Limau kasturi Fruit
Citrus x microcarpa Bunge
Rutaceae




Figure 1 : Citrus × microcarpa. (a) Fully grown plant; (b) a bunch of unripe fruits; (c) 5-white petals flower; (d) cross-section of ripe fruit (Photos courtesy of UKM, 2024)
DEFINITION
Limau kasturi fruit consists of the powder of dried fruit of Citrus × microcarpa Bunge (Rutaceae). Limau kasturi or calamansi is a hybrid (×) between C. reticulata and C. japonica. [1]
SYNONYM
× Citrofortunella microcarpa (Bunge) Wijnands, × Citrofortunella × mitis (Blanco) J.W Ingram & H.E. Moore, Citrus × mitis Blanco, Citrus × mitis f. gekkitsu Hayata, Citrus × mitis f. shikikitsu Hayata. (The × symbol is placed before a genus name to indicate intergeneric hybrids while before a specific epithet to indicate interspecific hybrids.) [2, 3]
VERNACULAR NAMES
Calamansi (English); limau kasturi (Malay) [4, 5].
CHARACTER
| Colour | Yellowish-green powder |
| Odour | Sweet |
| Taste | Bitter |
IDENTIFICATION
Plant morphology
C. microcarpa is an evergreen, small or shrub, often spiny, growing to a height of 3-5 m. Stems numerous branches with variable spines, 5 cm long on young growth but some only a few mm on flowering shoots. Leaves 1-foliolate or sometimes mixed with simple, alternate, blade elliptic to oblong-elliptic, 4-8 cm long, aromatic, dark-green, glossy on the upper surface, yellowish-green on the lower surface, base rounded to broadly cuneate, margin dentate near apex or rarely entire, apex rounded and rarely mucronate. Flowers axillary, solitary, rarely in pairs, 5 mm or less of 5 white petals, short-stalked, calyx 4- or 5-lobed, 3- or 4-loculed ovary as long as style with 3 or 4 ovules per locule and filaments cohering into 4 or 5 bundles. Fruits yellow when ripe, nearly spherical, 2.0-3.5 cm in diameter, 6- to 7-celled and thin skinned, skin or peel is green to yellowish green or yellow, loosely adhering to the flesh, 3- or 4-seeded, pericarp sweet and edible, and acidic sarcocarp. Seeds broadly ovoid, apex mucronate, smooth seed coat, embryos sometimes numerous, green cotyledons. Petioles narrowly and scarcely winged [6].
Microscopy
Powdered material consists of elongated parenchyma cells, epidermal cells, paracytic stomata, druse and prism crystals, macrosclereids, annular vessels and oil globules.
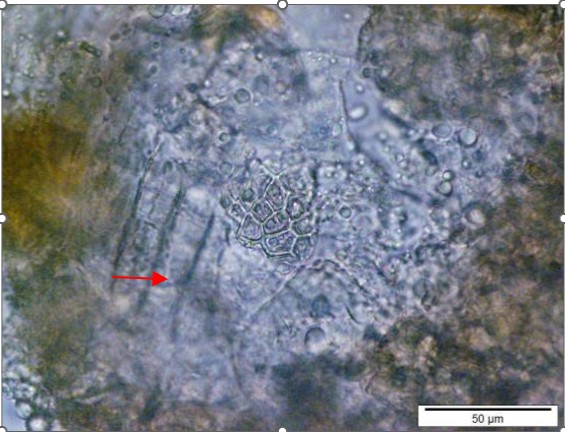
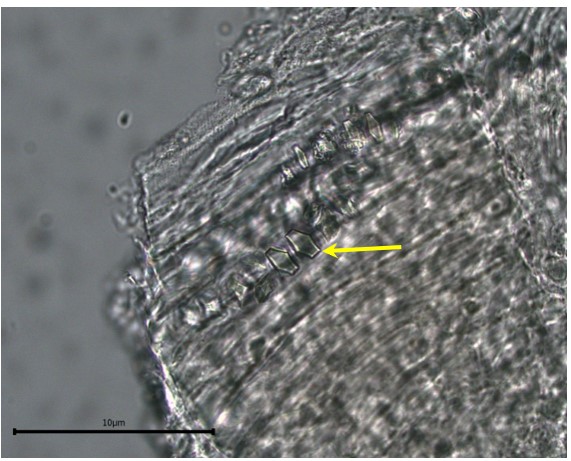
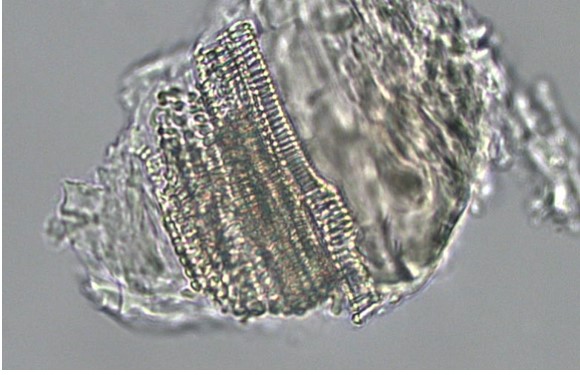
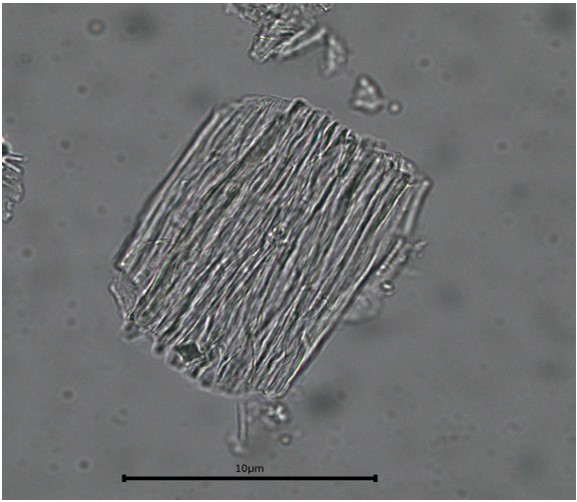
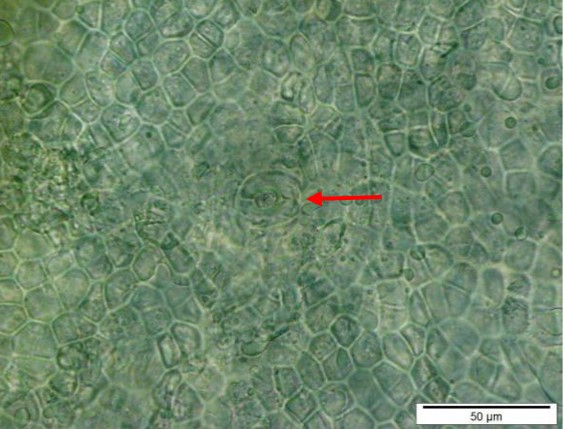
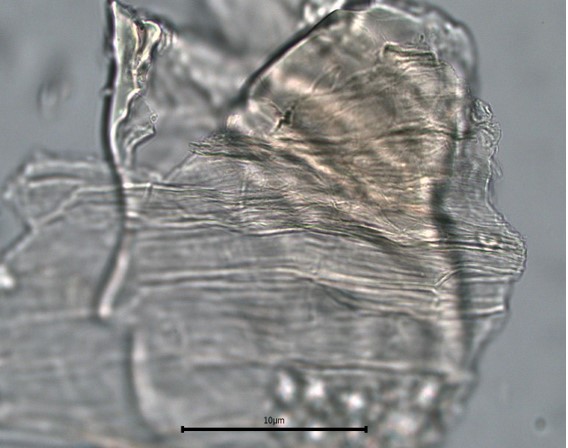
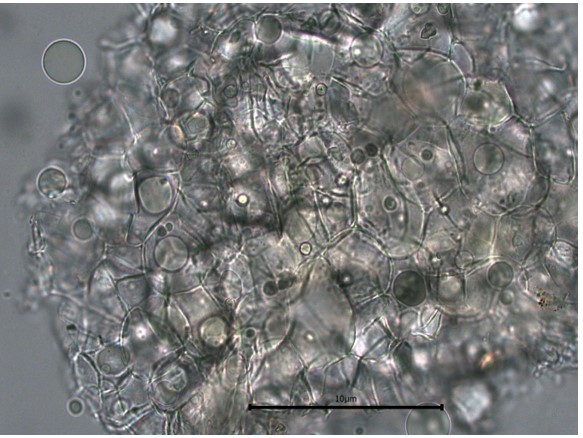
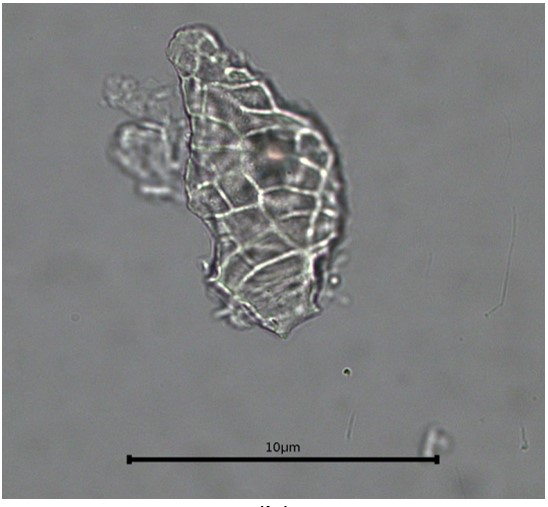
Figure 2 : Microscopic characters of C. microcarpa fruit powder of 0.355 mm size. (a) elongated parenchyma cell (red arrow) and epidermal cell (magnification 40×); (b) epidermal cell straight to curve and paracytic stomata (red arrow) (magnification 20×); (c) druse (yellow arrow) (magnification 40×); (d) macrosclereids (magnification 40×); (e) spiral vessels (magnification 40×); (f) oil globules (magnification 40×); (g) fibres (magnification 40×); (h) epidermal cells (magnification 40×). [Scale bars: a = 50 µm; b = 50 µm; c = 10 µm; d = 10 µm; e = 50 µm; f = 10 µm; g = 10 µm; h = 10 µm]
Chemical Test
Observed colour of solution after treatment with various reagents:
| Test of presence of alkaloids | White precipitates |
| Test for the presence of steroids | Bluish green |
| Test for the presence of saponins | Stable foams |
| Test for the presence of flavonoids | Yellow to colourless |
Thin Layer Chromatography (TLC)
| Test solution : | Weigh about 1 g of C. microcarpa dried whole fruit powder of 0.355 mm size in a 50-mL conical flask. Add 20 mL of methanol and sonicate for 1 hour at 40°C. Filter the extract and repeat twice. Evaporate the solvent using a rotary evaporator. Weigh 100 mg of the resultant dried extract and add 1mL of methanol. Vortex and heat in a water bath at 40°C until precipitates are fully dissolved. Filter the mixture with 0.22 µm syringe filter . Use the filtrate as a test solution. |
| Standard solution : | Dissolve 0.2 mg of hesperidin standard [CAS no.: 520-26-3] in 1mL methanol, and heat in a water bath at 40°C until it dissolves to give a standard concentration of 0.2 mg/mL. |
| Stationary phase : | HPTLC silica gel 60 F254,10 × 10 cm. |
| Mobile phase : | Ethyl acetate : methanol : water; (15 : 3 : 2) (v/v/v) |
| Application : | (a)Hesperidin standard solution (S); 10 μL, as a band (b)Methanol extract of C. microcarpa dried whole fruit powder (L); 10 μL, as a band |
| Development distance : |
|
| Drying : | Oven drying |
| Detection : | (a) UV at 254 nm before derivatisation with NP/PEG; (b) Visible light after derivatisation with NP/PEG; (c) UV at 366 nm after derivatisation with NP/PEG. |
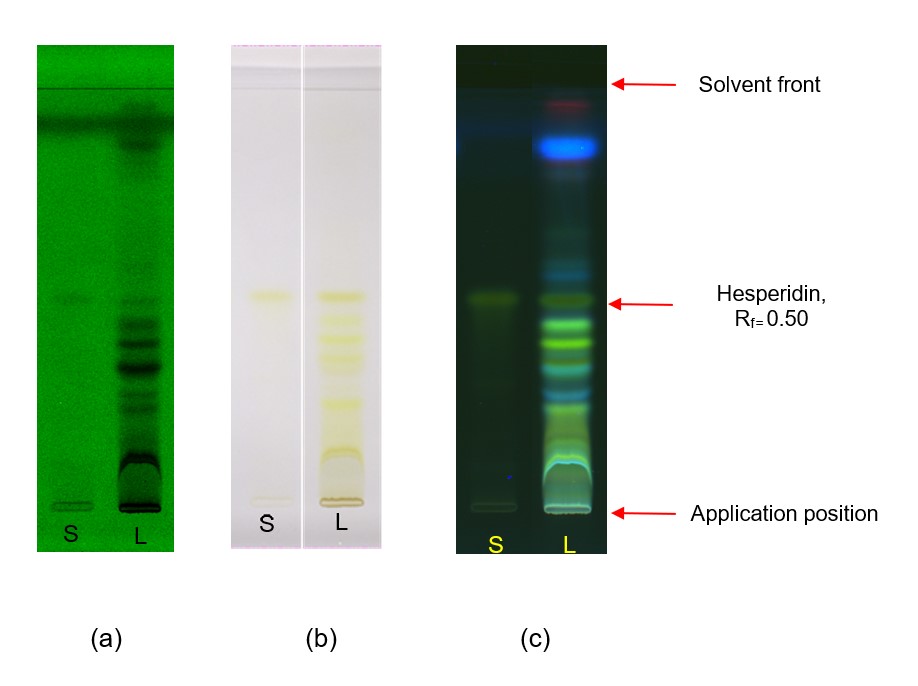
Figure 3 : HPTLC chromatogram of hesperidin (S1) , methanol extracts of Citrus microcarpa dried fruit powder (L) observed under (a) visible light after derivatisation (b) UV at 254 nm before derivatisation and (c) UV at 366 nm after derivatisation
High Performance liquid Chromatography (HPLC)
| Test solution | Weigh about 1 g of C. microcarpa dried whole fruit powder of 0.355 mm size in a 50-mL conical flask. Add 20 mL of methanol and sonicate for 1 hour at 40°C. Filter the extract and repeat twice. Evaporate the solvent using a rotary evaporator. Weigh 100 mg of the resultant dried extract and add 1mL of methanol. Vortex and heat in a water bath at 40°C until precipitates are fully dissolved. Filter the mixture with 0.22 µm syringe filter . Use the filtrate as a test solution. | |||||||||||||||||||||
| Standard solution | Dissolve 0.2 mg of hesperidin standard [CAS no.: 520-26-3] in 1mL methanol, and heat in a water bath at 40°C until it dissolves to give a standard concentration of 0.2 mg/mL. | |||||||||||||||||||||
| Chromatographic system |
Detector: Photodiode Array (PDA) |
|||||||||||||||||||||
| Mobile Phase (gradient mode) |
|
|||||||||||||||||||||
| System suitability requirements |
Perform at least five replicate injections of the standard solutions (0.2 mg/mL). The requirements of the system suitability parameters are as follow:
|
|||||||||||||||||||||
| Acceptance criteria |
|
(a)
(b)
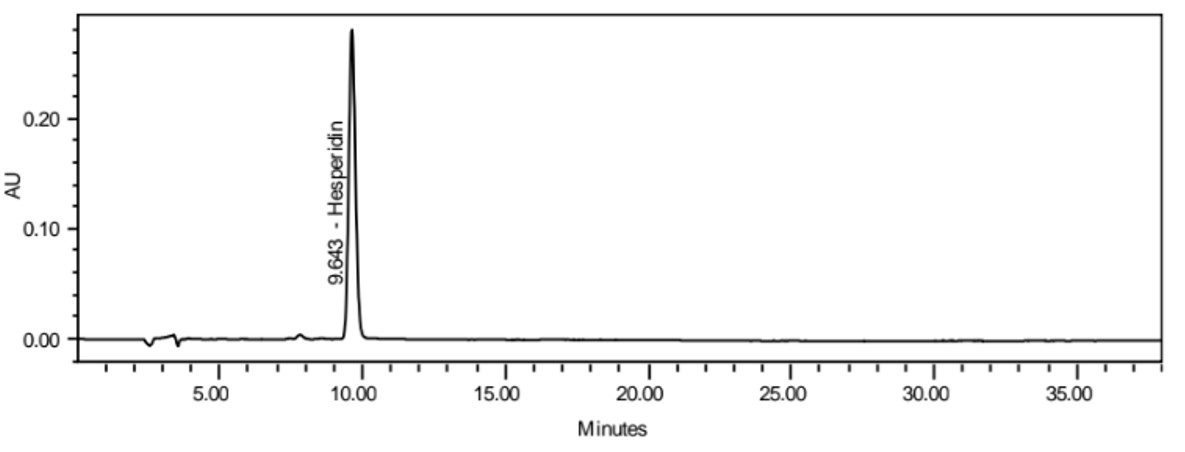
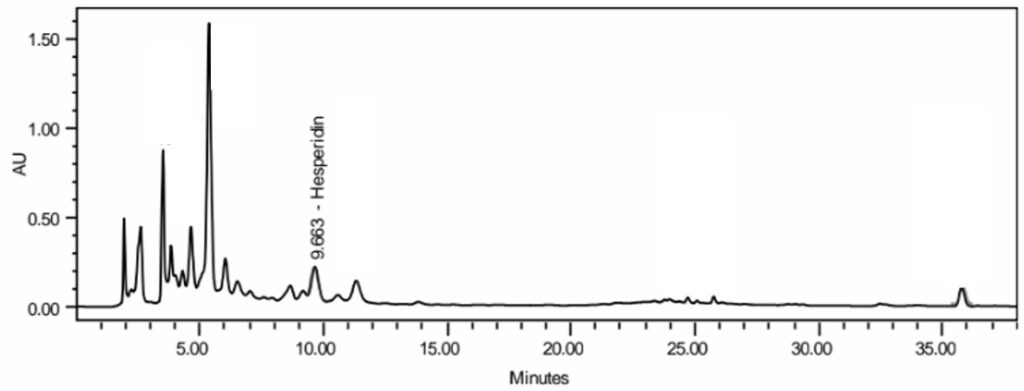
Figure 4 : Whole HPLC chromatogram of (a) hesperidin standard solution (0.2 mg/mL) at tr = 9.643min, (b) methanol extract of Citrus microcarpa dried whole fruit powder showing peak corresponding to hesperidin standard solution at tr = 9.663min.
(a)
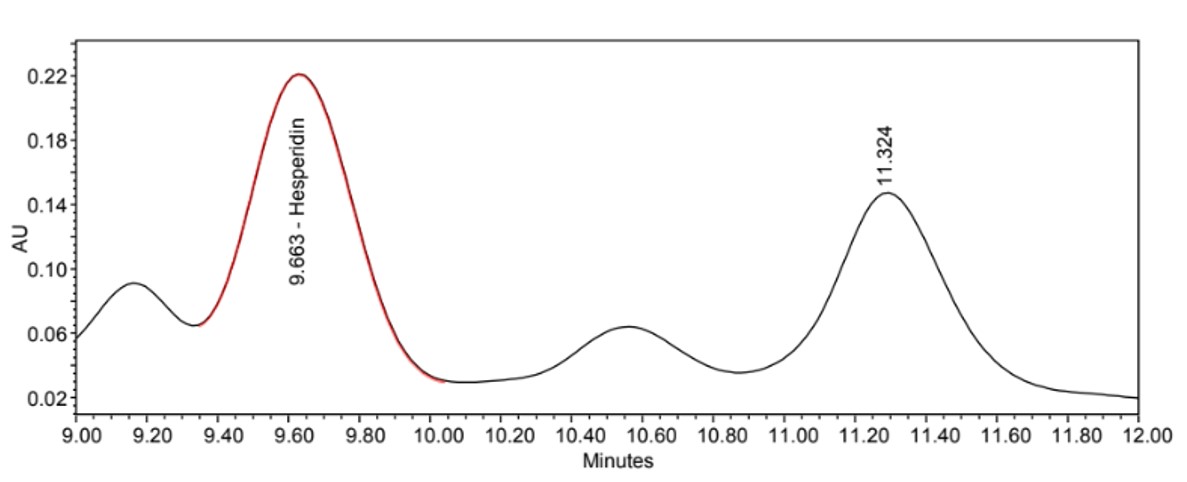
(b)
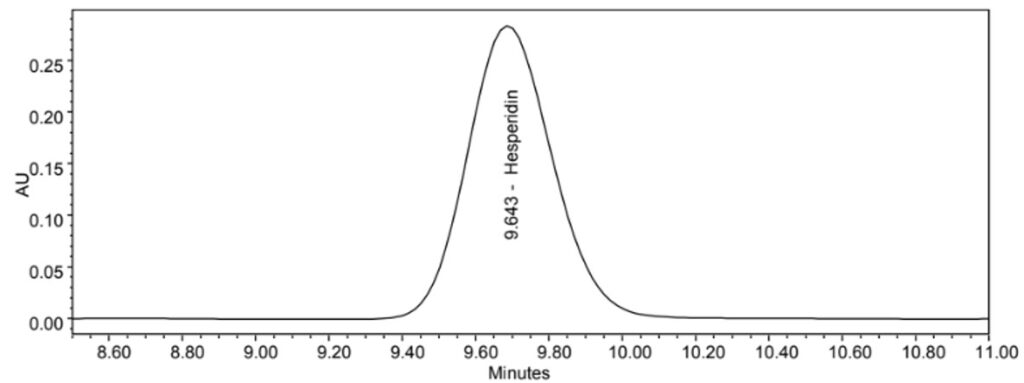
Figure 5 : HPLC chromatogram highlighting the elution region of hesperidin in (a) hesperidin standard solution and (b) methanol extract of C. microcarpa dried whole fruit powder showing peak corresponding to hesperidin standard solution at tr = 9.663min.
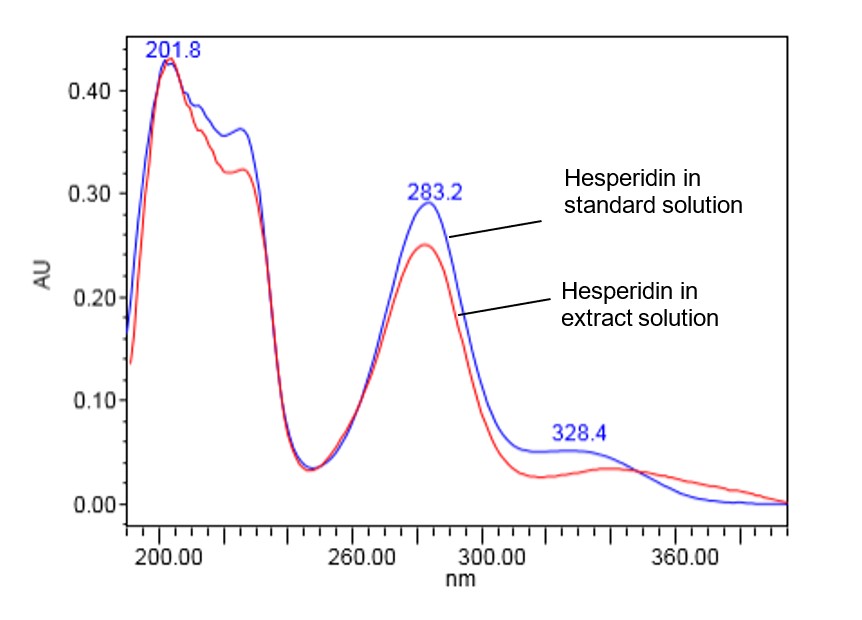
Figure 6 : UV spectrum of hesperidin standard solution (0.2 mg/mL) and methanol extract of C. microcarpa dried whole fruit powder.
PURITY TEST
The purity tests except for foreign matter test, are based on C. microcarpa dried whole fruit powder of 0.355 mm particle size.
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 7% |
| Acid-insoluble ash | Not more than 1% |
| Loss on Drying |
| Not more than 15% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 39% |
| Cold method | Not less than 42% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 21% |
| Cold method | Not less than 19% |
SAFETY TEST
The safety tests are based on C. microcarpa dried whole fruit powder of 0.355 mm particle size.
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total aerobic microbial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Whole fruits
Ethanol (96%) extract of C. microcarpa dried powdered whole fruit was found to contain flavonoids (e.g., hesperidin, kushenol J, malvidin 3,5-diglucoside, neokurarinol, noranhyoicaritin, typhaneoside, 3,5,6,7,8,3’,4’-heptemethoxy flavone, 3’,4’,4’,5,7,8-hexamethoxy flavone, 4’,5,6,7-tetramethoxyflavone and 5,7,8,3’,4’-pentamethoxy flavone), triterpene glucoside (e.g., chebuloside II), flavonoid glycosides (e.g., apigenin–6,8-di-C-glucoside, narcissoside and isoliquiritigenin-4’-O-β-D-glucopyranoside), flavanone glycoside (e.g., liquiritin), flavone glycosides (e.g., spinosin and patuletin-7-O-6-(6”-isobutyryl)-glucoside)), anthocyanidin-5-O-glycoside (e.g., malvidin 3,5-diglucoside) and others (e.g., (-)-epiafzelechin-3-O-(6”-O-acetyl)-β-D-allosepyranoside) [7].
Dichloromethane fraction of C. microcarpa freshly-squeezed fruit juice was found to contain monoterpenes (e.g., α-pinene, α-terpineol, β-myrcene, β-phellandrene, β-pinene, citronellyl acetate, geranyl acetate, isopiperitenone, limonene, linalool,, p-cymene, perilla aldehyde, sabinene, terpinolene and trans-β-ocimene), sesquiterpenes (e.g., α-cadinene, α-eudesmol, α-farnesene, α-humulene, α-selinene, β-caryophyllene, β-cubebene, β-elemene, β-elemol, β-farnesene, β-selinene, δ-elemene, γ-cadinene, γ-elemene, γ-eudesmol, bicycloelemene, germacrene B and germacrene D), sesquiterpenoids (e.g., β-bourbonene and bicyclogermacrene), diterpene (e.g., phytol), esters (e.g., decyl acetate, geranyl propionate, heptyl acetate, octyl acetate and perillyl acetate), fatty acids (e.g., decanoic acid, linoleic acid, palmitic acid and stearic acid), aldehydes (e.g., decanal, octanal, nonanal, trans-2-decenal and undecanal), fatty aldehydes (e.g., trans,cis-2,4-decadienal and trans,trans-2,4-decadienal), alcohol (e.g., cis-3-hexen-1-ol) and fatty alcohols (e.g., 1-nonanol and 1-octanol) [8].
Diethyl ether fraction of C. microcarpa freshly-squeezed fruit juice was found to contain monoterpenes (e.g., α-pinene, α-terpineol, α-thujene, β-pinene, γ-terpinene, D-limonene, dehydro-p-cymene, geranyl acetate, linalool, myrcene, neral, ocimene, perilla aldehyde, sabinene, trans-carveol, terpinolene and 4-terpineol), sesquiterpenes (e.g., α-humulene, β-elemene, δ-cadinene, δ-elemene and germacrene D), monoterpenoids (e.g., cis-carveol, citronellal, citronellol, geranial, L-carvone and neryl acetate), esters (e.g., ethyl acetate, ethyl butyrate, ethyl isobutyrate and ethyl propanoate), alkatetraene (e.g., 2,6-dimethyl-1,3,5,7-octatetraene), tetrahydrofurans (e.g., cis-linalool oxide and trans-linalool oxide), aldehydes (e.g., acetaldehyde, decanal, dodecanal, hexanal, heptanal, nonanal, octanal, undecanal and (E)-2-hexanal), alcohols (e.g., ethanol), fatty alcohol (e.g., 1-octanol), menthofuran (e.g., 3,9-epoxy-p-mentha-1,8(10)-diene) and others (e.g., 7-endo-ethenyl-bicyclo[4,2,0]-oct -1-ene) [9].
Essential oil obtained from steam distillation of C. microcarpa fresh whole fruit was found to contain monoterpenes (e.g., α-phellandrene, α-pinene, α-terpinene, α-terpinolene, β-myrcene, β-ocimene, β-pinene, γ-terpinene, camphene, citral, limonene, linalool and geranyl acetate), sesquiterpenes (e.g., α-caryophyllene, α-eudesmol, β-caryophyllene, β-eudesmol, β-terpineol, δ-elemene and germacrene B), monoterpenoid (e.g., neryl acetate, α-fenchol, carveol, myrcenol and perillal), sesquiterpenoids (e.g., muurolol and 10-epi-γ-eudesmol), esters (e.g., heptyl acetate, nonyl acetate and octyl acetate), ketone (e.g., carvone), alcohol (e.g., 3-hexene-1-ol), aldehydes (e.g., hexanal, 2-hexenal, heptanal, octanal, decanal, 2-decenal, 4,4-decadienal, undecanal and dodecanal), flavonoids (e.g., naringin, hesperidin, diosmin, quercetin and hesperitin), phytosterols (e.g., amyrin, campesterol, sitosterol and stigmasterol) and limonoids (e.g., limonin and nomilin) [10].
Freshly-squeezed juice from C. microcarpa fresh fruits was found to contain monoterpenes (e.g., β-myrcene, β-phellandrene, β-pinene, m-mentha-1,8-diene, limonenel, linalyl alcohol, terpieol and 4-terpineol), sesquiterpenes (e.g., α-guaiene, β-caryophyllene, β-cubebene, β-elemene, β-eudesmol, β-farnese, (-)-β-elemene, δ-cadinene, δ-elemene, germacrene D, germacrene B, calarene, eudesmene, elixene, muurolene, elemol, (+)-aromadendrene, (-)-aristolene and (+-)-cadinene), aliphatic hydrocarbon (e.g., docosane), esters (e.g., decyl acetate, dibutyl phthalate, octyl acetate, octyl formate and phenylethyl benzoate), aldehydes (e.g., decanal, hydroxymethylfurfurol, octanal, tridecanal, undecanal, 2-furaldehyde, 3-furaldehyde, 5-methylfurfural and (E)-2-decenal), alcohol (e.g., nonyl alcohol), fatty alcohol (e.g., 1-octanol), ketones (e.g., methylmaleic anhydride and 2,3-dihydro-3,5-dihydroxy-6-methyl- 4H-pyran-4-one) and other (e.g., 6-methyl-2-pyrazinyl methanol) [5].
Fruit Peels
Methanol extract of C. microcarpa dried peels was found to contain flavanones (e.g., hesperidin, naringin and neohesperidin), flavones (e.g., diosmin and sinensetin), flavonols (e.g., kaempferol, quercetin and rutin), phenolic acids (e.g., caffeic acid, chlorogenic acid, ferulic acid, sinapic acid and ρ-coumaric acid) and carotenoids (e.g., lutein, zeaxanthin, β-carotene and β-cryptoxanthin) [11].
Dichoromethane extract of C. microcarpa dried peels was found to contain monoterpenes (e.g., α-terpineol, β-pinene, γ-terpinene, geraniol, linalool, limonene, myrcene, pulegone and sabinene), sesquiterpenes (e.g., α-copaene, α-eudesmol, β-elemene, β-eudesmol, β-selinene, δ-elemene, calarane, germacrene B, germacrene D and trans-β-farnesene), monoterpenoids (e.g., carvone, cis-carveol, citronellol, elemol, geranial, geranyl acetate, limonene glycol, nerol, isogeranial, isopiperitenone, perillyl acetate, perillyl alcohol, perillyl aldehyde, p-menth-1-en-9-ol terpinen-4-ol, terpinolene, trans-ocimene, trans-2,8-p-mentha-dien-1-ol and 4,8-dimethyl-1,3,7-nonatriene), sesquiterpenoids (e.g., α-cubenene, α-farnesene, α-guaiene, α-humulene, α-ylangene, β-bourbonene, β-cubenene, β-phellandrene, δ-cadinene, γ-cadinene, γ-elemene, γ-eudesmol, γ-muurolene, τ-cadinol, farnesol, germacrene D-4-ol, guaiol, neophytadiene, nerolidol, isospathulenol and trans-β-copaene), diterpenoid (e.g., phytol), esters (e.g., citronellyl acetate, decyl acetate, ethyl linoleate, geranyl propionate, heptyl acetate and methyl stearate), aldehydes (e.g., hexadecanal, decanal, nonanal, octadanal and trans-2-hexenol), fatty aldehydes (e.g., trans-cis-2,4-decadienal, trans-trans-2,4-decadienal and 2,6-dodecadien-1-al), fatty acids (e.g., hexanoic acid, octanoic acid and palmitic acid), acyclic monoterpenoids (e.g., geranic acid), alcohols (e.g., benzyl alcohol, cis-3-hexanol, heptanol, hexanol, isophytol), fatty alcohols (e.g., nonanol and octanol), eremophilane (e.g., valerianol), carboxylic acid (e.g., acetic acid) and others (e.g., trans-2-decenol and trans-trans-2,4-decadienol) [12].
Flavedo
Hexane extract of C. microcarpa dried flavedo (pigmented exocarp) was found to contain monoterpenes (e.g., α-pinene, α-terpineol, β-myrcene, β-phellandrene, β-pinene, γ-terpinene, δ-3-carene, camphene, camphor, citronellyl acetate, isopiperitenone, linalool, limonene, neral, p-cymene, perillaldehyde, sabinene and terpinolene), sesquiterpenes (e.g., α-copaene, α-eudesmol, α-farnasene, α-humulene, α-seline, β-bourbonene, β-caryophyllene, β-cubebene, β-elemene, β-eudesmol, β-farnesene, β-selinene, δ-elemene, γ-elemene, γ-eudesmol, bicycloelemene, elemol, germacrene B and germacrene D), monoterpenoids (e.g., cis-β-ocimene, citronellol, geranial, geranyl acetate, limonene oxide, perilla alcohol, perillyl acetate, sabinene hydrate, trans-β-ocimene and trans-nerolidol), sesquiterpenoids (e.g., α-cubebene and bicyclogermacrene), diterpenoid (e.g., phytol), esters (e.g., cis-3-hexenyl acetate, decyl acetate, dodecyl acetate, ethyl octanoate, geranyl propionate, heptyl acetate, methyl-N-methyl anthranilate, methyl salicylate and octyl acetate), aldehydes (e.g., decanal, nonanal, octanal and undecanal), fatty acids (e.g., decanoic acid, linoleic acid, myristic acid, nonanoic acid, octanoic acid, palmitic acid and stearic acid), carboxylic acid (e.g., acetic acid), alcohols (e.g., hexanol, cis-3-hexen-1-ol), fatty alcohols (e.g., 1-nonanol and 1-octanol), fatty aldehydes (e.g., trans-2-dodecenal, trans-cis-2,4-decadienal, trans-trans-2,4-decadienal and trans-cis-2,6-dodecadienal) and others (e.g., trans-2-decenol) [8].
Seeds
Methanol extract of C. microcarpa dried seeds was found to contain triterpenoid (e.g., squalene), fatty acids (e.g., linoleic acid, methyl palmitate, palmitic acid and stearic acid), phenolic acids (e.g., methylparaben, p-hydroxybenzoic acid and vanillin acid), aldehyde (e.g., p-hydroxybenzaldehyde), phytosterols (e.g., β-sitostenne, β-sitosterol and stigmastenone) [14].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
The fruit juice of C. microcarpa is known as a cooling agent and an odorant. The juice is often used to treat respiratory problems, especially productive coughs. It is taken orally as a cough remedy, while warm dark kalamansi-ade serves as a laxative. If combined with pepper, it can promote sputum secretion. The juice is also applied directly to freckles to help lighten the spots, treat acne and itching in women’s genital areas [16,17].
The cut fruit of C. microcarpa is applied to insect bites to relieve itching and irritation. Crushed fruits with saponaceous bark of Entada phaeseoloides used as a shampoo to relieve itching and promote hair growth [14]. Grilled fruits are eaten with honey to treat cough and phlegm [17].
The roots of C. microcarpa used for treatment at childbirth meanwhile the distilled oil of the leaves serves as a carminative with more potency than peppermint oil [15].
Biological and pharmacological activities supported by experimental data
Antioxidant activity
Methanol extract of C. microcarpa dried fruit peels showed antioxidant activity (EC50 = 94.01 μg/mL) compared to a positive control, ascorbic acid (EC50 = 18.74 μg/mL) using a DPPH free radical-scavenging assay [18].
Methanol extract of C. microcarpa fruit peels showed antioxidant activity with IC50 of 12.91 ± 3.31 mg/mL compared to ascorbic acid (IC50 = 0.10 ± 0.03 mg/mL) using a DPPH free radical-scavenging assay [19].
Antibacterial activity
Ethanol (90%) extract of C. microcarpa pulp with extraction period of 6 days (10 mg/disc) showed comparable antibacterial activities using the parameter of inhibition zone diameter, against Staphylococcus aureus (14.15 mm) compared to streptomycin (0.01 mg/disc; 14.00 mm), Salmonella enteritidis (17.49 mm) compared to streptomycin (0.2 mg/disc; 11.06 mm), and Listeria monocytogenes (18.53 mm) compared to streptomycin (0.01 mg/disc; 16.16 mm) using a paper disc diffusion assay [20].
Larcividal activity
Essential oils of C. microcarpa fruit peels that were dissolved in acetone and ethanol (95%) showed larvicidal activity of LC90 of 0.58 ppm and LC90 of 0.57 ppm, respectively, against third and early fourth instar of Aedes aegypti larvae after 24-hour exposure period compared to a positive control, mosquito larvicide product containing 1% temephos with LC90 of 0.40 ppm [21].
Anticoagulant activity
Hot water extract of C. microcarpa fresh peels at 50% concentration had an anticoagulant effect observed at an average time of 17.38 minutes using blood drawn from the tails of albino mice that were mounted onto slides compared to a positive control, heparin (20 μL/mL), which had an instant anticoagulant effect [22].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Information and data have not been established.
Others (Adverse reactions, contraindications, side effects, warning, precautions)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The World Flora Online. [Internet] Citrus × microcarpa Bunge ; 2023 [cited on 4 December 2024]. Available from: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:927792-1
- The International Plant Names Index and World Checklist of Vascular Plants 2024. Published on the Internet at http://www.ipni.org
- Greuter, Werner, et al. International Code of Botanical Nomenclature (Saint Louis Code). Electronic version, 2000. International Association for Plant Taxonomy. [cited on 4 December 2024]. Available from: https://www.bgbm.org/iapt/nomenclature/code/saintlouis/0071AppendixINoHa003.htm
- Malaysia Biodiversity Information System (MyBIS). [Internet] Citrus microcarpa Calamansi [cited on 19 October 2024]. Available from: https://www.mybis.gov.my/sp/49483
- Yo SP and Lin CH. Qualitative and Quantitative Composition of the Flavour Composition of Taiwan Calamondin and Philippine Calamansi Fruit. European Journal of Horticultural Science. 2004;69(2):117-124.
- Quisumbing E. Medicinal Plants of the Philippines. Katha Publication, Quezon City. 1978:454-455
- Yunita E, Kurniati T, Rosa FL, Yani DF. LC-QTOF-MSMS Analysis: Metabolite Compounds from The Crude Extract of Kalamansi Oranges Fruit (Citrofortunella microcarpa). Journal of the Austrian Society of Agricultural Economics. 2022;18(10):1309-1315.
- Cheong MW, Zhu D, Sng J, Liu SQ, Zhou W, Curran P, Yu B. Characterisation of calamansi (Citrus microcarpa). Part II: Volatiles, physicochemical properties and non-volatiles in the juice. Food Chemistry. 2012;134:696-703.
- Dharmawan J, Kasapis S, Curran P, Johnson JR. Characterization of volatile compounds in selected citrus fruits from Asia. Part I: freshly-squeezed juice. Flavour and Fragrance Journal. 2007;22:228-232.
- Chen MH, Yang KM, Huang TC, Wu ML. Traditional Small-Size Citus from Taiwan: Essential Oils, Bioactive Compounds and Antioxidant Capacity. Medicines. 2017;4(2):28-38.
- Wang YC, Chuang YC, Hsu HW. The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chemistry. 2008;106:277-284
- Goh RMV, Pua A, Liu SQ, Lassabliere B, Leong KC, Sun J, Tan LP, Yu Bin. Characteristization of Volatile Compounds in Kumquat and Calamansi Peel Oil Extracts. Journal of Essential Oil Bearing Plants. 2020;23(4):953-969.
- Cheong MW, Chong ZS, Liu SQ, Zhou W, Curran P, Yu B. Characterisation of calamansi (Citrus microcarpa). Part I: Volatiles, aromatic profiles and phenolic acids in the peel. Food Chemistry. 2012;134:686-695.
- Chen CY, Kao CL, Yeh HC, Li HT, Yuan LT. Chemical Constituents of the seeds of Citrus microcarpa. Chemistry of Natural Compounds. 2020;56(3):521-522
- Julia FM. Fruits of warm climates. Echo Point Books and Media. 2012:176-178.
- Norhayati I, Zhari I, Muzlifah AM. Indeks tumbuhan ubat Malaysia. Victus Semulajadi (M) Sdn. Bhd. 1999;15.
- Tantengco OAG, Condes MLC, Estadilla HHT, Ragragio EM. Ethnobotanical Survey of Medicinal Plants used by Ayta Communities in Dinalupihan, Bataan, Philippines. Pharmacognosy Journal. 2018;10(5):859-870.
- Wulandari M, Idiawati N, Gusrizal. Aktivitas Antioksidan Ekstrak n-Heksana, Etil Asetat dan Metanol Kulit Buah Jeruk Sambal (Citrus microcarpa Bunge). Jurnal Kedokteran dan Kesehatan 2013;2:90–4.
- Lim S.M., Loh S.P. In Vitro antioxidant capacities and anti-diabetic properties of phenolic extracts from selected citrus peels. Inter. Food Res. J. 2016;23:211–219.
- Cho JW, Barido FH, Kim HJ, Kim HJ, Kim DW, Shin DJ, Jang A. Effect of Calamansi Pulp Ethanol Extracts on the Meat Quality and Biogenic Amine Formation of Pork Patty during Refrigerated Storage. Food Science of Animal Resources 2023;43(1):25-45.
- Carigaba MAE, Leonida M, Masculino CJC, Joy C, Mediodia A, Garbo AG. Larvicidal activity of Citrofortunella microcarpa (calamansi) peels essential oil against third and early fourth instar Aedes aegypti. Publiscience 2020;3(1):37-41.
- Rocha ICN, Roque SJR, Tanyag LG. Effect of Citrofortunella microcarpa (Calamansi) Peelings on Whole Blood Coagulation Using Blood Samples from Albino Mice. Journal of Complementary and Alternative Medical Research 2020;12(1):51-56.

