Scientific Name
Curcuma longa L.
Synonyms
Amomum curcuma Jacq., Curcuma brog Valeton, Curcuma domestica Valeton, Curcuma euchroma Valeton, Curcuma ochrorhiza Valeton, Curcuma soloensis Valeton, Curcuma tinctoria Guibourt, Kua domestica Medik. [Illegitimate], Stissera curcuma Giseke. [1]
Vernacular Name
| Malaysia | Kunyit, temu kunyit (Malay); tius (Semang) [2] |
| English | Turmeric, indian saffron [2] [3] |
| China | Jiang huang huang jiang (Cantonese Wòhng gèung, Wong keong); yu jin (yu chin), yu chiu [3] |
| India | Halud (Bengali); haldi (Hindi); arishina (Kannada); haldar, halja (Punjabi); ameshta, bahula, bhadra (Sanskrit); manjal (Tamil) [3] |
| Indonesia | Kunyit (Indonesian); kunir (Javanese); koneng (Sundanese)[2] |
| Thailand | Khamin (General); khamin-kaeng (Northern); khamin-chan (Central) [2] |
| Laos | Khminz khünz [2] |
| Philippines | Dilaw (Tagalog); duwaw (Cebuano); kalawag (Ilocano) [2] |
| Cambodia | Rômiêt, lômiêt [2] |
| Vietnam | Ngh[eej], u[aas]t kim [2] |
| France | Curcuma [2]. |
Geographical Distributions
Curcuma longa is presumed to originate from South or South-East Asia, most probably from India. It is not known in a true wild state although in some places it appears to have become naturalised (e.g. in teak forests of East Java). C. longa has been grown in India since time immemorial. It reached China before the 7th century, East Africa in the 8th century and West Africa in the 13th century. It was introduced into Jamaica in the 18th century. Currently turmeric is widely cultivated throughout the tropics but cultivation on a considerable scale is largely confined to India and South-East Asia. [2]
Botanical Description
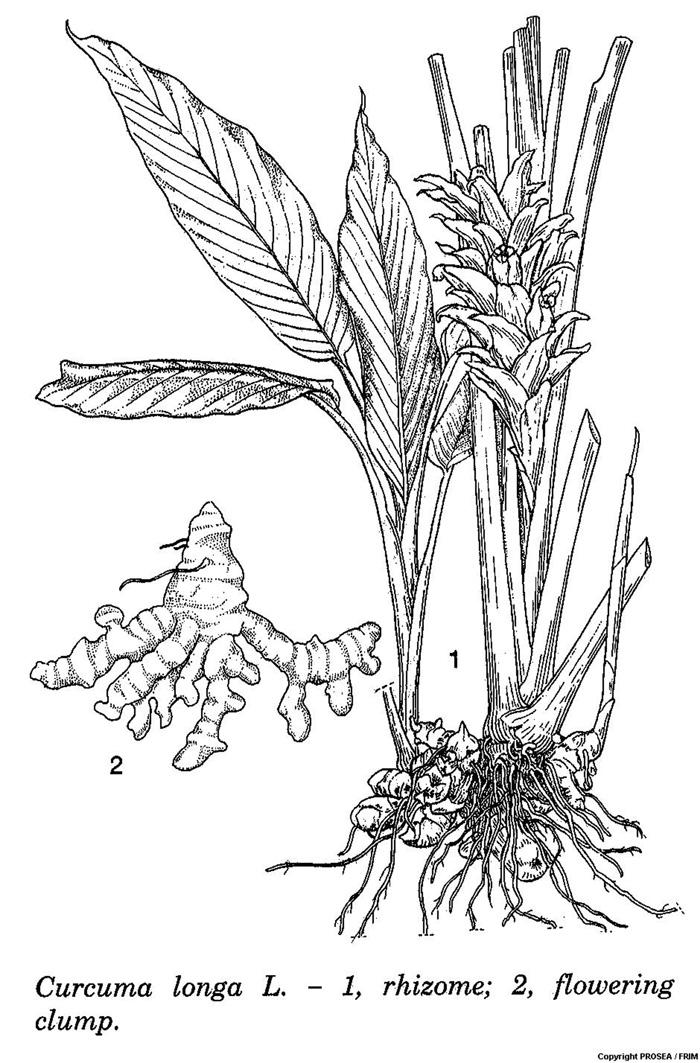
C. longa is classified as a member of the Zingiberaceae family. It is a robust, perennial, erect, strongly tillering herb (often cultivated as an annual), up to 1(-1.5) m tall. [2]
The rhizome is a fleshy complex with an ellipsoidal primary tuber (about 5 cm x 2.5 cm), ringed with the bases of old scale leaves and when mature bearing numerous cylindrical, lateral rhizomes (called fingers), 5-10 cm x 1-1.5 cm, the whole forming a dense clump. The rhizomes’ colour is deep orange inside and yellowish orange outside. , Its young tips are white, and it smells spicy when bruised. [2]
The leaves alternate, distichous, with sheaths forming a short pseudostem. The petiole 0.5 – 10 cm long, broadly furrowed and narrowly winged. Its blades are oblong-lanceolate to ovate-lanceolate, 7-70 cm x 3-20 cm, base cuneate to rounded, apex acute or acuminate, above dark green, below very pale green, densely studded with pellucid dots. [2]
The inflorescence is a terminal spike up to 20 cm long, erect, appearing between the leaf sheaths. The peduncle is 3-20 cm long, densely hairy, and covered by bladeless sheaths or scales. The bracts are elliptical-lanceolate, 5-7.5 cm x 2.5 cm, densely hairy, pale green to white colour. [2]
The flowers are in pairs in axils of bracts, bisexual, zygomorphic, 5-6 cm long, opening one at a time. The calyx is tubular, short, with 3 unequal teeth. The corolla is tubular at base, upper half much widened and with 3 unequal lobes, which are white in colour. The labellum (central staminode) is orbicular to obovate, 12-17 mm in diameter, with 2 small lateral lobes and a large emarginate central lobe, white with a yellow central streak, lateral staminodes 2, elliptical-oblong, 1 cm x 6 cm, creamy-white, functional stamen 1, for larger part connate with staminodes, 5-6 mm x 3 mm, anther with a broad, curved large spur at base. Ovary inferior, 3-celled, with 2 erect glands at top, style slender, passing between and held by the anther thecae, stigma expanded. [2]
The fruit is never produced. [2]
The roots are filiform, tough, sometimes very long, often swollen into an ellipsoidal tuber at the apex (2-4cm x 1-2 cm). [2]

Cultivation
Soil Suitability and Climatic Requirement
C. longa thrives well in tropical and subtropical areas. The total annual rainfall of 1,000-2,000 mm or more is required for optimum growth. The plant can grow on many soil types. Sandy loam with high organic matter content is preferred. [4]
Field preparation
Land Preparation
Like other crops, C. longa needs well-prepared planting bed. This includes ploughing using disc plough and disc harrow. Pre-germination herbicide is applied prior to planting to avoid weeds smothering the area. [4]
Production of Planting Materials
C. longa is propagated using mature rhizomes. The rhizome is cut into 4-5 cm segments with 2-3 buds per segment. The rhizomes can be planted directly or after buds start to sprout. The planting material of 600-1,500 kg/ha is needed for single crop planting and 400-500 kg/ha for mixed crop planting. To reduce the incidence of fusarium disease, the planting material should be soaked in a fungicide (benomyl) solution containing 0.25% active ingredient, or 1% Bordeaux mixture for 5-10 minutes, and dried before field planting. [4]

Field Planting
On bris sandy soil, C. longa is usually planted at a planting distance of 40-60 cm on 120 cm or wider beds, with a bed height of 25-30 cm. The length of beds depends on the size of the planting area. The recommended planting distance for an area without beds is 40 cm within a row and 60 cm between rows. The rhizome is planted 5-7 cm deep in the soil. Rhizome formation starts in the 5th month and continue forming actively until it is ready for harvest at 7-9 months after field planting. Hilling at the base of the plant is recommended at 8-10 weeks after planting to encourage rhizome formation and to make sure it does not grow above the ground. [4]

Field maintenance
Fertilisation
C. longa or ‘Kunyit’ requires organic fertiliser such as cow manure or chicken manure. For bris soil, chicken manure at 10 t/ha is recommended and for mineral/inland soil, 5 t/ha is recommended. Apart from organic fertiliser, semi-organic fertiliser like Kokei (NPK=8:6:16) at the rate of 750 kg/ha is used to obtain a good growth. [4]
Weed Control
Prior to planting, pre-germination herbicide with active ingredient, alachlor, at the rate of 5.6 litres/ha should be sprayed. Weed control after germination and during growth can be done manually or by spraying cautiously. [4]
Water Management
To avoid water stress, field planting should be done at the onset of the rainy seasons. A supplementary irrigation by using sprinkler irrigation system is needed during the dry seasons. [4]
Pest and Disease Control
The main disease for the crop is leaf spot caused by the fungus Taphrina maculans. The leaf spot is 1-2 mm in diametre and causes the leaves to dry. The infection can become more serious when humidity is high. Bacterial wilt is also of significance. To avoid pest and disease, continuous planting of the crop on the same piece of land is not recommended. [4]
Harvesting
C. longa reaches maturity 7-9 months after planting. Maturity depends on the soil fertility, but maturity can be determined when the leaves dry up. The crop is harvested manually using a hoe. The rhizome is cut from the stem. The fresh weight of the harvest is 13–25 t/ha. [4]


Postharvest Handling
To market as fresh produce, the rhizomes have to be cleaned before packaging. For processing into powder, the rhizomes have to be boiled or steamed until soft. The rhizomes are then sun-dried for 10-15 days or artificially dried at 60ºC before finely ground. About 20–30% of dried C. longa is produced from the fresh rhizomes. [4]
Estimated Cost of Production
The estimated production cost per hectare of C. longa on bris soil is RM12,300. Based on fresh yield at 13,000-25,000 kg/ha, the production cost estimated to be RM0.49-RM0.95/kg. The production cost was estimated based on the cost of current inputs during writing of this article. [4]
Chemical Constituent
C. longa has been reported to contain curcuminoids (e.g. curcumin (diferuloylmethane), demethoxycurcumin, and bisdemethoxycurcumin), various volatile oils(e.g. turmerone, atlantone, and zingiberene) and. other constituents (e.g. sugars, proteins and resins) [5][6]. The curcuminoids give turmeric its bright yellow colour [5]. Turmeric contains 0.3-5.4 percent of curcumin content, which has been shown to be a primary active constituent for medicinal purposes [6].
Plant Part Used
Leaves, roots/rhizomes. [2]
Traditional Use
C. longa is cultivated for its rhizome, primarily as a dye source, as well as a culinary spice. The rhizome is usually boiled, cleaned, and dried, yielding a yellow powder. Rhizomes are also used in Africa and Asia as a cosmetic for body and face. In Asia, turmeric is widely used as an important constituent of curry powder (contains up to 25% turmeric). In Western countries, ground turmeric rhizome is widely used in the food industry, in particular as a colouring agent (E 100 in the European Union) in processed food and sauces. It is also applied as a colouring agent in pharmaceuticals, confectionery and textile dyes, and is a cheaper substitute for the true saffron (Crocus saticus L.). [2]
The young shoots and young rhizomes can be eaten fresh as a spicy vegetable. [2]
The leaves are used in preparing special medicinal bread in Nepal and India. [2]
The C. longa rhizomes are part of numerous traditional compound medicines used as stomachic, stimulant and blood purifier, and to treat liver complaints, biliousness and jaundice. Mixed with warm milk they are used to cure common cold, bronchitis and asthma. Juice from fresh rhizomes is applied against many skin infections, while a decoction is effective against eye infections. [2]
C. longa can also be applied topically in poultices to relieve pain and inflammation. [6] Turmeric also has insecticidal, fungicidal and nematicidal properties. In Madagascar ground rhizomes are mixed with grains to protect them from storage pests. [2]
Preclinical Data
Pharmacology
Pharmacokinetic study
Pharmacokinetic studies in animals have demonstrated that 40-85 percent of an oral dose of curcumin passes through the gastrointestinal tract unchanged, with most of the absorbed flavonoid being metabolized in the intestinal mucosa and liver. Due to its low rate of absorption, curcumin is often formulated with bromelain for increased absorption and enhanced anti-inflammatory effect. [7][8]
Antioxidant activity
Water- and fat-soluble extracts of turmeric and its curcumin component exhibit strong antioxidant activity, comparable to vitamins C and E [9]. Curcumin was shown to be eight times more potent than vitamin E in lipid peroxidation, and three times more powerful than vitamin C in neutralizing free radicals [10][11].
A study of ischemia in the feline heart demonstrated that curcumin pretreatment decreased ischemia-induced changes in the heart. [12]
An in vitro study measuring the effect of curcumin on endothelial heme oxygenase-1, an inducible stress protein, was conducted utilizing bovine aortic endothelial cells. Incubation (18 hours) with curcumin resulted in enhanced cellular resistance to oxidative damage. The antioxidant activity of curcumin could be mediated through antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase. Curcumin has been shown to serve as a Michael acceptor, reacting with glutathione and thioredoxin. [13]
Anti-inflammatory activity
Curcumin works to inhibit the inflammatory activity and synthesis of the enzymes implicated in inflammation, such as cyclooxygenase-2 (COX-2) [14][15][16][17] and lipoxygenase [18][19][20].
It is also involved in inhibition of incorporation of arachadonic acid into membrane lipids, inhibition of prostaglandin E2, leukotriene B4, leukotriene C4, inhibition of secretion of collagenase, elastase, hyaluronidase by macrophages. [21]
It reduces inflammation by lowering histamine levels and possibly by increasing the production of natural cortisone by the adrenal glands. [18]
Oral administration of curcumin in instances of acute inflammation was found to be as effective as cortisone or phenylbutazone, and half as effective in cases of chronic inflammation. [22]
In monkeys, curcumin inhibited neutrophil aggregation associated with inflammation. [23]
A trial was performed on 18 patients with rheumatoid arthritis where all the patients received 1200 mg/d of curcumin for 2 weeks and 300 mg/d of phenylbutazone for another 2 weeks. But the sequence of administration of the two medications was randomly allocated. Compared to baseline, statistically there were significant improvement in morning stiffness, walking time, and joint swelling at the end of both treatment regimens, however the magnitude of the improvement was higher after the phenylbutazone regimen. [24]
Another trial was conducted on 45 postsurgical patients (hernia or hydrocele) where all the patients were randomly assigned to receive curcumin (1200 mg/d), phenylbutazone (300 mg/d), or placebo for 5 days. Four clinical parameters of inflammation and pain were evaluated: spermatic cord edema, spermatic cord tenderness, pain at operative site, and tenderness at operative site. These were evaluated using a semi-quantitative scale of 0, absent; 1, mild; 2, moderate; and 3, severe. Addition of these gave a total intensity scale ranging from 0 to 12. Mean reduction in total intensity score at day 6 for curcumin was 2.38 (standard error [SE] 0.59, p , 0.01), for phenylbutazone was 1.57 (SE: 0.55, p , 0.05), and for placebo was 1.0 (SE: 0.79, not significant [NS]). Curcumin was demonstrated to be efficacious in reducing these parameters of postoperative inflammation. [25]
Anticarcinogenic activity
Animal studies involving rats and mice as well as in vitro studies utilizing human cell lines have demonstrated curcumin’s ability to inhibit carcinogenesis at three stages: Tumour promotion [26], angiogenesis [27] and tumour growth [28]. In two studies of colon and prostate cancer, curcumin inhibited cell proliferation and tumour growth.
Turmeric and curcumin compounds have been found to induce apoptosis in lung and colon tumour cell lines [29][30]. Curcumin inhibited AK-5 tumor growth and induced apoptosis in AK-5 cells [31].
Curcumin’s ability to protect against damage to DNA was demonstrated in a study in a community with a high content of groundwater arsenic [32]. Arsenic is extremely carcinogenic because it causes severe oxidative damage to DNA. Blood samples before curcumin supplementation showed severe DNA damage, with increased levels of free radicals and lipid peroxidation. Three months of curcumin intervention reduced the DNA damage, retarded free radical formation and lipid peroxidation, and raised the level of antioxidant activity. In another study, cigarette smokers receiving turmeric demonstrated a significant reduction in the level of urinary-excreted mutagens – an indication of the ability of the body to rid cancer-causing compounds via detoxification mechanisms. For many reasons, curcumin is emerging as a very important agent in the battle against cancer [33].
Hepatoprotective activity
Turmeric’s hepatoprotective effect is mainly a result of its antioxidant properties as well as its ability to decrease the formation of proinflammatory cytokines. It protects the liver from a number of toxic compounds such as carbon tetrachloride (CCl4) [34][35], galactosamine [36], acetaminophen (paracetamol) [37], and Aspergillus aflatoxin [38]. Sodium curcuminate, a salt of curcumin, also exerts choleretic effects by increasing biliary excretion of bile salts, cholesterol, and bilirubin as well as by increasing bile solubility, therefore, possibly preventing and treating cholelithiasis. Curcumin has choleretic activity that increases bile output and solubility, which may be helpful in treating gallstones [39].
Antimicrobial activity
Antibacterial activity
Both curcumin and the oil fraction suppress growth of several bacteria like Streptococcus, Staphylococcus, Lactobacillus, etc. [40]. The aqueous extract of turmeric rhizomes has antibacterial effects [41]. Curcumin also prevents growth of Helicobacter pylori CagA+ strains in vitro [42].
Antiviral activity
Curcumin acts as an efficient inhibitor of Epstein-Barr virus (EBV) key activator Bam H fragment z left frame 1 (BZLF1) protein transcription in Raji DR-LUC cells. EBV inducers such as 12-0-tetradecanoylphorbol- 13-acetate, sodium butyrate and transforming growth factor-beta increase the level of BZLF1 m-RNA at 12–48 h after treatment in these cells, which is effectively blocked by curcumin. [43]
Curcumin also shows anti-HIV (human immunodeficiency virus) activity by inhibiting the HIV-1 integrase needed for viral replication [44][45]. It also inhibits UV light induced HIV gene expression [46].
Antiprotozoan activity
The ethanol extract of the rhizomes has anti-Entamoeba histolytica activity. Curcumin has anti-Leishmania activity in vitro. [47] Several synthetic derivatives of curcumin have anti-L. amazonensis effect [48]. Anti-Plasmodium falciparum and anti-L. major effects of curcumin have also been reported [49].
Antifungal activity
Ether and chloroform extracts and oil of C. longa have antifungal effects [50][51][52]. Crude ethanol extract also possesses antifungal activity [53]. Turmeric oil is also active against Aspergillus flavus, A. parasiticus, Fusarium moniliforme and Penicillium digitatum [54].
Cardiovascular effects and its effects on lipid metabolism
Curcumin decreases the severity of pathological changes and thus protects from damage caused by myocardial infarction [55]. Curcumin improves Ca2+-transport and its slippage from the cardiac muscle sarcoplasmic reticulum, thereby raising the possibility of pharmacological interventions to correct the defective Ca2+ homeostasis in the cardiac muscle [56]. Curcumin has significant hypocholesteremic effect in hypercholesteremic rats [57]. It protective effects on the cardiovascular system include lowering cholesterol and triglyceride levels, decreasing susceptibility of low density lipoprotein (LDL) to lipid peroxidation [58], and inhibiting platelet aggregation [59]. These effects have been noted even with low doses [58]. Turmeric extract’s effect on cholesterol levels may be due to decreased cholesterol uptake in the intestines and increased conversion of cholesterol to bile acids in the liver [60].
Gastrointestinal effects
It increases mucin secretion in rabbits and may thus act as gastro protectant against irritants [61]. Both antiulcer [62] and ulcerogenic [63][64] effects of curcumin have been reported but detailed studies are still lacking. It also protects from 5-hydroxytryptamine- induced ulceration at 20 mg/kg dose [65][66]. In fact, at higher doses of 50 mg/ kg and 100 mg/kg, it produces ulcers in rats [64]. It also enhances intestinal lipase, sucrase and maltase activity [67].
Anticoagulant effect
Inhibition of platelet aggregation by curcumin constituents is thought to be via potentiation of prostacyclin synthesis and inhibition of thromboxane synthesis. [59]
Curcumin shows anticoagulant activity by inhibiting collagen and adrenaline-induced platelet aggregation in vitro as well as in vivo in rat thoracic aorta. [68]
Toxicity
No documentation.
Clinical Data
Poisonous Management
No documentation.
Line Drawing
References
- The Plant List. Ver1.1. Curcuma longa L. [homepage on the internet]. c2013 [updated 2012 Mar 23; cited 2015 Mar 11]. Available from: http://www.theplantlist.org/tpl/record/kew-235249
- Dahal KR, Idris S. Curcuma longa L. In: de Guzman CC, Siemonsma JS, editors. Plant Resources of South-East Asia No. 13: Spices. Leiden, Netherlands: Backhuys Publisher, 1999; p. 111-116.
- Porcher MH. Multilingual Multiscript Plant Name Database. Sorting Curcuma names. [homepage on the Internet]. Melbourne: The University of Melbourne; c1995-2020 [updated 2006 Jan 29; cited 2015 Mar 11]. Available from: http://www.plantnames.unimelb.edu.au/Sorting/Curcuma.html#longa
- Aminah A, Kunyit (Cucurma longa). In: Musa Y, Muhammad Ghawas M, Mansor P, editors. Penanaman tumbuhan ubatan & beraroma. Serdang, Selangor: MARDI, 2005; p. 43-49.
- Ruby J, Kuttan G , Babu KD, Rajashekharan KN, Kuttan R. Antitumor and oxidant activity of natural curcumnoids. Cancer Lett. 1995;94(1):79–83.
- Leung A. Encyclopedia of common natural ingredients used in food, drugs, and cosmetics. New York: John Wiley, 1980; p. 313-314.
- Wahlstrom B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol. 1978;43(2):86-92.
- Ravindranath V, Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicol. 1980;16(3):259-265.
- Toda S, Miyase T, Arich H, et al. Natural antioxidants. Antioxidative compounds isolated from rhizome of Curcuma longa L. Chem Pharmacol Bull. 1985;33(4):1725-1728.
- Higdon J. Micronutrient Information Center. Curcumin. [homepage on the Internet]. Oregon: Linus Pauling Institute, Oregon State University; c2016 [updated 2005 Nov; cited 2015 Mar 11]. Available from: http://lpi.oregonstate.edu/mic/dietary-factors/phytochemicals/curcumin
- Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of Curcumin. Adv Exp Med Biol. 2007;595:105-125.
- Dikshit M, Rastogi L, Shukla R, Srimal RC. Prevention of ischaemia-induced biochemical changes by curcumin and quinidine in the cat heart. Indian J Med Res. 1995;101:31-35.
- Mortellini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and antiinflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28(8):1303-1312.
- Goel A, Boland CR, Chauhan DP. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary cur- cumin in HT-29 human colon cancer cells. Cancer Lett. 2001;172(2):111–118.
- Plummer SM, Holloway KA, Manson MM, et al. Inhibition of cyclooxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF- kB activation via the NIK/IKK signalling complex. Oncogene. 1999;18(44):6013–6020.
- Ramsewak RS, DeWitt DL, Nair MG. Cytotoxicity, antioxidant and anti-inflammatory activities of curcumins I-III from Curcuma longa. Phytomedicine. 2000;7(4):303–308.
- Zhang F, Altorki NK, Mestre JR, Subbaramaiah K, Dannenberg AJ. Curcumin inhibits cyclooxygenase-2 transcription in bile acid-and phorbol ester treated human gastrointestinal epithelial cells. Carcinogenesis. 1999;20(3):445–451.
- Ammon H, Safayhi H, Mack T, Sabieraj. Mechanism of antiinflammatory actions of curcumin and boswellic acids. J Ethnopharmacol. 1993;38(2-3):113-119.
- Began G, Sudharshan E, Appu Rao AG. Inhibition of lipooxygenase 1 (LOX1) by phosphatidylcholine micelles-bound curcumin. Lipids. 1998;33(12):1223–1228.
- Skrzypczak-Jankun E, McCabe NP, Selman SH, Jankun J. Curcumin inhibits lipoxygenase by binding to its central cavity: Theoretical and X-ray evidence. Int J Mol Med. 2000;6(5):521–526.
- Joe B, Lokesh BR. Effect of curcumin and capsaicin on arachadonic acid metabolism and lysosomal enzyme secretion by rat peritoneal macrophages. Lipids. 1997;32(11):1173–1180.
- Mukhopadhyay A, Basu N, Ghatak N. Anti-inflammatory and irritant activities of curcumin analogues in rats. Agents Actions. 1982;12(4):508–515.
- Srivastava R. Inhibition of neutrophil response by curcumin. Agents Actions 1989;28(3-4):298-303.
- Deodhar SD, Sethi R, Srimal RC. Preliminary studies on antirheumatic activity of curcumin. Ind J Med Res. 1980;71:632–634.
- Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol. 1986;24(12):651–654.
- Kawamori T, Lubet R, Steele VE. Chemopreventative effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59(3):597-601.
- Thaloor D, Singh AK, Sidhu GS, Prasad PV, Kleinman HK, Maheshwari RK. Inhibition of angiogenic differentiation of human umbilical vein endothelial cells by curcumin. Cell Growth Differ. 1998;9(4):305-312.
- Limtrakul P, Lipigorngoson S, Namwong O, Apisariyakul A, Dunn FW. Inhibitory effect of dietary curcumin on skin carcinogenesis in mice. Cancer Lett. 1997;116(2):197–203.
- Radhakrishna Pillai G, Sriastava AS, Hassanein TI, et al. Induction of apoptosis in human lung cancer cells by curcumin. Cancer Lett. 2004;208(2):163-170.
- Samaha HS, Kelloff GJ, Steele, et al. Modulation of apoptosis by sulindac, curcumin, phenylethyl-3-methylcaffeate, and 6-phenylhexyl isothiocyanate: apoptotic index as a biomarker in colon cancer chemoprevention and promotion. Cancer Res. 1997;57(7):1301-1305.
- Khar A, Ali AM, Pardhasaradhi BV, Begum Z, Anjum R. Antitumour activity of curcumin is mediated through the induction of apoptosis in AK-5 tumour cells. FEBS Lett. 1999;445(1):165-168.
- Biswas J, Sinha D, Mukherjee S, et al. Curcumin protects DNA damage in a chronically arsenic-exposed population of West Bengal. Hum Exp Toxicol. 2010;29(6):513-524.
- Shehzad A, Wahid F, Lee YS. Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pham (Weinheim). 2010;343(9):489-499.
- Deshpande UR, Gadre SG, Raste AS, et al. Protective effect of turmeric (Curcuma longa L.) extract on carbon tetrachloride-induced liver damage in rats. Indian J Exp Biol. 1998;36(6):573-577.
- Park EJ, Jeon CH, Ko G, et al. Protective effect of curcumin in rat liver injury induced by carbon tetrachloride. J Pharm Pharmacol. 2000;52(4):437-440.
- Kiso Y, Suzuki Y, Watanabe N, et al. Antihepatotoxic principles of Curcuma longa rhizomes. Planta Med. 1983;49(3):185-187.
- Donatus IA, Sardjoko, Vermeulen NP. Cytotoxic and cytoprotective activities of curcumin. Effects on paracetamol induced cytotoxicity, lipid peroxidation and glutathione depletion in rat hepatocytes. Biochem Pharmacol. 1990;39(12):1869-1875.
- Soni KB, Rajan A, Kuttan R. Reversal of aflatoxin induced liver damage by turmeric and curcumin. Cancer Lett. 1992;66(2):115-121.
- Ramprasad C, Sirsi M. Curcuma longa and bile secretion. Quantitative changes in the bile constituents induced by sodium curcuminate. J Sci Ind Res. 1957;16(C):108–110
- Bhavani Shankar TN, Sreenivasa Murthy V. Effect of turmeric (Curcuma longa) fractions on the growth of some intestinal and pathogenic bacteria in vitro. Indian J Exp Biol. 1979;17:1363-1366.
- Kumar S, Narain U, Tripathi S, Misra K. Synthesis of curcumin bioconjugates and study of their antibacterial activities against betalactamase-producing microorganisms. Bioconjug Chem. 2001;12(4):464-469.
- Mahady GB, Pendland SL, Yun G, Lu ZZ. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, of group 1 carcinogen. Anticancer Res. 2002;22(6C):4179-4181.
- Hergenhahn M, Soto U, Weninger A, et al. The chemopreventive compound curcumin is an efficient inhibitor of Epstein-Barr virus BLZF1 transcription in Raji DR-LUC cells. Mol Carcinogen. 2002;33(3):137-145.
- Mazumdar A, Raghavan K, Weinstein J, Kohn KW, Pommer Y. Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem Pharmacol. 1995;49(8):1165-1170.
- De Clercq E. Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med Res Rev. 2000;20(5):323-349.
- Taher MM, Lammering G, Hershey C, Valerie K. Curcumin inhibits ultraviolet light induced human immunodeficiency virus gene expression. Mol Cell Biochem. 2003;254(1-2):289-297.
- Koide T, Nose M, Ogihara Y, Yabu Y, Ohta N. Leishmanicidal effect of curcumin in vitro. Biol Pharm Bull. 2002;25(1):131-133.
- Gomes Dde C, Alegrio LV, de Lima ME, Leon LL, Araujo CA. Synthetic derivatives of curcumin and their activity against Leishmania amazonensis. Arzneimittelforschung. 2002;52(2):120-124.
- Rasmussen HB, Christensen SB, Kuist LP, Karazmi A. A simple and effective separation of the curcumins, the antiprotozoal constituents of Curcuma longa. Planta Med. 2000;66(4):396-398.
- Banerjee A, Nigam SS. Antimicrobial efficacy of the essential oil of Curcuma longa. Indian J Med Res. 1978;68:864-866.
- Misra SK, Sahu KC. Screening of some indigenous plants for antifungal activity against dermatophytes. Indian J Pharmacol. 1977;9(4):269-272.
- Apisariyakul A, Vanittanakomm N, Buddhasukh D. Antifungal activity of turmeric oil extracted from Curcuma longa (Zingiberaceae). J Ethnopharmacol. 1995;49(3):163-169.
- Wuthi-Udomler M, Grisanapan W, Luanratana O, Caichompoo W. Antifungal activity of Curcuma longa grown in Thailand. Southeast Asian J Trop Med Public Health. 2000;31 Suppl 1:178-182.
- Jayaprakasha GK, Negi PS, Anandharamakrishnan C, Sakariah KK. Chemical composition of turmeric oil – a byproduct from turmeric oleorsin industry and its inhibitory activity against different fungi. Z. Naturforsch. C. 2001;56(1-2):40-44.
- Nirmala C, Puvanakrishnan R. Protective role of curcumin against isoproterenol-induced myocardial infarction in rats. Mol Cell Biochem. 1996;159(2):85-93.
- Sumbilla C, Lewis D, Hammerschmidt T, Inesi G. The slippage of the Ca2+ pump and its control by anions and curcumin in skeletal and cardiac sarcoplasmic reticulum. Biol Chem. 2002;277(16):13900-13906.
- Patil TN, Srinivasan M. Hypocholesteremic effect of curcumin in induced-hypercholesteremic rats. Indian J Exp Biol. 1971;9(2):167-169.
- Ramirez-Tortosa MC, Mesa MD, Aguilera MC, et al. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis. 1999;147(2):371-378.
- Srivastava R, Puri V, Srimal RC, Dhawan BN. Effect of curcumin on platelet aggregation and vascular prostacyclin synthesis. Arzneimittelforschung. 1986;36(4):715-717.
- Ramprasad C, Sirsi M. Curcuma longa and bile secretion. Quantitative changes in the bile constituents induced by sodium curcuminate. J Sci Indust Res. 1957;16:108-110.
- Lee C.J, Lee JH, Seok JH, et al. Effects of baicalein, berberine, curcumin and hespiridin on mucin release from airway goblet cells. Planta Med. 2003;69(6):523-526.
- Sinha M, Mukherjee BP, Mukherjee B, Sikdar S, Dasgupta SR. Study of the mechanism of action of curcumin; An antiulcer agent. Indian J Pharmacol. 1975;7:98.
- Prasad DN, Gupta B, Srivastava RK, Satyavati GV. Studies on ulcerogenic activity of curcumin. Indian J Physiol Pharmacol. 1976;20:92.
- Gupta B, Kulshrestha VK, Srivastava RK, Prasad DN. Mechanisms of curcumin induced gastric ulcer in rats. Indian J Med Res. 1980;71:806-814.
- Dasgupta SR, Sinha M, Sahana CC, Mukherjee BP. A study of the effect of an extract of Curcuma longa Linn. on experimental gastric ulcers in animals. Indian J Pharmacol. 1969;1(3):49- 54.
- Sinha M, Mukherjee BP, Mukherjee B, Dasgupta SR. Study on the 5- hydroxytryptamine contents in guinea pig stomach with relation to phenylbutazone induced gastric ulcers and the effects of curcumin thereon. Indian J Pharmacol. 1974;6(2):87-96.
- Platel K, Srinivasan K. Influence of dietary spices or their active principles on digestive enzymes of small intestinal mucosa in rats. Int J Food Sci Nutr. 1996;47(1):55-59.
- Srivastava R, Dikshit M, Srimal RC, Dhawan BN. Antithrombotic effect of curcumin. Thromb. Res. 1985;40(3):413-417.
- Lal B, Kapoor AK, Asthana OP, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999 Jun;13(4):318-322.
- Allegri P, Mastromarino A, Neri P. Management of chronic anterior uveitis relapses: efficacy of oral phospholipidic curcumin treatment. Long-term follow-up. Clin Ophthalmol. 2010;4:1201-1206.
- Kanai M, Yoshimura K, Asada M, et al. A phase I/II study of gemcitabine based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol. 2011;68(1):157-164.
- Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar-Sela G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer. 2010;62(8):1137-1141.
- He ZY, Shi CB, Wen H, Li FL, Wang BL, Wang J. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest. 2011;29(3):208-213.
- Carroll RE, Benya RV, Turgeon DK, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila). 2011;4(3):354-364.
- Golombick T, Diamond TH, Manoharan A, Ramakrishna R. Monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, and curcumin: A randomized, double-blind placebo-controlled cross-over 4g study and an open-label 8g extension study. Am J Hematol. 2012;87(5):455-460.
- Sugawara J, Akazawa N, Miyaki A, Choi Y, Tanabe Y, Imai T, Maeda S. Effect of endurance exercise training and curcumin intake on central arterial hemodynamics in postmenopausal women: Pilot study. Am J Hypertens. 2012;25(6):651-656.
- Belcaro G, Cesarone MR, Dugall M, et al. Efficacy and safety of Meriva®, a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern Med Rev. 2010;15(4):337-344.
- Usharani P, Mateen AA, Naidu MU, Raju YS, Chandra N. Effect of NCB-02, atorvastatin and placebo on endothelial function, oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus: A randomized, parallel-group, placebo-controlled, 8-week study. Drugs R D. 2008;9(4):243-250.
- Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: A pilot study. Dig Dis Sci. 2005;50(11):2191-2193.
- Prucksunand C, Indrasukhsri B, Leethochawalit M, Hungspreugs K. Phase II clinical trial on effect of the long turmeric (Curcuma longa Linn) on healing of peptic ulcer. Southeast Asian J Trop Med Public Health. 2001;32(1):208-215.
- Bundy R, Walker AF, Middleton RW, Booth J. Turmeric extract may improve irritable bowel syndrome symptomology in otherwise healthy adults: A pilot study. J Altern Complement Med. 2004;10(6):1015-1018.
- Di Mario F, Cavallaro LG, Nouvenne A, et al. A curcumin-based 1-week triple therapy for eradication of Helicobacter pylori infection: Something to learn from failure?. Helicobacter. 2007;12(3):238-243.
- Rasyid A, Lelo A. The effect of curcumin and placebo on human gall-bladder function: An ultrasound study. Aliment Pharmacol Ther. 1999;13(2):245–249.
- Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–2900.
- James JS. Curcumin trial results: Antiviral effect reported. AIDS Treat News. 1996;242:1-2.
- Pavithra BH, Prakash N, Jayakumar K. Modification of pharmacokinetics of norfloxacin following oral administration of curcumin in rabbits. J Vet Sci. 2009;10(4):293-297.
- Somasundaram S, Edmund NA, Moore DT, et al. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002;62(13):3868-3875.
- Shah BH, Nawaz Z, Pertani SA. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-actiating factor and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling. Biochem Pharmacol. 1999;58(7):1167-1172.
- PDR for herbal medicines, 2nd ed. Montvale, New Jersey: Medical Economics Company, 2000; p. 776.

