Telang flower
Clitoria ternatea Linn.
Fabaceae
DEFINITION
Telang flower consists of the powder of dried flower with pedicel of Clitoria ternatea Linn (Fabaceae).
SYNONYM
Clitoria ternatea L., Clitoria ternatea var. albiflora Voigt, Clitoria ternatea var. angustifolia Baker f., Clitoria ternatea var. pilosula (Benth.) Baker, Clitoria ternatea var. ternatea and Clitoria ternatea f. ternatea [1]; Clitoria zanzibarensis Vatke, Clitoria tanganicensis Micheli, Clitoria mearnsii De Wild. [2]; Clitoria albiflora Matteiand Clitoria bracteate Poir [3]; Clitoria coelestris Siebert and Voss, Clitoria parviflora Raf., Clitoria philippensis Perr., Clitoria pilosula Benth., Clitoria ternatensium Crantz, Lathyrus spectabilis Forssk., Nauchea ternatea (L.) Descourt., Ternatea ternatea (L.) Kuntze, Ternatea vulgaris Kunth, Ternatea vulgaris Kuntze [4].
VERNACULAR NAMES
Blue pea, bluebellvine, butterfly-pea, cordofan-pea, Darwin-pea, Asian pigeon-wings (English); bunga telang, kacang telang, bunga biru (Malay); die dou (Chinese); kakkattan, kakkanam (Tamil) [5, 6, 7].
CHARACTER
| Colour | Bluish green |
| Odour | Aromatic |
| Taste | Flavourless |
IDENTIFICATION
Plant morphology
C. ternatea is a perennial climbers, scrambler or trailing herb with woody woodstock. Stems are slender, measuring 0.5–3 m long, mostly pubescent or glabrescent, and sometimes suberect at the base. Leaves are pinnate, 5–7 leaflets, leaflets elliptical, oblong, oblong-lanceolate, or almost round, measuring 1–7 cm x 0.3–4 m, acute, rounded or emarginated at the apex, acute to rounded at the base, glabrescent above and appressed pubescent beneath. Petiole is about 1–3 cm long; rachis 1–6 cm long; petiolules 1–3 mm long; stipules persistent, lanceolate, (2-)4–10 mm long, veined. Flowers axillary, solitary or paired; peduncle 3–10 mm long; pedicel 6–9 mm long and twisted 180º; bracteoles ovate or round, 4–17 mm x 2.5–15 mm, veined; calyx pubescent, veined, tube 8–12 mm long, lobes oblong-lanceolate or triangular, 7–10 mm x 2.5–3 mm, acute or acuminate, the upper pair joined for less than one third of their length; standard oblong-obovate, 25–50 mm x 15–35 mm, white or greenish-white often blue margined or entirely blue, basal central area often yellow or greenish, very finely puberulous, margins sometimes finely ciliate. Pod is linear-oblong, flattened, 6–12.5 cm x 7–12 mm, margined, apiculate, glabrous or with a mixture of sparse adpressed long hairs and very short hairs. Seeds are 8–10, ellipsoid, oblong or oblong-reniform, sometimes truncate at one end, 4.5–7 mm x 3–4 mm, 2–2.5 mm, olive, pale brown or deep reddish-brown with dark mottling, or almost black, minutely pitted. [8]
Microscopy
Powdered material consists of straight anticlinal walls on adaxial surface, sinuous anticlinal walls on abaxial surface, anomocytic stomata found on the epidermis, thin-walled and elongated rectangular epidermal cells, pitted vessel cells, pollen grain and two types of trichomes; warty trichome with apical tip and uniseriate hooked tip, and fibre. [9, 10]












Figure 2 : Microscopic characters of C. ternatea flowers powder of 0.355 mm size. (a) straight anticlinal walls on adaxial surface (magnification 40x); (b) sinuous anticlinal walls on abaxial surface (magnification 40x); (c) Anomocytic stomata (magnification 40x); (d) thin-walled and elongated parenchyma rectangular epidermal cells (magnification 40x); (e) pitted vessel (magnification 40x); (f) pollen grain from polar view (magnification 40x); (g) pollen grain from equatorial view (magnification 40x); (h) capitate glandular trichome (echinate ornamentation) (magnification 40x); (i) simple unicellular trichome (hooked) (magnification 40x); (j) simple unicellular trichome (magnification 40x); (k) solitary calcium oxalate crystal (magnification 40x); (l) calcium oxalate crystal (red arrow) (magnification 20x). [Scale bars: a-j = 50 µm; k = 20 µm; l = 100 µm].







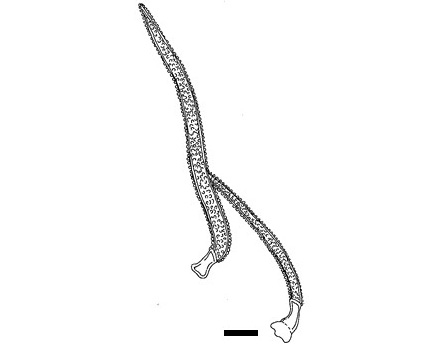



Figure 3 : Line drawing of microscopic characters of C. ternatea flowers powder of 0.355 mm size. (a) Straight anticlinal walls on adaxial surface (magnification 40x); (b) sinuous anticlinal walls on abaxial surface (magnification 40x); (c) anomocytic stomata (magnification 40x); (d) thin-walled and elongated parenchyma rectangular epidermal cells (magnification 40x); (e) pitted vessel (magnification 40x); (f) pollen grain from polar view (magnification 40x); (g) pollen grain from equatorial view (magnification 40x); (h) capitate glandular trichome (echinate ornamentation) (magnification 40x); (i) simple unicellular trichome (hooked) (magnification 40x); (j) simple unicellular trichome (magnification 40x); (k) solitary calcium oxalate crystal (magnification 40x). [Scale bars: a-j = 50 µm; k = 20 µm].
Chemical Test
Observed colour of solution after treatment with various reagents:
| Test of presence of flavonoids | Yellowish |
Thin Layer Chromatography (TLC)

Figure 3 : TLC chromatogram of rutin (S) and methanol extract of C. ternatea dried flower powder (L) observed under UV at 254 nm before derivatisation.
| Test Solutions | Weigh about 1.0 g of C. ternatea dried flower powder of 0.355 mm size in a 20 mL vial. Add 10 mL of methanol : water : formic acid (70 : 29 : 1, v/v/v) and sonicate the mixture for 30 min at 40°C. Filter the mixture with filter paper into vials. Use the filtrate as a test solution. |
| Standard solution | Dissolve rutin standard [CAS no.: 207671-50-9] in methanol to give a standard concentration of 1 mg/mL. |
| Stationary Phase |
HPTLC silica gel pre-coated plate 60 F254, 10 x 10 cm |
| Mobile phase | Ethyl acetate : acetic acid : formic acid : water (10 : 1.1 : 1.1 : 2.3, v/v/v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
High Performance liquid Chromatography (HPLC)
| Test solution | Weigh about 1.0 g of C. ternatea dried flower powder of 0.355 mm size in a 20 mL vial. Add 10 mL of methanol : water : formic acid (70 : 29 : 1) and sonicate the mixture for 30 min at 40–50°C. Filter the mixture with 0.22 µm nylon membrane syringe filter into vials. Use the filtrate as a test solution | ||||||||||||||||||||||||
| Standard solution | Dissolve rutin standard [CAS no.: 207671-50-9] in methanol to produce a standard concentration of 1 mg/mL. This standard solution was diluted to 0.1 mg/mL in methanol before being injected into the HPLC system. | ||||||||||||||||||||||||
| Chromatographic system |
Detector: UV 355nm |
||||||||||||||||||||||||
| Mobile Phase (gradient mode) |
|
||||||||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the standard solutions (1 mg/mL). The requirements of the system suitability parameters are as follow:
|
||||||||||||||||||||||||
| Acceptance criteria |
|

(a)

(b)
Figure 5 : : WholeHPLC chromatogram of (a) rutin standard solution (0.1 mg/mL) at tr = 22.281 min and (b) methanol extract of C. ternatea dried flower powder showing peak corresponding to rutin standard solution at tr = 22.286 min.

(a)

(b)
Figure 6 : HPLC chromatogram highlighting the elution region of (a) rutin standard solution (0.1 mg/mL) at tr = 22.281 min and (b) methanol extract of C. ternatea dried flower powder showing peak corresponding to rutin standard solution at (tr = 22.286 min).
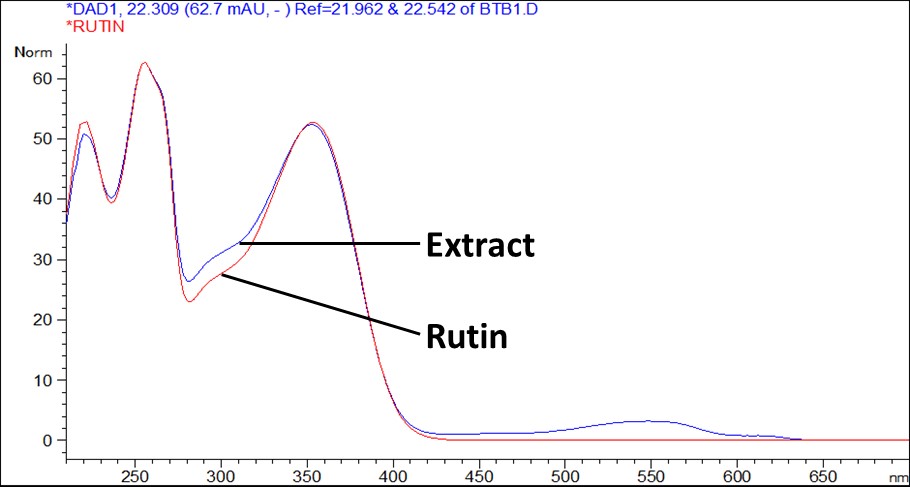
Figure 7 : UV spectrum of rutin standard solution (0.1 mg/mL) and methanol extract of C. ternatea dried flower powder.
PURITY TEST
The purity tests, except for foreign matter test, are based on C. ternatea dried flower powder of 0.355 mm particle size.
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 8% |
| Acid-insoluble ash | Not more than 1% |
| Water-soluble ash | Not less than 2% |
| Loss on Drying |
| Not more than 12% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 47% |
| Cold method | Not less than 42% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 29% |
| Cold method | Not less than 19% |
SAFETY TEST
The safety tests are based on C. ternatea dried flower powder of 0.355 mm particle size.
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total aerobic microbial count | Not more than 5 x 105 cfu/g* |
| Total yeast and mould count | Not more than 5 x 104 cfu/g* |
| Bile-tolerant gram negative | Not more than 104 cfu/g* |
* Values are based on ASEAN guidelines [11]
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Press juice of fresh petals of C. ternatea was found to contain pentaacylated anthocyanin, ternatin B1 [12].
The 5% of acetic acid in water extract was found to contain anthocyanins, ternatin C1-C5 and D3 and preternatins A3 and C4 from young C. ternatea flowers [13].
The 1% TFA–50% aqueous methanol extracts of the petals has been found to contain flavonol glycosides, i.e. quercetin 3-(2G-rhamnosylrutinoside); kaempferol 3-(2G-rhamnosylrutinoside) (clitorin); kaempferol 3-neohesperidoside; quercetin 3-neohesperidoside; myricetin 3-neohesperidoside; kaempferol 3-rutinoside (nicotiflorin); quercetin 3-rutinoside; myricetin 3-rutinoside; kaempferol 3-glucoside; quercetin 3-glucoside; myricetin 3-glucoside; kaempferol 3-O-(2”-O-α-rhamnosyl-6”-O-malonyl)-β-glucoside; quercetin 3-O-(2”-O-α-rhamnosyl-6”-O-malonyl)-β-glucoside and myricetin 3-O-(2”,6”-di-O-α-rhamnosyl)-β-glucoside [14].
A mixture of 1% TFA in methanol (50%) extract of the petals was found to contain anthocyanin; delphinidin 3-(2’’-rhamnosyl-6’’-malonyl)-glucoside; delphinidin 3-(6’’-malonyl)-glucoside; delphinidin 3-neohesperidoside and delphinidin 3-glucoside [15].
Methanol (80%) extract of the flowers was found to contain anthocyanin; ternatins A3, B4, B3, B2, B1, A2, A1, D2 and D1 [16].
Aqueous extract of the flower was found to contain acylated anthocyanins; ternatins (A1, A2, B1, B2, D1 and D2) [17].
Aqueous extract containing 5% acetic acid of the flower was found to contain anthocyanins (ternatins C1, C2, C3, C4, C5 and D3 and preternatins A3 and C4) [13].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
In India, the juice of C. ternatea traditionally is used as treatment for insect bite and skin disease [18]. While in Malaysia, the flowers are sometimes used to colour rice, as the rice when boiled with them turns blue. However, in Java, the juice of the flowers is used for inflamed eyes [5].
Biological and pharmacological activities supported by experimental data
Antioxidant activity
Methanol crude extract (95%), chloroform fraction and ethyl acetate fraction (1.0, 0.5, 0.25 and 0.125 mg/mL) inhibited 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging with IC50 of 95.30 µg/mL, 132.50 µg/mL, 107.42 µg/mL respectively compared to L-ascorbic acid (IC50 = 70.80 µg/mL). [19]
Antidiabetic activity
Methanol crude extract (95%) and ethyl acetate fraction of C. ternatea flower (300 mg/kg) were administered orally to alloxan-induced diabetic male Wistar rats (200–250 g) for 12 days. Results showed that methanol crude extract (from 382 mg/dL to 171.42 mg/dL; p < 0.001) and ethyl acetate fraction (from 385.67 mg/dL to 169.67 mg/dL; p < 0.01) significantly reduced blood glucose level after 12 days as compared to normal control (from 97.67 mg/dL to 94.33 mg/dL). [19]
Clinical studies
A randomised, crossover study involving 15 healthy men (aged 22.53±0.30 years; with body mass index of 21.57±0.54 kg/m2) was conducted to study the effects of aqueous extract of C. ternatea flower (CTE) on glycaemic response. Three days before treatment, the subjects had to avoid any consumption of phytochemical-rich food. After overnight fasting, each group of subjects (n = 3) were given one treatment in beverage form to be consumed within 5 min, either (1) 50 g sucrose in 400 mL of water; (2) 1 g CTE in 400 mL of water; (3) 2 g CTE in 400 mL of water; (4) 50 g sucrose and 1 g CTE in 400 mL of water; or (5) 50 g sucrose and 2 g CTE in 400 mL of water. For 3 hours, no ingestion was allowed after treatment. Their blood samples were collected at 15-, 30-, 60-, 90-, and 120-min after treatment. All groups then underwent a week of washout period before receiving other treatments. All collected blood samples were analysed to determine the levels of plasma glucose and insulin. Significant difference analysis was conducted on area under curve (AUC) for changes in the measured parameters. A significant lower AUC for plasma glucose level (p < 0.05) was observed in subjects that received 1 g and 2 g of CTE plus sucrose in water (0.67- and 0.60-fold) compared to the subjects that received sucrose in water. Lower AUC was observed for insulin level (p < 0.05) only in subjects that received 2 g of CTE plus sucrose in water (0.67-fold) compared to the subjects that received sucrose in water. [20]
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute toxicity
Methanol crude extract (95%) and its two fractions (chloroform and ethyl acetate fraction) of C. ternatea flower (300 mg/kg) administered orally once to Wistar rats (200–250 g body weight; three rats per treatment) were observed for 72 hours. No mortality nor changes in behavioural pattern were observed. [19]
Others (Adverse reactions, contraindications, side effects, warning, precautions)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The plant list. [Internet] Clitoria ternatea Linn; 2013 [cited on 19 October 2020]. Available from: http://www.theplantlist.org/tpl1.1/search?q=Clitoria+ternatea
- Chukwuma EC, Soladoye MO, Abdus Salam KRP. Taxonomic value of the leaf micro-morphology and quantitative phytochemistry of Clitoria ternatea and Centrosema pubescens (Papilionoideae, Fabaceae). Phytologia balcanica. 2014;20(1):3–8.
- USDA, ARS, National genetic resources program. Germplasm resources information network-(GRIN). National germplasm Resources Laboratory, Beltsville, Maryland. [Internet] Clitoria ternatea Linn; 2020 [cited on 01 November 2020]. Available from: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomydetail?id=10942
- Clitoria ternatea Linn. [Internet] CABI; 2020 [cited on 19 October 2020]. Available from: cabi.org/isc/datasheet/55416
- Burkill LH. A dictionary of the economic products of Malay Peninsula. Jilid 1 (A-H). Kuala Lumpur: Ministry of Agriculture Malaysia. 1935;p.588–589.
- Ridley HN. Flora of Malay Peninsular. Jilid 1. London. L. Reeve & Co. Ltd. 1922;p.569
- Clitoria ternatea Linn. [Internet] The plants database, National Plant Data Center, NRCS, USDA; 2019 [cited on 01 November 2020]. Available from: https://plants.sc.egov.usda.gov/core/profile?symbol=CLTE3
- Staples IB. Clitoria ternatea L. In. Mannetje L’t, Jones RM. Plant Resources of South-East Asia No. 4: Forages. Bogor: Prosea Foundation. 1992;p.94–97.
- Chauhan D, Daniel M. Foliar micromorphological studies on some members of the family Fabaceae. International Journal of Pharma and Bio Sciences. 2011;2(4):603–611.
- Khatijah H, Mohamad-Ruzi AR, Nurulnahar E. Malaysian medicinal plants. Volume 4. Bangi: Penerbit Universiti Kebangsaan Malaysia (UKM). 2006;p.67–71.
- ASEAN Guidelines on limits of contaminants for health supplements. Version 2.0. [Internet] asean.org [cited on 23 December 2021]. Available from: http://asean.org/wp-content/uploads/2017/09/ASEAN-Guidelines-on-Limits-of-contaminants-HS-V2.0-with-disclaimer.pdf
- Kondo T, Ueda M, Goto T. Structure of ternatin B1, a pentaacylated anthocyanin substituted on the B-ring asymmetrically with two long chains. Tetrahedron. 1990;46(13-14):4749–4756.
- Terahara N, Toki K, Saito N, Honda T, Matsui T, Osajima Y. Eight new anthocyanins, ternatin C1-C5 and D3 and preternatins A3 and C4 from young Clitoria ternatea flowers. Journal of Natural Products. 1998;61:1361–1367.
- Kazuma K, Noda N, Suzuki M. Malonylated flavonol glycosides from the petals of Clitoria ternatea. Phytochemistry. 2003a;2(2):229–237.
- Kazuma K, Noda N, Suzuki M. Flavonoid composition related to petal color in different lines of Clitoria ternatea. Phytochemistry. 2003b;64:1133–1139.
- Terahara N, Oda M, Matsui T, Osajima Y, Saito N, Toki K, Honda T. Five new anthocyanin, Ternatin A3, B4, B3, B2 and D2. Journal of Natural Products. 1996;59:139–144.
- Terahara N, Saito N, Honda T, Toki K, Osajima Y. Acylated anthocyanins of Clitoria ternatea flowers and their acyl moieties. Phytochemistry. 1990;29(3):949–953.
- Agrawal P, Deshmukh S, Ali A, Patil S, Magdum CS, Mohite SK, Nandgude TD. Wild flower as medicine. International Journal of Green Pharmacy. 2007;1(1):12–13.
- Manivannan R, Prabakaran K, Ilayaraja S. Evaluation of anti-oxidant and anti-diabetic activity of flower extract of Clitoria ternatea L. Journal of Applied Pharmaceutical Science. 2015;5(08):131–138.
- Chusak C, Thilavech T, Henry CJ, Adisakwattana S. Acute effect of Clitoria ternatea flower beverage on glycemic response and antioxidant capacity in healthy subjects: a randomized crossover trial. BMC Complementary and Alternative Medicine. 2018;18:6.






