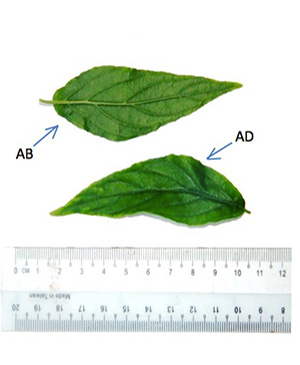
Belalai gajah Leaves
Clinacanthus nutans Lindau
Acanthaceae
DEFINITION
Belalai gajah leaves consist of the leaves and shoots of C. nutans (Burm. f.) Lindau. (Acanthaceae).
SYNONYM
Clinacanthus nutans var. robinsonii Benoist, Clinacanthus burmanni Nees, Justicia nutans Burm. f [ 1 , 2 ].
VERNACULAR NAMES
Sabah snake grass (English), belalai gajah (Malay), ezuihua (Chinese) [ 3 ].
CHARACTER
| Colour | Green (fresh leaves), Green to dark green (powder) |
| Odour | Slight acrid smell |
| Taste | Light bitter taste |
IDENTIFICATION
Plant Morphology
C. nutans is a shrub 1-3 m high with pubescent branches. The stems are terete, striate and glabrescent. The leaves are simple, opposite, narrowly elliptic oblong or lanceolate, measuring 2.5-13 cm long and 0.5-4 cm wide, apex acute or acuminate, margin exsculptate-dentate or subentire, base cuneate, obtuse, rounded or truncate often oblique, pubescence on the nerves, the leaf blade are lanceolate-ovate, lanceolate or linear-lanceolate; surfaces pubescent when young then glabrescent except abaxially pilose along veins, secondary veins 4-6 on each side of midvein and abaxially elevated; the petioles are 0.3-2 cm long, sulcate, bifariously pubescent. Flowers are in dense cymes at the top of the branches and their branchlets; cymes 5–8 flowered, often terminating with drooping horizontal branches but themselves erect, subsecund, combined into a large lax, leafy panicle; each flower has calyx densely patently glandular-pubescent, about 1 cm long; corolla glandular-pubescent, about 3.5 cm, dull red with green base; lower lip (turned upwards) with yellow streaks, apically sordidly yellow or greenish yellow; stamens 2, inserted in the throat, more or less appressed against the upper lip; ovary is compressed, 2-celled, 2 ovules in each cell; having style filiform, shortly bidentate. Capsule is oblong, basally contracted into a short, solid stalk, 4-seeded [ 2 , 4 ].
Microscopy
The leaf powder shows fragment of diacytic stomata found on the lower epidermis was surrounded by two subsidiary cells at right angle to it; covering trichomes exhibits multicellular and glandular on the epidermis which was interpose sparsely with lithocysts; parenchyma cells has thin-walled, rounded to oval with small intercellular spaces; spiral thickened vessels are more abundant [ 5 , 6 ].
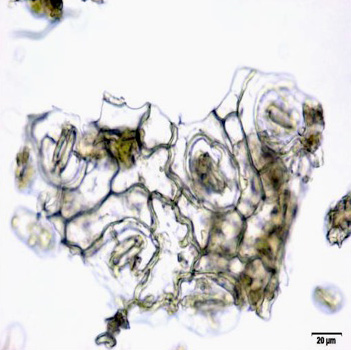

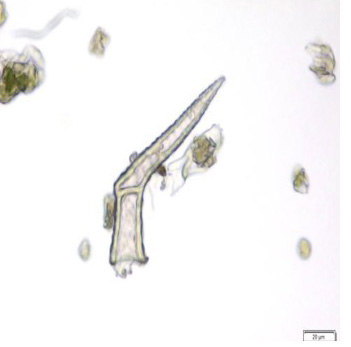
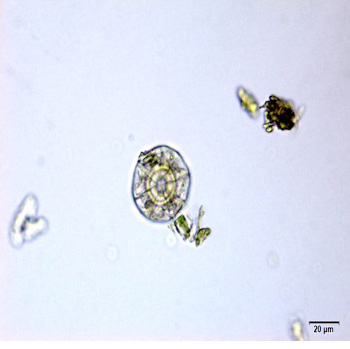
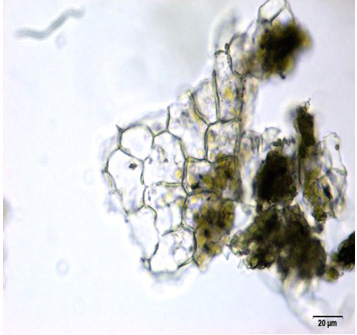
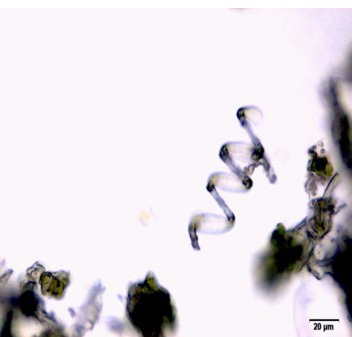
Figure 2 : Microscopic characters of C.nutans leaf powder. (a) Fragments of epidermal cells with diacytic stomata (magnification 20x); (b) a group of spiral and annular vessels (magnification 20x); (c) simple multicellular trichome (magnification 20x); (d) glandular trichome (magnification 20x); (e) parenchyma cells (magnification 20x); (f) fragments of spiral vessel (magnification 20x). [Scale bars: a-f = 20 µm]
Colour Tests
Observed colour changes on treatment with various reagents:
| H2SO4 (conc.) | Green |
| 5% KOH | Yellow |
| 5% FeCI3 | Yellow |
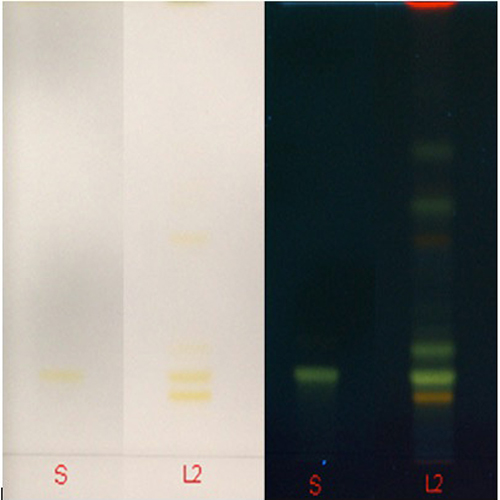
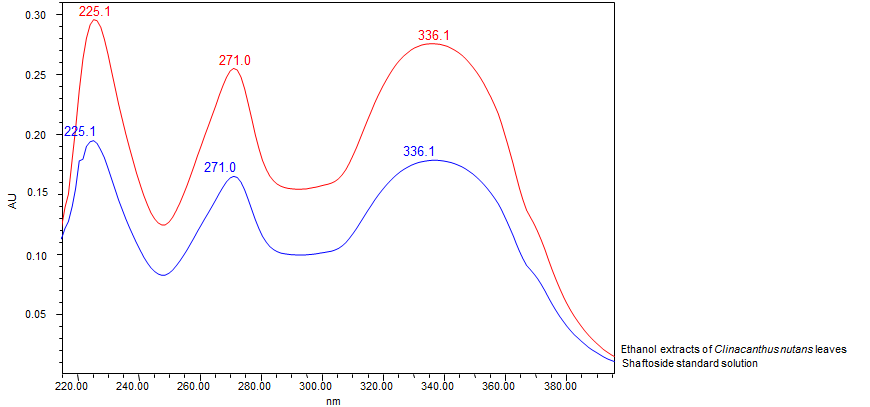
Thin Layer Chromatography (TLC)
| Test Solutions |
Weigh 0.5 g dried powdered leaf of C. nutans in a test tube. Add 5 mL of absolute ethanol and sonicated for 30 min at room temperature. Filter the sample (Whatman no. 1) and use the supernatant for TLC analysis. |
| Standard solution | Prepare 1 mg shaftoside standard [CAS no.: 51938-32-0] in 10 mL of absolute ethanol to produce a standard concentration 100 µg/mL. |
| Stationary Phase | HPTLC Silica gel 60 F254, 10 x 10 cm |
| Mobile phase | Ethyl acetate: acetic acid: formic acid: water;(100:11:11:27) (v/v/v/v) |
| Application |
|
| Development distance | 7 cm |
| Drying | Air drying |
| Detection |
|
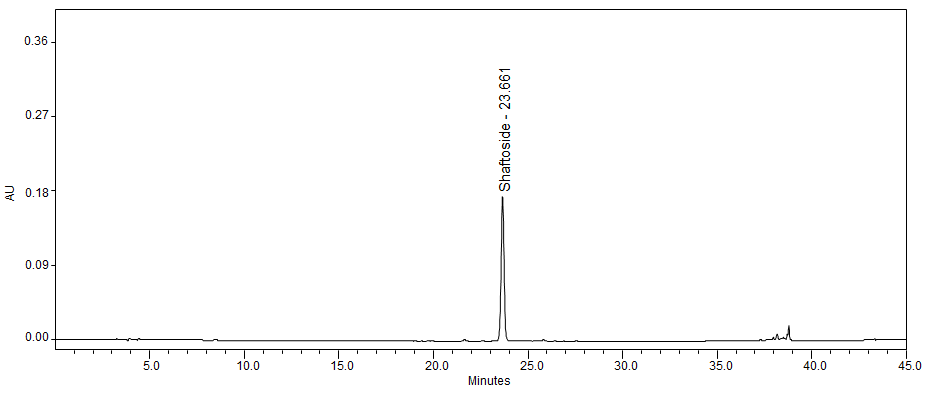
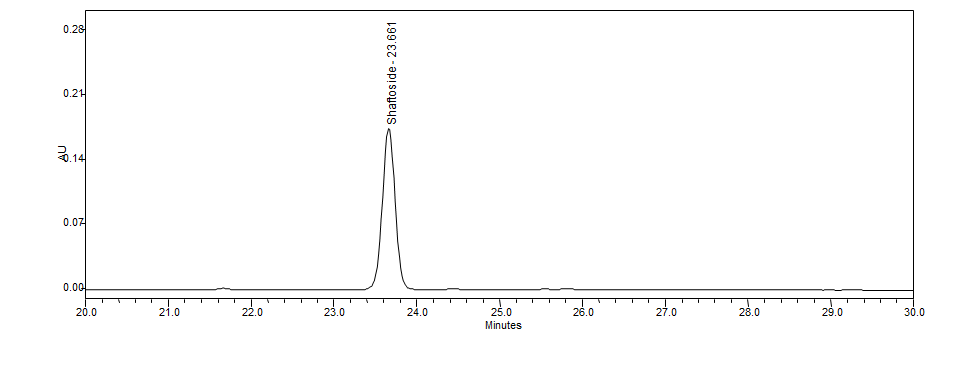
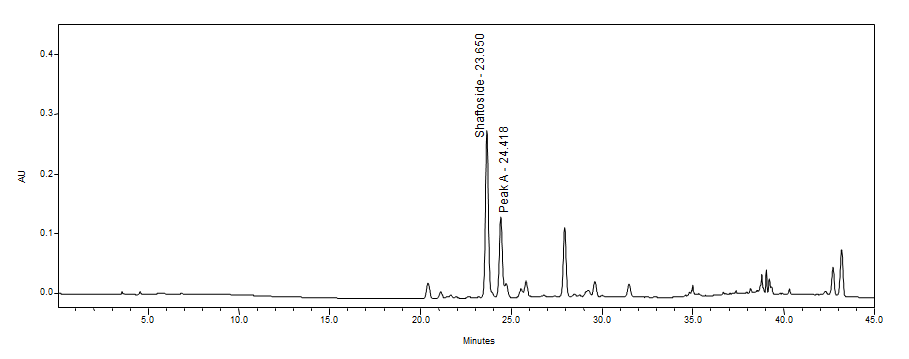
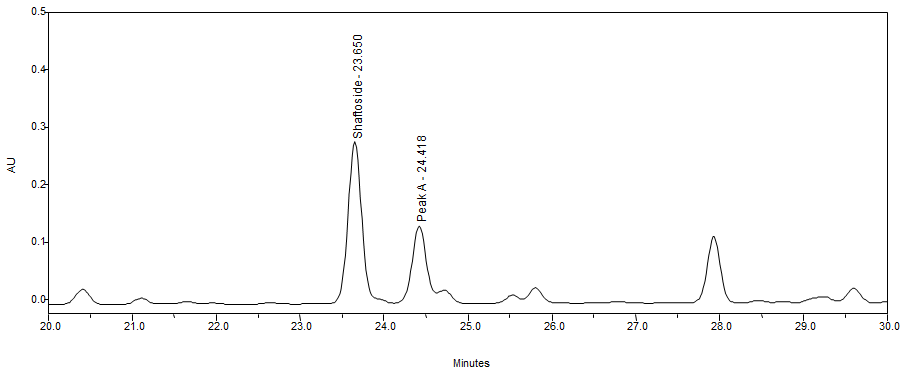
High Performance Liquid Chromatography (HPLC)
| Test solution | Weigh 0.5 g dried powdered leaf of C. nutans in a test tube. Add 5 mL of absolute ethanol and sonicate for 30 min at room temperature. Filter the sample through a 0.45 µm syringe filter and use the filtrate for HPLC analysis. | |||||||||||||||||||||
| Standard solution | Prepare 1 mg shaftoside standard [CAS no.:51938-32-0] in 10 mL of absolute ethanol to produce a standard concentration 100 µg/mL. | |||||||||||||||||||||
| Chromatographic system |
Detector: UV 330 nm Column: Polar-RP C18 4µm (250×4.6 mm) (preferably Phenomenex Synergi) Column oven temperature: 35˚C Flow rate: 1 mL/min Injection volume: 10 µL |
|||||||||||||||||||||
| Mobile Phase (gradient mode) |
|
|||||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the standard solutions (0.1 mg/mL). The requirements of the system suitability parameters are as follow:
|
|||||||||||||||||||||
| Acceptance criteria |
|
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 10.0% |
| Acid-insoluble ash | Not more than 1.0% |
| Loss on Drying |
| Not more than 12% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 30.0% |
| Cold method | Not less than 28.0% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 14.0% |
| Cold method | Not less than 5.0% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Methanol extracts of C. nutans stems and leaves have been found to contain flavonoids (e.g. vitexin, isovitexin, shaftoside, isomollupentin 7-O-β-glucopyranoside, orientin and isoorientin) and sulfurous glycosides (e.g. clinacosides A-C, cycloclinacosides A1 and A2) [ 7 , 8 ].
Ethanol extracts of C. nutans leaves have been found to contain nine cerebrosides and monoacylmonogalactoylglycerol [ 9 ].
Chloroform extracts of C. nutans leaves was found to contain chlorophyll derivatives (e.g. 132-hydroxy-(132-R)-phaeophytin b, 132-hydroxy-(132-S)-phaeophytin a, 132-hydroxy-(132-R)-phaeophytin a, 132-hydroxy-(132-S)-phaeophytin b, 132-hydroxy-(132-S)-chlorophyll b, 132-hydroxy-(132-R)-chlorophyll b, purpurin 18 phytyl ester, phaeophorbide a) and fatty acids (e.g. n-pentadecanol, eicosane, 1-nonadecene, heptadecane, dibutylphthalate, n-tetracosanol-1, heneicosane, behenic alcohol, 1-heptacosanol, 1,2-benzenedicarboxylic acid, mono(2-ethylhexyl)-ester, nonadecyl heptafluorobutyrate, eicosayl trifluoroacetate, dinonyl ester, phthalic acid, dodecyl nonylester) [ 10 , 11 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally used for dysentery by Javanese [ 13 ].
Biological and pharmacological activities supported by experimental data
Immunomodulatory activity
Ethanol extract of C. nutans dried leaves (0.5-5.0 µg/mL) significantly (p < 0.05) increased the proliferation of lymphocytes from peripheral blood mononuclear cells (PMBCs) of healthy adult human (stimulation index of 1.14-1.36) compared to untreated control (stimulation index of 1.00). However, at dose of 2.5 and 5.0 mg/mL, the extract significantly (p < 0.05) reduced the lymphocyte proliferation (stimulation index of 0.69-0.75) compared to untreated control (stimulation index of 1.00) [ 14 ].
Ethanol extract of C. nutans dried leaves (1 and 5 mg/mL) significantly (p < 0.05) reduced the activity of natural killer cells (from PMBCs of healthy adult human) (0.01 and 42.04 lytic units x 10-7 PMBCs) compared to untreated control (103.10 ±56.64 lytic units x 10-7 PMBCs) [ 14 ].
Ethanol extract of C. nutans dried leaves (2.5 and 5 mg/mL) induced the production of interleukin 4 (from PMBCs of healthy adult human) (40 and 1200 pg/mL) compared to untreated control (<16 pg/mL) by using enzyme linked immunosorbent assay [ 14 ]. Cytotoxic activity Compounds (132-hydroxy-(132-R)-phaeophytin b, 132-hydroxy-(132-S)-phaeophytin a and 132-hydroxy-(132-R)-phaeophytin a) isolated from crude extract of C. nutans dried leaves (5.89-6.21 µM) showed no cytotoxicity effect on African green monkey kidney cell line (Vero cells) using crystal violet assay [ 10 ].
Ethanol extract of C. nutans leaves (1 and 100 µg/mL) significantly (p < 0.05) increased the percentage of human keratinocyte HaCaT cells survival (pre-treated with interferon-γ and tumor necrosis factor-α) compared to untreated control using MTT assay [ 15 ].
Fractions (CL03 and CL21) from ethanol extract of C. nutans leaves showed no cytotoxicity effect on human laryngeal carcinoma cells (HEp-2) (50% cytotoxic concentration (CC50) > 300 µg/mL) compared to untreated control [ 15 ].
Compounds (1-4) isolated from extract of C. nutans dried leavesshowed cytotoxicity effect on A549 cells (adenocarcinomic human alveolar basal epithelial cells) with CC50 of 25-50 µg/mL using MTT assay compared to untreated control [ 17 ].
Chloroform extract of C. nutans leaves (25-100 µg/mL) significantly inhibited the growth of cultured human cancer cell lines in a dose-dependent manner (p < 0.05), i.e. liver hepatocellular carcinoma (29.97-38.76%), lung cancer cells (39.14-55.82%), gastric cancer cells (25.05-31.25%), colon adenocarcinoma (22.25-56.73%), erythroleukemia (36.52-41.97%, cervical cancer cells (32.32-91.28%) and Burkitt’s lymphoma cells (38.01-88.97%) using MTT assay compared to normal cells [ 12 ].
Methanol extract of C. nutans leaves (12.5-100 µg/mL) significantly inhibited the growth of cultured human cancer cell lines in a dose-dependent manner (p < 0.05), i.e. liver hepatocellular carcinoma (25.00-41.88%) and colon adenocarcinoma (13.21-30.33%) using MTT assay compared to normal cells. The same extract at a concentration of 100 µg/mL also significantly inhibited the growth of neuroblastoma cancer cells (22.08 ±1.22%) and gastric cancer cells (21.32 ±1.43%) using the same assay [ 12 ].
Aqueous extract of C. nutans leaves (12.5-100 µg/mL) significantly inhibited the growth of cultured human cancer cell lines in a dose-dependent manner (p < 0.05), i.e. liver hepatocellular carcinoma (14.89-27.42%), colon adenocarcinoma (16.56-27.57%), erythroleukemia (19.56-36.31%) and Burkitt’s lymphoma cells (12.48-40.94%) using MTT assay compared to normal cells. The same extract at concentrations of 50 and 100 µg/mL also significantly inhibited the growth of neuroblastoma cancer cells (13.80 ±0.70% and 23.22 ±0.63%) using the same assay. [12] Anti-oxidant activity Chloroform, methanol and aqueous extracts of C. nutans leaves were tested for their scavenging activity on 1,1-diphenyl-2-picrylhydrazyl radicals. Chloroform extract showed the highest scavenging activity (7852.63 ±449.90 µg Trolox equivalent/g) compared to other extracts (<6000 µg Trolox equivalent/g) [ 12 ].
Chloroform, methanol and aqueous extracts of C. nutans leaves were tested for their scavenging activity on galvinoxyl radicals. Chloroform extract showed the highest scavenging activity (12248.82 ±173.50 µg Trolox equivalent/g) compared to other extracts (<3000 µg Trolox equivalent/g) [ 12 ].
Aqueous extract of C. nutans leaves (100 µg/mL) showed nitric oxide scavenging activity with 32.33 ±0.97% inhibition [ 12 ]. Antiviral activity Compounds (132-hydroxy-(132-R)-phaeophytin b, 132-hydroxy-(132-S)-phaeophytin a and 132-hydroxy-(132-R)-phaeophytin a) isolated from chloroform extract of dried leaves of C. nutans completely inhibited activity of pre-compound-treated HSV-1 strain F (the HSV-1Fwas pre-treated for 60 min) on Vero cells after 72 hours incubation (IC50 = 1.96-3.11 nM) compared to pre-acyclovir-treated (5 µg/mL anti-viral drug; also pre-treated for 60 min) using plaque reduction assay. Pre-compound-treated Vero cells (was pre-treated for 60 min) showed 100% inhibition after 72 hours incubation than post-compound-treated cells (the Vero cells initially infected with HSV-1F and then was pre-treated for 60 min) [ 10 ].
Both fractions CL03 (50-200 µg/mL) and CL21 (5-80 µg/mL) from ethanol extract of C. nutans leaves reduced quantity of HSV-2’s DNA in a dose-dependent manner (12.99-70.96% and 7.65-62.94% DNA intensities respectively) compared to untreated control. These fractions also reduced the quantity of eight HSV-2’s proteins (20, 40, 44, 55, 69, 78, 125 and 146 KDa proteins) in a dose-dependent manner (144-15946 µg/mL and 50-15657 µg/mL) compared to untreated control (2396-16799 µg/mL) [ 16 ].
Compound 2 isolated from crude extract (hexane and chloroform) of dried leaves of C. nutans (5-34 µg/mL) inhibited the replication of dengue virus 2 (DV2) on pre-treated type II human lung alveolar epithelial cell carcinoma A549 [ 17 ].
Crude extract (organic solvents) of C. nutansleaves actively inhibited multiplication of varicella-zoster virus (VZV) in inactivation treatment (5000 PFU/mL cell-free VZV mixed with growth media containing extract or control) with 50% inhibitory dose of 1:9600 compared to acyclovir control (ID50 = 30 µM) using plaque reduction assay [ 18 ].
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute toxicity
Methanol extract of C. nutans leaves (0.9 and 1.8 g/kg body weight) given orally to male albino Swiss mice (25-35 g of body weight) respectively for 14 days showed no toxic effect (LD50 > 1.8 g/kg body weight), neither death nor significant changes on body weights and relative organ weights [ 19 ].
Oral single dose acute toxicity study using aqueous extract of C. nutans leaves on female Sprague Dawley rats (aged between 8 and 12 weeks old) showed no toxic effect on the parameters observed, which include behaviours, body weight, food and water intakes. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. Half lethal dose (LD50) is more than 2,000 mg/kg body weight[ 22 ].
Clinical studies
A randomized placebo-controlled trial involving 51 patients (excluded dropouts of nine patients) was conducted to study the antiviral activity of C. nutans against herpes simplex virus and varicella zoster virus. The treatment group was given 5% C. nutans extract to apply on infected skin area five times daily for 14 days and were followed-up on day 0, 3, 7, 10 and 14. Twenty five patients receiving C. nutans extract had lesion crusting on day 3 and lesion healing on day 7 (p < 0.01) compared to none in placebo group. On day 10, 28 treated patients had lesion healing compared only three patients in placebo group (p < 0.01). The pain scores were reduced rapidly for treatment group (score 0 on day 7) compared to placebo group (score 1 on day 7). No side effect was observed [ 20 ].
A randomized double-blind trial involving 75 patients (excluded dropouts of 45 patients) was conducted to study the antiviral activity of C. nutans cream against herpes zoster. These patients were randomly given C. nutans cream or placebo to apply on infected skin area thrice daily for 28 days and were followed-up for another month. Eleven patients receiving C. nutans cream had full crusting within three days compared to only three patients in placebo group (p < 0.05). The mean number of days for patients receiving C. nutans cream to heal completely (14.2 ±5.2 days) was significantly (p < 0.05) shorter than placebo group (18.0 ±7.5 days) [ 21 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The plant list. [Internet] Clinacanthus nutans (Burm.f.) Lindau; 2013 [cited on 6th February 2014]. Available from: http://www.theplantlist.org/tpl1.1/record/kew-2727901.
- Flora of China. [Internet] Clinacanthus nutans (Burm. f.) Lindau; Vol. 19, 2011 [cited on 6th February 2014]. Available from: http://www.eflora.cn/spfoc/Clinacanthus%20nutans.
- Complementary and Alternative Healing University. [Internet] Clinacanthus nutans (Burm. f.) Lindau; 2011 [cited on 6th February 2014]. Available from: http://alternativehealing.org/Sabah_snake_grass.html
- Asean herbal and medicinal plants. [Internet] Clinacanthus nutans (Burm. f.) Lindau; 2010 [cited on 6th February 2014]. Available from: http://www.scribd.com/doc/112146724/ASEAN-Herbal-and-Medicinal-Plants-2010
- Nainggolan M. Pemeriksaan mikroskopis dan analisis kandungan senyawa kimia daun Thailand (Clinacanthus nutans (Burm. f.) Lindau). Jurnal Komunikasi Penelitian. 2004;5(16):40-44.
- Kunsorn P, Ruangrungsi N, Lipipun V, Rungsihirunrat K. The identities anti-herpes simplex virus activity of Clinacanthus nutans and Clinacanthus siamensis. Asian Pacific Journal of Tropical Biomedicine. 2013;3(4):284-290.
- Teshima KI, Kaneko T, Ohtani K, Kasai R, Lhieochaiphant S, Picheansoonthon C, Yamasaki K. C-glycosyl flavones from Clinacanthus nutans. Natural Medicines. 1997;51(6):557.
- Teshima KI, Kaneko T, Ohtani K, Kasai R, Lhieochaiphant S, Picheansoonthon C, Yamasaki K. Sulfur-containing glucosides from Clinacanthus nutans. Phytochemistry. 1998;48(5):831-835.
- Tuntiwachwuttikul P, Pootaeng-On Y, Phansa P, Taylor WC. Cerebrosides and a monoacylmonogalactosylglycerol from Clinacanthus nutans. Chemical and Pharmaceutical Bulletin (Tokyo). 2004;52(1):27-32.
- Sakdarat S, Shuyprom A, Pientong C, Ekalaksananan T, Thongchai S. Bioactive constituents from the leaves of Clinacanthus nutans Lindau. Bioorganic & Medicinal Chemistry. 2009;17(5):1857-1860.
- Sakdarat S, Shuyprom A, Na Ayudhya TD, Waterman P, Karagianis G. Chemical composition investigation of the Clinacanthus nutans Lindau leaves. Thai Journal of Phytopharmacy. 2006;13(2):13-24.
- Yong YK, Tan JJ, Teh SS, Mah SH, Ee GC, Chiong HS, Ahmad Z. Clinacanthus nutans extracts are antioxidant with antiproliferative effect on cultured human cancer cell lines. Evidence-Based Complementary and Alternative Medicine. 2013;2013:462751.
- Burkill IH. A dictionary of the economic products of the Malay peninsula. Vol. 1. London; Published on behalf of the governments of the Straits settlements and Federated Malay states by the Crown agents for the colonies. 1935;p.587.
- Sriwanthana B, Chavalittumrong P, Chompuk L. Effect of Clinacanthus nutans on human cell-mediated immune response in vitro. Thai Journal of Pharmaceutical Sciences. 1996;20(4):261-267.
- Thongrakard V, Tencomnao T. Modulatory effects of Thai medicinal plant extract on proinflammatory cytokines-induced apoptosis in human keratinocyte HaCaT cells. African Journal of Biotechnology. 2010;9(31):4999-5003.
- Vachirayonstien T, Promkhatkaew D, Bunjob M, Chueyprom A, Chavalittumrong P, Sawanpanyalert P. Molecular evaluation of extracellular activity of medicinal herb Clinacanthus nutans against herpes simplex virus type-2. Natural Product Research. 2010;24(3):236-245.
- Sittiso S, Ekalaksananan T, Pientong C, Sakdarat S, Charoensri N, Kongyingyoes B. Effects of compounds from Clinacanthus nutans on dengue virus type 2 infection. Srinagarind Medical Journal. 2010;25(Suppl):272-275.
- Thawaranantha D, Balachandra K, Jongtrakulsiri S, Chavalittumrong P, Bhumiswasdi J, Jayavasu C. In vitro antiviral activity of Clinacanthus nutans on varicella-zoster virus. Siriraj Hospital Gazette. 1992;44(4):284-291.
- P’ng XW, Akowuah GA, Chin JH. Acute oral toxicity study of Clinacanthus nutans in mice. International Journal of Pharmaceutical Sciences & Research. 2012;3(11):4202.
- Sangkitporn S, Chaiwat S, Balachandra K, Na-Ayudhaya TD, Bunjob M, Jayavasu C. Treatment of herpes zoster with Clinacanthus nutans (bi phaya yaw) extract. Journal of the Medical Association of Thailand. 1995;78(11):624-627.
- Charuwichitratana S, Wongrattanapasson N, Timpatanapong P, Bunjob M. Herpes zoster: treatment with Clinacanthus nutans cream. International Journal of Dermatology. 1996;35:665–666.
- Teh BP, Hamzah NF, Norazila Z, Norliyana MY, Wan Abdul Hakim WL. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2014. Report no.: HMRC 11-045/01/CN/L/J.