Limau purut Fruit
Citrus hystrix DC
Rutaceae
Definition
Limau purut fruit consists of the powder of dried fruits of Citrus hystrix DC. (Rutaceae).
Synonym
Citrus hystrix subsp. acida Engl., Citrus hystrix var. annamensis (Yu.Tanaka) Guillaumin, Citrus hysrix var. boholensis Wester, Citrus hystrix var. micrantha (Wester) Merr., Citrus hystrix var. microcarpa (Wester) Merr., Citrus hystrix var. southwickii (Wester) Merr., Citrus hystrix var. torosa (Blanco) Wester, Citrus auraria Michel, Citrus echinata Saint-Lager, Citrus hyalopulpa Yu.Tanaka, Citrus kerrii (Swingle)Tanaka, Citrus macroptera Montrouzier var. kerrii Swingle, Citrus papeda Miquel, Fortunella sagittifolia F.M.Feng & P.I.Mao, Papeda rumphii Hasskarl [ 1 , 2 ].
Vernacular names
Kaffir lime (English); Limau purut, limau hantu, limau suwangi (Malay); Ma feng gan, mao li qiu si ku cheng, ma feng cheng, ma feng mao gan fatt-fung-kam, thai-ko-kam (Chinese); Kolumichai (Tamil) [ 2 ].
Character
Identification
Plant Morphology
C. hystrix is a small tree that grows up to about 3–6 m high, crooked with glabrous spiny branches. Leaves are alternate, unifoliolate, broadly ovate to ovate-oblong, 7.5–10 cm long, dark green adaxial, light green abaxial, very fragrant; base is cuneate, or rounded, apex obtuse or slightly acuminate or notched; petiole is long and expanded into prominent wings, 15 cm long, 5 cm wide. Inflorescences are axillary and terminal, 3–5 flowered. Flowers are small, fragrant, white; calyx cuspidate 4–lobed, white with violet fringe; petals 4–5, ovate-oblong, yellowish white with pink tinge; stamens 24–30 free. Fruits are large, warty or bumpy, verrucose, globose, ovoid to elliptic, green turning yellowish-green when ripe, 5–7 cm diameter; pulp yellowish, rind thick, very acid and bitter. Seeds are numerous, ridged, ovoid-oblong, 1.5–1.8 long, 1–1.2 cm wide, 0.5 cm thick; monoembryonic with white cotyledons [ 2 ].
Microscopy
Powdered material consists of solitary oval-shaped starch grains, fibres, scalariform vessels, lignified round parenchyma cells, oil globules and prism calcium oxalate crystals.
Figure 2 : Microscopic characters of Citrus hystrix dried fruit powder of 0.355 mm size. (a) Oval starch grains (magnification 100x); (b) starch grains stained blue-black (magnification 100x); (c) fibre (magnification 100x); (d) scalariform vessels (magnification 100x); (e) round lignified parenchyma (magnification 100x); (f) oil globules stained orange-pink (magnification 400x); (g-h) prism calcium oxalate crystals (magnification 100x). [Scale bars: f = 20 µm; a-e, g-h = 100 µm]
Colour Tests
Observed colour of solution after treatment with various of reagents
| KOH (5%) | Dark Yellow |
| NH4OH (25%) | Dark Yellow |
| NaOH (5%) | Dark Yellow |
Thin Layer Chromatography (TLC)
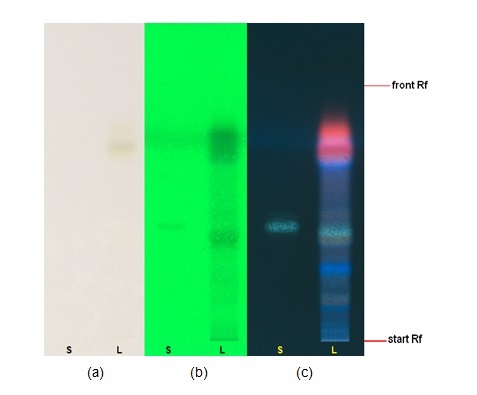
Figure 3 : TLC chromatogram of hesperidin (S) and methanol extract of Citrus hystrix dried fruit powder (L) observed under (a) UV visible light after derivatization (b) UV at 254 nm after derivatization and (c) UV at 366 nm after derivatization.
| Test Solutions | Weigh 5.0 g of C. hystrix dried fruit powder of 0.355 mm size in a 250 mL round bottom flask and add 80 mL of methanol (> 99.9%). Reflux the mixture at 60˚C for 30 min, cool and filter. Transfer the filtrate into evaporating dish and evaporate to dryness on water bath at 60˚C. Dissolve with 5 mL HPLC grade methanol. Use the filtrate as the test solution. |
| Standard solution | Dissolve hesperidin standard (trc Canada) [CAS no.: 520-26-3] in methanol to produce 1.0 mg/mL solution by sonication for 30 min. |
| Stationary Phase | HPTLC Silica gel 60 F254, 10 x 10 cm |
| Mobile phase | n-butanol : acetic acid : water; (9:1:1) (v/v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Dry in an oven at 60°C for 5 min. |
| Detection |
|
High Performance Liquid Chromatography (HPLC)
| Test Solutions | Weigh 5.0 g of C. hystrix dried fruit powder of 0.355 mm size in a 250 mL round bottom flask and add 80 mL of methanol (> 99.9%). Reflux the mixture at 60°C for 30 min, cool and filter. Transfer the filtrate in an evaporating dish and evaporate to dryness over a boiling water bath at 60°C. Reconstitute the residue with 5 mL HPLC grade methanol and filter through a 0.22 µm nylon syringe filter. Use the filtrate as the test solution. |
| Standard solution | Dissolve hesperidin standard (trc Canada) [CAS no.: 520-26-3] in methanol to give 1.0 mg/mL solution by sonication for 30 min. |
| Chromatographic system | Detector: UV 280 nm Column: C18 column (5 µm, 4.6 mm I.D x 250 mm) (Phenomenex Luna if necessary) Column oven temperature: 32°C Flow rate: 1 mL/min Injection volume: 20 µL |
| Mobile phase(isocratic mode) | 3.7% acetic acid in water : acetonitrile; (81 : 19) (v/v) |
| Run Time | 40 min |
| System suitability requirements |
Perform at least five replicate injections of hesperidin standard solution (1.0 mg/mL). The requirements of the system suitability parameters are as follow:
|
| Acceptance criteria |
|
Figure 4 : Whole HPLC chromatogram of (a) hesperidin standard solution (1.0 mg/mL) at tr= 16.221 min and (b) methanol extract of Citrus hystrix dried fruit powder showing peak corresponding to hesperidin standard solution at tr= 16.098 min.
(b)

(a)
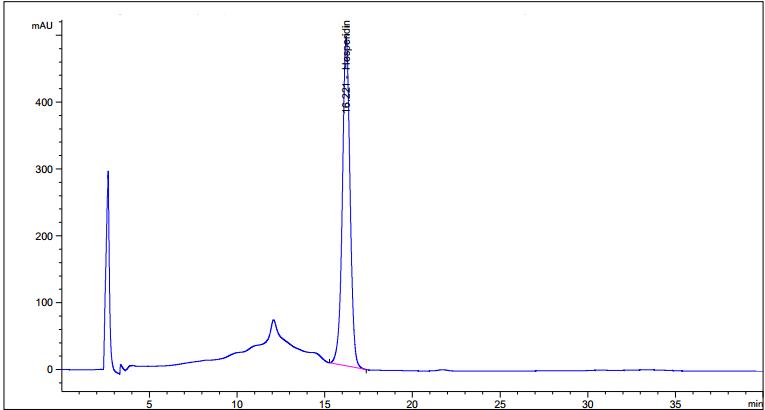
Figure 5 : HPLC chromatogram highlighting the elution region of hesperidin in (a) hesperidin standard solution (1.0 mg/mL) at tr= 16.221 min and (b) methanol extract of Citrus hystrix dried fruit powder showing peak corresponding to hesperidin standard solution at tr= 16.098 min.
(b)
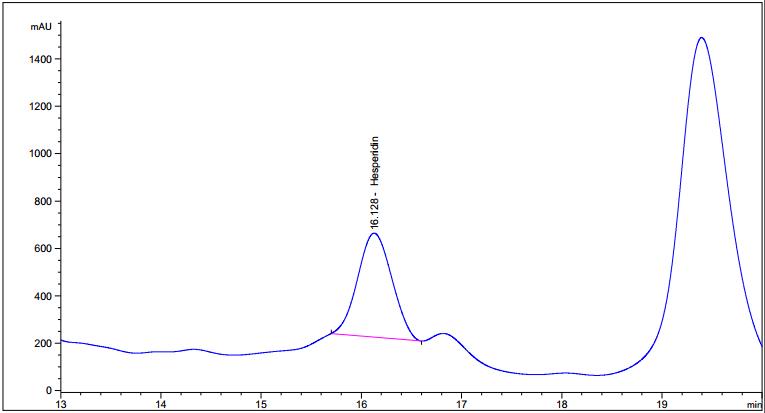
(a)
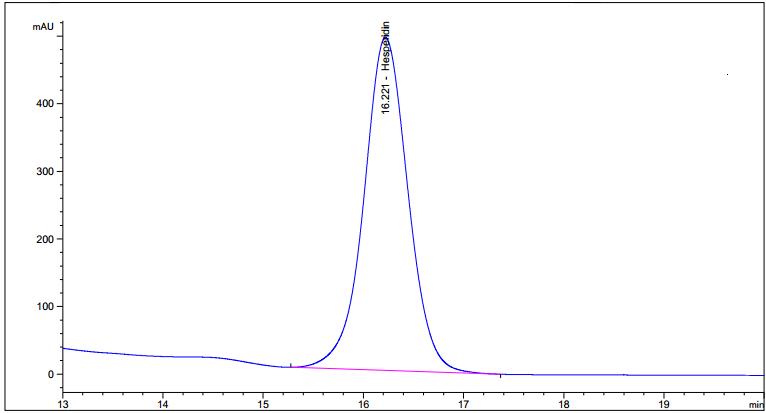
Figure 6 : UV spectrum of hesperidin standard (1.0 mg/mL) and methanol extract of Citrus hystrix dried fruit powder.

Purity Test
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
| Foreign Matter | |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 6% |
| Acid-insoluble ash | Not more than 1% |
| Loss on Drying | |
| Not more than 13% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot Method | Not less than 40% |
| Cold Method | Not less than 30% |
| Ethanol-soluble extracts | |
| Hot Method | Not less than 20% |
| Cold Method | Not less than 12% |
Safety Test
| Heavy Metals | |
| Arsenic | Not More than 5.0mg/kg |
| Mercury | Not More than 0.5 mg/kg |
| Lead | Not More than 10mg/kg |
| Cadmium | Not More than 0.3mg/kg |
| Microbial limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1g |
Chemical Constituents
Fruits
The methanol extract of C. hystrix fruits was found to contain coumarins (e.g. bergamotin, oxypeucedanin, 5-[(6’,7’-dihydroxy-3’,7’dimethyl-2-octenyl)oxy-]-psoralen) [ 3 ].
The acidified methanol (0.1% HCl) extract was found to contain flavonoids (e.g. apigenin-6,8-di-C-glucoside, eiodictyol-7-O-rutinoside, eriodictyol-7-O-neohesperidoside, phloretin-3’,5’-di-C-glucoside, quercetin-3-O-rutinosyl-7-O-glucoside, quercetin-3-O-rutinoside, luteolin-7-O-rutinoside, hesperetin-7-O-rutinoside, diosmetin-7-O-rutinoside, hesperetin-7-O-neohesperidoside, diosmetin-O-rutinoside) [ 4 ].
The acetone extract was found to contain coumarin-monoterpenoid type (e.g. auraptene) [ 5 ].
Fruit peels
The water extract (juice) of C. hystrix fruit peels was found to contain limonin, flavanones (e.g. naringin, hesperidine) and polymethoxyflavones (e.g. sinensetin, nobiletin, tangeretin) [ 6 ].
The dichloromethane extract was found to contain furanocoumarins (e.g. (+)-oxypeucedanin, (R)-(+)-6′,7′-dihydroxybergamottin), citrusosides (e.g. citrusoside A, citrusoside B, citrusoside C, citrusoside D), phytosterol (e.g. sitosteryl-β-D-glucopyranoside) and others (e.g. 1-O-isopropyl-β-D-glucopyranoside) [ 7 ].
The ethyl acetate extract was found to contain essential oil (e.g. limonene, citronellal, β-pinene, sabinene, citronellol, citronellyl acetate, δ-cadinene, α-copaene, trans-caryophyllene, 1-isopulegol, trans-sabinene hydrate, germacrene D, myrcene) [ 8 ].
The hexane extract was found to contain furanocoumarins (eg. (R)-(+)-oxypeucedanin hydrate, (+)-isoimparatorin, (R)-(+)-6′-hydroxy-7′-methoxybergamottin, (R)-(+)-6′,7′dihydroxybergamottin, (+)-bergomottin), monoterpenes (e.g. citronellal, citronellol, citronellol acetate, isopulegol, neo-isopulegol), eudesmane sesquiterpene (e.g. (+)-4-epi-crytomeridiol (eudesmane-4β,11-diol)) and phytosterol (e.g. β-sitosterol) [ 7 ].
The essential oils was found to contain monoterpenoids (e.g. α-pinene, β-pinene, camphene, sabinene, trans-sabinene hydrate, δ-3-carene, myrcene, β-myrcene, α-phellandrene, α-terpinene, limonene, L-limonene, 1,8-cineole, octanal, β-phellandrene, β-ocimene, cis-ocimene, γ-terpinene, p-cymene, m-cymene, terpinolene, α-terpinolene, 2,6-dimethyl-5-heptenol, cis-linalool-3,6-oxide, trans-sabinene hydrate, citronellal, L-citronellal, (-)-citronellal, α-copaene, linalool, cis-linalool oxide, epoxy linalool oxide, trans-linalool oxide, nonanal, β-citronellal, β-citronellol, citronellol p-menth-2-en-1-lol, decanal, cis-sabinene hydrate, isopulegol, neo-isopulegol, terpinen-4-ol, 4-terpineol, citronellyl acetate, α-terpineol, L-α-terpineol, borneol, terpinene-4-ol, neryl acetate, camphor, neral, limonen-4-ol, geranial, nerol, geranyl, geranyl acetate, citronellol, β-citronellol, geraniol, perillaldehyde, trans,trans-2,4-decanienal, p-mentha-1,8-diol, indole, epoxy-isopulegol, citronellic acid, α-thujene, exo-fenchol, ρ-menthan-8-ol, dihydro-α-terpineol, guaiol, 4-ρ-menthene, ρ-menthan-8-ol, 2,6-dimethyl-5-heptenol, β-bisabolene), sesquiterpenoids (e.g. α-humulene, α-muurolene, cis–α-farnesene, cubenol, epi-cubenol, α-cubebene, β-cubebene, δ-cadinene, γ-cadinene, cadinol, (+)-4-epi-crytomeridiol, trans-nerolidol, nerolidol, elemol, α-eudesmol), germacrane-type sesquiterpenoid (e.g. germacrene D), caryophyllane-type sesquiterpenoids (e.g. caryophyllene, β-caryophyllene, caryophyllene oxide), and elemane-type sesquiterpenoid (e.g. β-elemene) [ 8 , 9 , 10 , 11 , 12 , 13 , 14 ].
Fruit seeds
The methanol extract of C. hystrix seeds was found to contain limonoid (e.g. limonin, nomilin, obacunone, deacetylnomilin) and limonoid glucosides (e.g. limonin-17-β-D-glucopyranoside, nomonilic acid-17-β-D-glucopyranoside, nomilin-17-β-D-glucopyranoside, obacunone-17-β-D-glucopyranoside) [ 15 ].
The acetone extract (after defatted with hexane) was found to contain limonoid (e.g. limonin, deacetylnomilin) [ 16 ].
Medicinal Uses
Uses described in folk medicine, not supported by experimental or clinical data
Biological and pharmacological activities supported by experimental data
Antioxidant activity
Ethanol extract of C. hystrix fruit peels demonstrated antioxidant activity based on oxygen consumption measurement using oxygen depletion in a linoleic acid emulsion system. The extract gave protective index of 2.9, significantly better antioxidant activity than the commercial antioxidants, rosemary (7.4) and sage (6.6) [ 18 ].
Antimicrobial activity
Oil extract of C. hystrix peels inhibited Bacillus cereus (25 mm), Salmonella typhi (27 mm) and Staphylococcus aureus (28 mm) compared to deoxytetracycline (42, 56 and 45 mm for the respective microbes). Meanwhile, fresh extract of C. hystrix peels was found to inhibit Bacillus cereus (25 mm), Salmonella typhi (25 mm) and Staphylococcus aureus (24 mm) using agar diffusion method [ 19 ].
Essential oil of C. hystrix fruit peels exhibited minimum inhibitory concentration (MIC) value of 4.2 µL/mL against Salmonella typhimurium (non-DT 104 strain) and E. coli compared to the positive control, penicillin G (MIC = 0.5 unit/mL). The oil also inhibited growth of S. agona, S. anatum, S. choleraesuis, S. senflenberg and Klebsiella pneumonia (MIC = 4.2 µL/mL) compared to penicillin G (MIC = 50 unit/mL); S. enteritidis and Citrobacter freundii (MIC = 4.2 µL/mL) compared to penicillin G (MIC = 500 unit/mL); S. derby, S. typhimurium DT104 and Enterobacter aerogenes (MIC = 4.2 µL/mL) compared to penicillin G (MIC = 5000 unit/mL); as well as S. risen (MIC = 8.3 µL/mL) compared to penicillin G (MIC = 50 unit/mL) [ 20 ].
Anti-inflammatory activity
Bergamottin isolated from methanol extract of C. hystrix fruits inhibited lipopolysaccharide/interferon-γ-induced nitric oxide generation in murine macrophage cell line RAW 264.7 with inhibition concentration at 50% of growth (IC50) value of 14 µM compared to a synthetic inducible nitric oxide synthase inhibitor, N-(iminoethyl)-L-ornithine (IC50 = 7.9 µM) [ 3 ].
Cholinesterase inhibitory activity
Three compounds isolated from hexane extract of C. hystrix dried fruit peels showed inhibition of butyrylcholinesterase with inhibitory activity (IC50) (R)-(+)-6’-hydroxy-7’-methoxybergamottin (IC50 = 11.2 ± 0.1 µM), (R)-(+)-6’,7’-dihydroxybergamottin (IC50 = 15.4 ± 0.3 µM) and (+)-isoimparatorin (IC50 = 23.1 ± 0.2 µM) compared to positive control, galanthamine (IC50 = 3.2 ± 0.2 µM) [ 7 ].
Anti-implantation activity
Ethanol extracts of C. hystrix dried fruit peels (0.1, 1.0 and 2.5 g/kg dose) was administered orally to pregnant adult female Wistar rats (on day 2 of pregnancy) twice daily (at hour 0900 and 1700) for four days and sacrificed on day 9 of pregnancy. The extract inhibited implantation in the rats in a dose-dependent manner (23.0% – 86.1%) compared to DMSO vehicle-treated group (0.5 mL/kg) [ 21 ].
Chloroform extracts of C. hystrix dried fruit peels (0.5 and 1.0 g/kg dose) was administered orally to pregnant adult female Wistar rats (on day 2 of pregnancy) twice daily (at hour 0900 and 1700) for four days and sacrificed on day 9 of pregnancy. The extract inhibited implantation in the rats in a dose-dependent manner (0.5 g/kg: 34.4% and 1.0 g/kg: 62.2%) compared to DMSO vehicle-treated group (0.5 mL/kg) [ 21 ].
Abortifacient effect
Ethanol extracts of C. hystrix dried fruit peels (0.2, 1.0 and 2.5 g/kg dose) was administered orally to adult female Wistar rats (on day 8 of pregnancy) twice daily (at hour 0900 and 1700) for five days and sacrificed on day 20 of pregnancy. The extract interrupted pregnancy in the rats in a dose-dependent manner (38.6% – 96.9%) compared to DMSO vehicle-treated group (0.5 mL/kg) [ 21 ].
Chloroform extracts of C. hystrix dried fruit peels (0.5 and 1.0 g/kg dose) was administered orally to adult female Wistar rats (on day 8 of pregnancy) twice daily (at hour 0900 and 1700) for five days and sacrificed on day 20 of pregnancy. The extract interrupted pregnancy in the rats in a dose-dependent manner (0.5 g/kg: 62.2% and 1.0 g/kg: 91.9%) compared to DMSO vehicle-treated group (0.5 mL/kg) [ 21 ].
Effect on foetus
Ethanol extract of C. hystrix dried fruit peels (1 g/kg) was administered orally to adult female Wistar rats (on day 15 of pregnancy) twice daily (at hour 0900 and 1700) until labour. The fetal weight in extract-treated group was significantly (p < 0.05) lower (4.9 ± 0.1) compared to DMSO vehicle-treated group (5.4 ± 0.1) [ 21 ].
Uterotrophic activity
Ethanol extract of C. hystrix dried fruit peels (1 g/kg body weight; oral route) and estradiol benzoate in corn oil (1 µg/kg body weight; subcutaneous route) were administered together to ovariectomized immature female rats (20 days old) twice daily for five days. The uterine wet weight in extract-treated group was significantly (p < 0.01) higher (≈ 130 mg/100 g body weight) compared to estradiol-treated group (≈ 110 mg/100 g body weight) [ 21 ].
Clinical studies
Information and data have not been established.
Safety Information
Preclinical studies (Toxicology study)
Acute toxicity
Ethanol extract of C. hystrix dried fruit peels given orally to the rats showed no toxic effect (LD50 > 100 g/kg body weight) with one death was reported at dose 100 g/kg [ 21 ].
Oral single dose acute toxicity study on female Sprague Dawley rats (aged between 8 and 12 weeks old) using aqueous extract of C. hystrix fruits showed no toxic effect on the parameters observed, including behaviours, body weight, food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. Half lethal dose (LD50) is more than 2,000 mg/kg body weight [ 22 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
Dosage
Information and data have not been established.
Storage
Store below 30°C. Protect from light and moisture.
Reference
- The Plant List 2010. [Internet] Citrus hystrix DC; 2013 [cited on 26 October 2012]. Available from: http://www.theplantlist.org/tpl1.1/search?q=citrus+hystrix.
- Lim TK. Edible Medicinal and Non Medicinal Plants. Volume 4. Springer Dordrecht Heidelberg London New York. 2012.
- Murakami, A., Gao, G., Kim, O.K., Omura, M., Yano, M., Ito, C., Furukawa, H., Jiwajinda, S., Koshimizu, K. & Ohigashi, H. Identification of coumarins from the fruit of Citrus hystrix DC as inhibitors of nitric oxide generation in mouse macrophage RAW 264.7 cells. Journal of Agricultural and Food Chemistry.1999; 47: 333-339.
- Roowi, S. & Crozier, A. Flavonoids in tropical Citrus species. Journal of Agricultural and Food Chemistry. 2011;59:12217-12225.
- Ogawa, K., Kawasaki, A., Yoshida, T., Nesumi, H., Nakano, M., Ikoma, Y. & Yano, M. Evaluation of auraptene content in Citrus fruits and their products. Journal of Agricultural and Food Chemistry. 2000;48:1763-1769.
- Chinapongtitiwat, V., Jongaroontaprangsee, S., Chiewchan, N. & Devahastin, S. Important flavonoids and limonin in selected Thai citrus residues. Journal of Functional Foods. 2013;5:1151-1158.
- Youkwan, J., Sutthivaiyakit, S. & Sutthivaiyakit, P. Citrusosides A-D and furanocoumarins with cholinesterase inhibitory activity from the fruit peels of Citrus hystrix. Journal of Natural Products. 2010;73:1879-1883.
- Chanthaphon, S., Chanthachum, S. & Hongpattarakere, T. Antimicrobial activities of essential oils and crude extracts from tropical Citrus spp. against food-related microorganisms. Songklanakarin Journal of Science and Technology. 2008;30:125-131.
- Sato, A., Asano, K. & Sato, T. The chemical composition of Citrus hystrix DC (Swangi). Journal of Essential Oil Research. 1990;2:179-183.
- Waikedre, J., Dugay, A., Barrachina, I., Herrenknecht, C., Cabalion, P. & Fournet, A. 2010. Chemical composition and antimicrobial activity of the essential oils from new Caledonia Citrus macroptera and Citrus hystrix. Chemistry & Biodiversity. 2010;7:871-877.
- Hongratanaworakit, T. & Buchbauer, G. Chemical composition and stimulating effect of Citrus hystrix oil on humans. Flavour and Fragrance Journal. 2007;22:443-449.
- Lawrence, B.M., Hogg, J.W., Terhune, S.J. & Podimuang, V. Constituents of the leaf and peel oils of Citrus hystrix D.C. Phytochemistry. 1971;10:1404-1405.
- Muhammad Nor, O. Volatile aroma compounds in Citrus hystrix oil. Journal of Tropical Agriculture and Food Science. 1999;27(2):225-229.
- Ibrahim, J., Abu Said, A., Abdul Rashih, A., Nor Azah, M.A. & Norsiha, A. Chemical composition of some Citrus oils from Malaysia. Journal of Essential Oil Research. 1995;8:627–632.
- Berhow, M.A., Fong, C.H. & Hasegawa, S. Limonoid and flavonoid composition in varieties of papeda and papedocitrus. Biochemical and Systematic Ecology. 1996;24:237-242.
- Dreyer, D.L. Citrus bitter principles-V. Botanical distribution and chemotaxonomy in the Rutaceae. Phytochemistry. 1966;5:367-378.
- Burkill IH. A Dictionary of the Economics Products of the Malay Peninsular. 2nd Ed. Kuala Lumpur: Ministry of Agriculture and Cooperative. 1966.
- Jaswir I, Hassan TH, Said MZM. Efficacy of Malaysian plant extracts in preventing peroxidation reaction in model and food oil systems. Journal of Oleo Science. 2004;53(11):525-529.
- Chaisawadi S, Thongbute D, Methawiriyasilp W. Preliminary study of antimicrobial activities on medicinal herbs of Thai food ingredients. Acta Hort (ISHS). 2005; 675:111-114.
- Nanasombat S. & Lohasupthawee P. Antibacterial activity of crude ethanolic extracts and essential oils of spices against Salmonellae and other enterobacteria. KMITL Science and Technology Journal. 2005;6(1):1-5.
- Piyachaturawat P, Glinsukon T, Chanjarunee A. Antifertility effect of Citrus hystrix DC. Journal of Ethnopharmacology. 1985;13(1):105–110.
- Syed Muhammad Asyraf ST, Norzahirah A, Nor Kasmini S, Wan Abdul Hakim WL, Wan Mohammad Adham Afiq WZ, Teh BP, Hussin M. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2015. Report no:. HMRC 11-045/01/CH/F/K.

