbs On Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2012. Report No .: HMRC 11-045/01/CA/WP/P.
- Laerum OD, Iversen OH. Reticuloses and epidermal tumors in hairless mice after topical skin applications of cantharidin and asiaticoside. Cancer Research. 1972;32:1463–1469.
- Izu R, Aguirre A, Gil N, Diaz-Perez JL. Allergic contact dermatitis from a cream containing Centella asiatica extract. Contact Dermatitis. 1992,26:192-193.
- Danese P, Carnevali C, Bertazzoni MG. Allergic contact dermatitis due to Centella asiatica extract. Contact Dermatitis. 1994;31:201.
- ESCOP Monographs the scientific foundation for herbal medicinal products. ed 2. Thieme. 2009;p37.
- Standard of ASEAN herbal medicine. Volume 1. ASEAN countries. 1993;p.152.