Kunyit Rhizome
Curcuma longa L.
Zingiberaceae
DEFINITION
Kunyit rhizome consists of dried rhizome powder of Curcuma longa L. (Zingiberaceae) [ 1 ].
SYNONYM
Amomum curcuma Jacq., Curcuma brog Valeton, Curcuma domestica Valeton, Curcuma euchroma Valeton, Curcuma ochrorhiza Valeton, Curcuma soloensis Valeton, Curcuma tinctoria Guibourt, Stissera curcuma Giseke, Stissera curcuma Raeusch [ 2 ].
VERNACULAR NAMES
Turmeric (English); kunyit (Malay); huang jiang, jiang huang, yu jin, yu jin xiang gen (Mandarin); manjal (Tamil) [ 1 , 3 , 4 , 5 ].
CHARACTER
IDENTIFICATION
Plant Morphology
C. longa is a perennial herbaceous plant which bears thick fleshy rhizomes, without stem or perhaps pseudo stem formed by the leaf sheaths; height of plant is up to 1 m. Leaf-shoots bearing a group of leaves surrounded by bladeless sheaths, leaves alternately arranged, usually 70 cm long, oblong, uniformly green, lanceolate, broad, with acute and caudate apex with tapering base; no ligule or sometimes very small; sheath near ligules has ciliated edges; petiole is blade like. Inflorescence apical on the leaf shoot of 10-20 cm long, 5-7 cm wide; bracts white or white streaked with green grading to light green, adnate for less than half their length, elliptic-lanceolate, length 5-6 cm. Flowers 5-5.5 cm long, petals white, staminoid and lip creamy-white with yellow median band on the lip. Rhizome bright yellow to orange in colour and horny fracture; primary rhizome is ovate, oblong or pear-shaped round (half as broad as long); secondary rhizome is short-branched long, 2-5 cm long, 1-1.8 cm thick; externally it has root scars and annulations of leaf base scars [ 1 , 9 ].
Microscopy
Powdered material consists of abundant parenchyma cells are thin-walled and round to oval in shape. Starch granules are less abundant, mostly simple, sometimes flat, oval or irregular in shape. Sometimes hilum and faint striations are present. Epidermal cells are thin-walled. Cork cells are light brown in colour and very abundant, quite large, polygonal in shape and thin-walled. Unicellular trichome is very scarce but easily identified. Vessels are fairly abundant, mostly are thickly reticulated. The pits are oval in shape and well arranged. Spirally thickened vessels are also fairly numerous with a few reticulate and annular thickening. Oil droplets and calcium oxalate crystals are rarely seen [ 1 , 9 ].
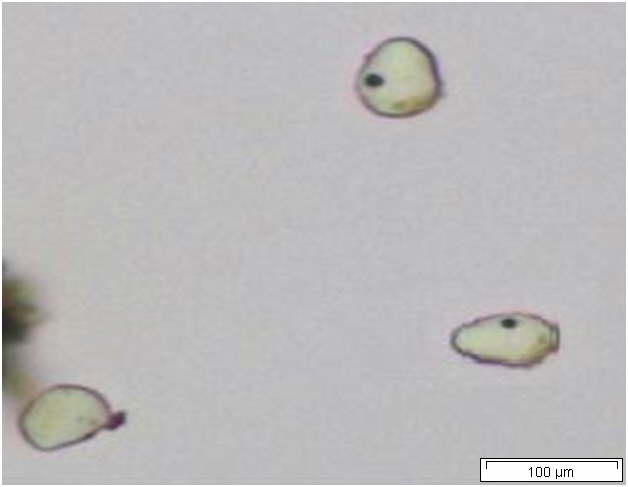
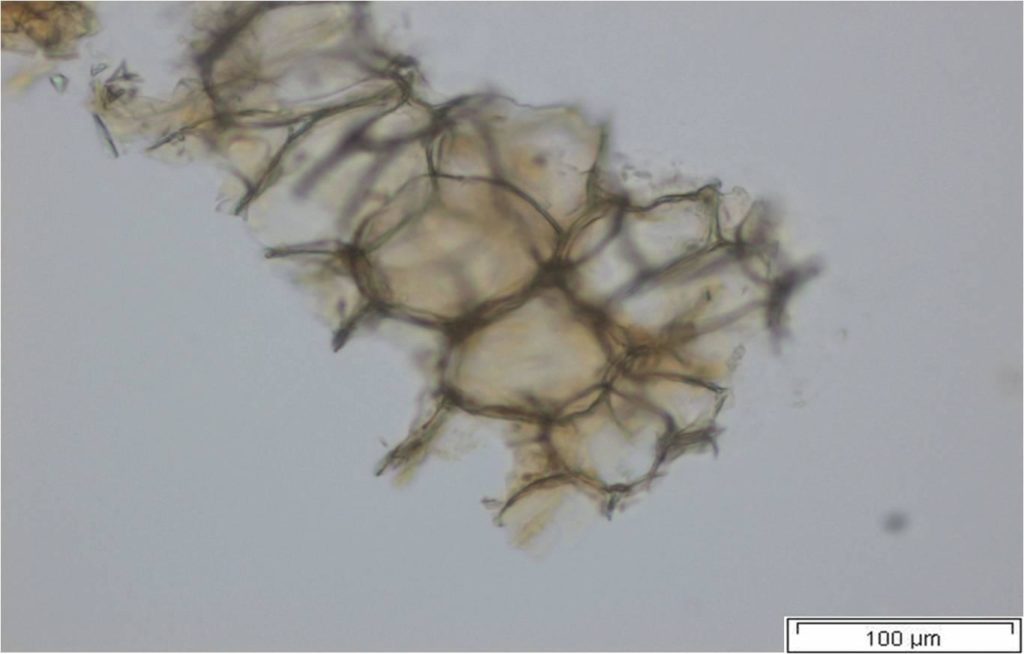
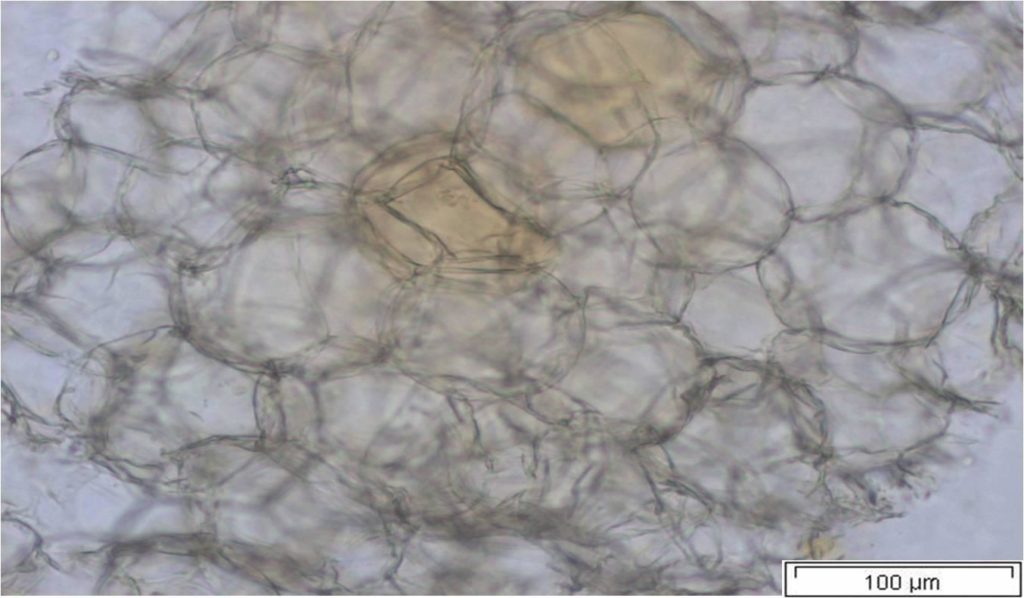


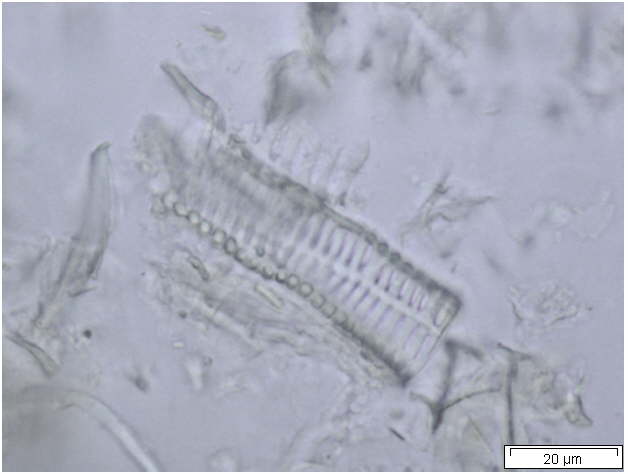

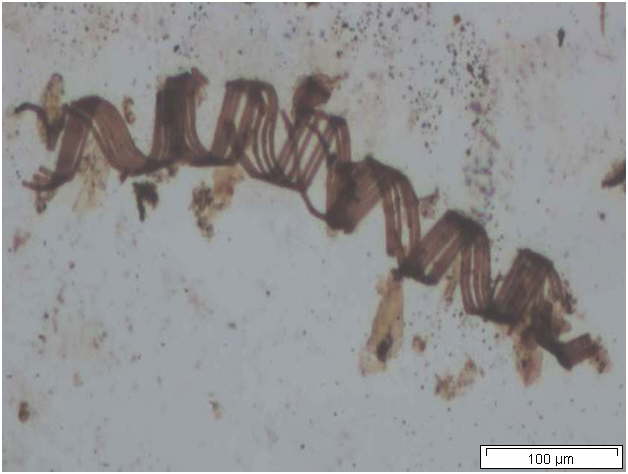
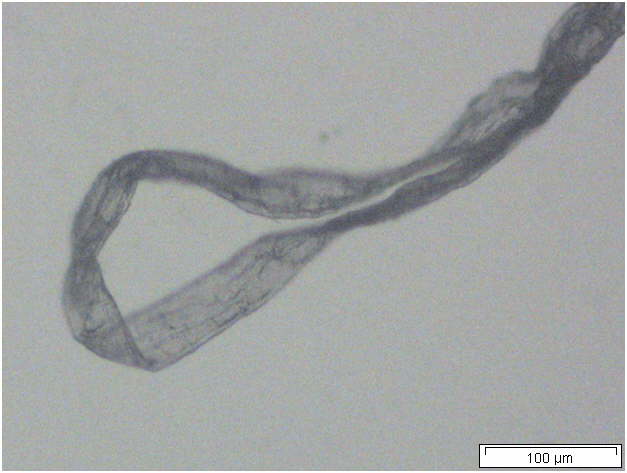
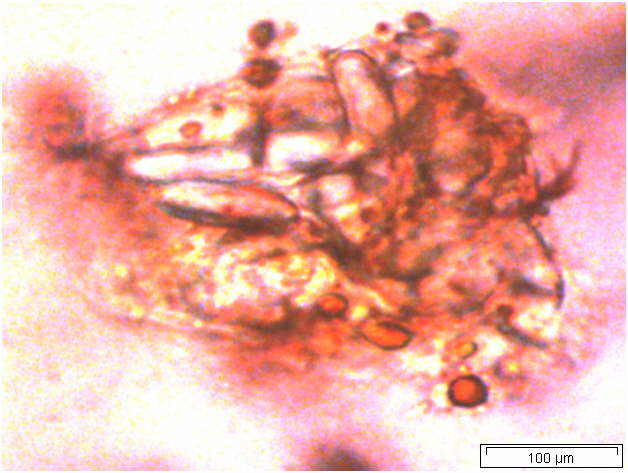

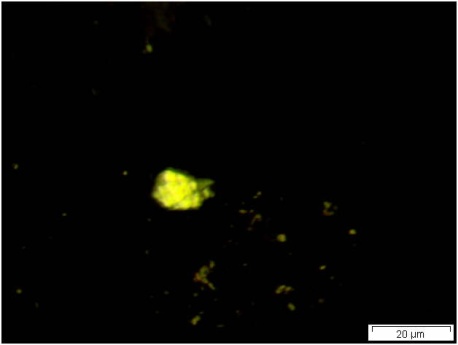
Figure 2 : Microscopic characters of C. longa rhizome powder. (a) Starch grains with hilum; (b) epidermal cells; (c) parenchyma cells; (d) lignified cork cells; (e) trichome; (f) reticulate vessel; (g) bordered pitted vessel; (h) spiral vessel; (i) fibre; (j) oil globules; (k-l) rosette calcium oxalate crystal. [Scale bars: a-e, g-j = 100 µm; f, k, l = 20 µm]
Colour Tests
Observed colour of solution after treatment with various reagents:
| KOH (5%) | Dark orange |
| NaOH (5%) | Dark orange |
Thin Layer Chromatography (TLC)
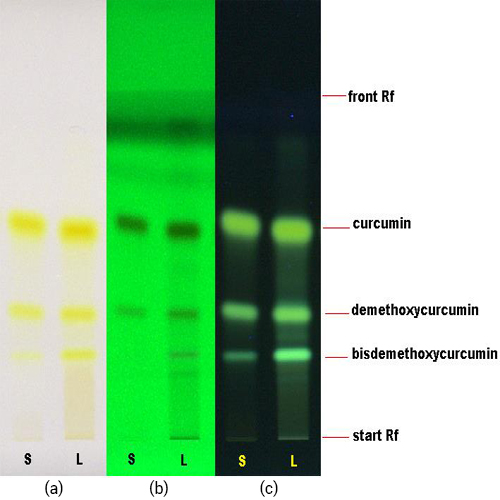
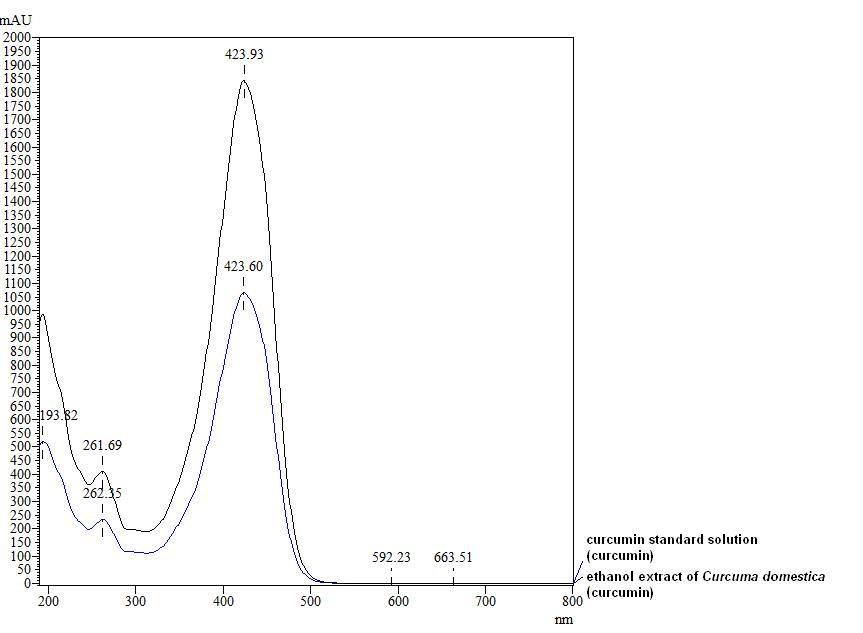
Figure 3 : TLC profiles of curcumin (S) and ethanol extract of C. longa dried rhizome powder (L) observed under (a) visible light, (b) UV at 254 nm and (c) UV at 366 nm.
| Test Solutions | Weigh 5 g of C. longa dried rhizome powder in a 250 mL round bottom flask and add 100 mL of ethanol (95%). Reflux the mixture for 1 hour, cool and filter. Transfer 20 mL of the filtrate in an evaporating dish and evaporate to dryness over a boiling water bath. Reconstitute the residue with 20 mL chloroform and filter. Use the filtrate as the test solution. |
| Standard solution | Dissolve 5 mg curcumin standard [CAS no.: 458-37-7, Sigma Aldrich] in 5 mL of chloroform to give 1 mg/mL solution. |
| Stationary Phase | HPTLC Silica gel 60 F254, 10 x 10 cm |
| Mobile phase | Dichloromethane-methanol, 25 : 1 (v/v) |
| Application |
|
| Development distance | 8 cm; manual chamber |
| Drying | Air drying |
| Detection |
|
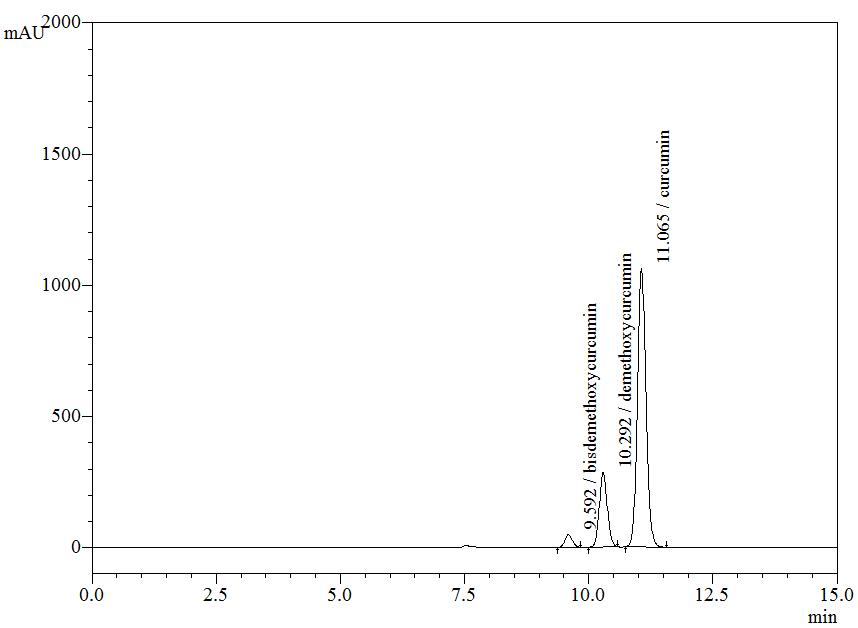
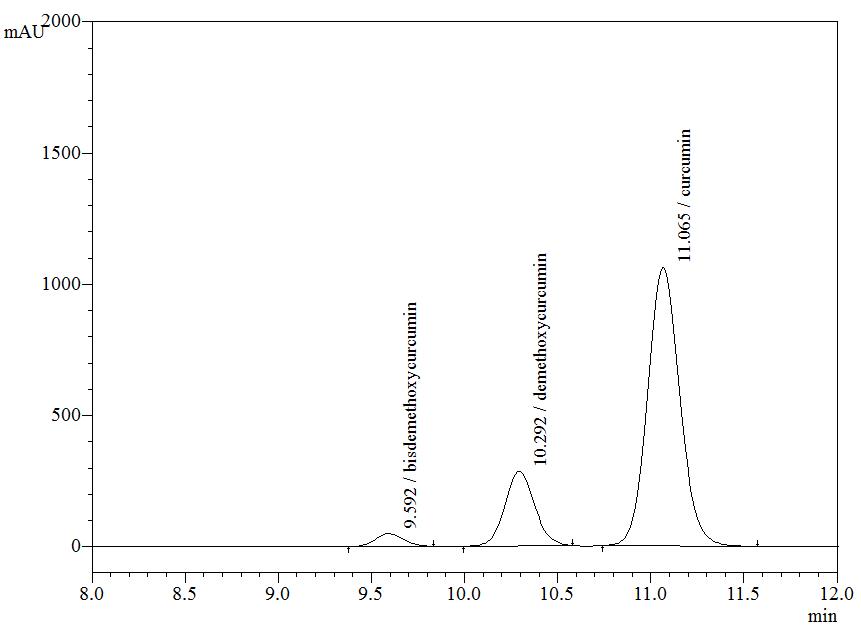
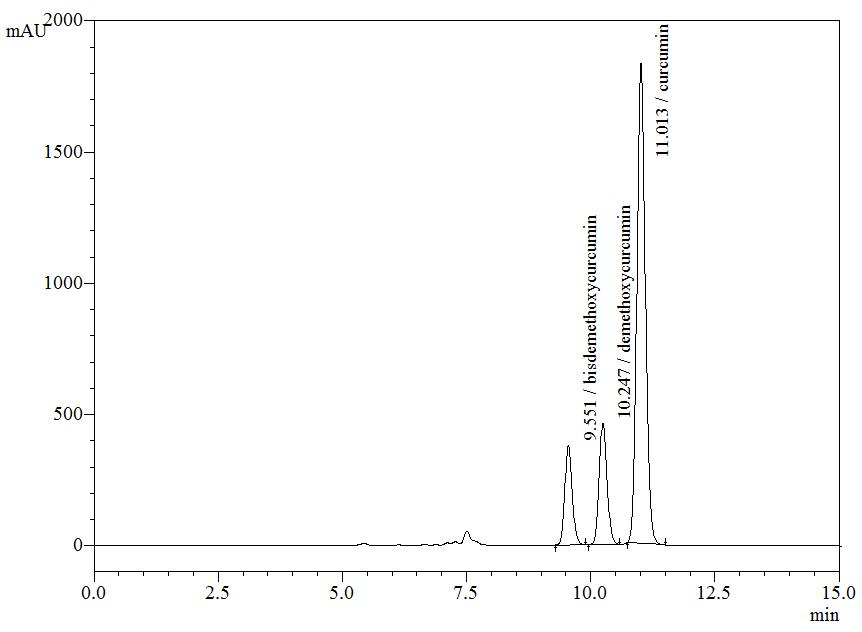
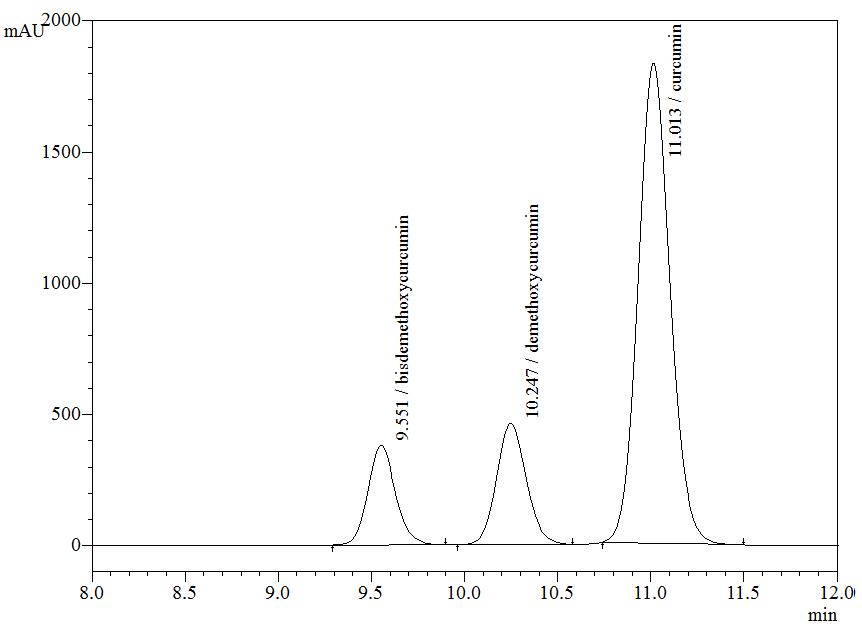
High Performance Liquid Chromatography (HPLC)
| Test solution | Weigh about 5 g of C. longa dried rhizome powder in a 250 mL round bottom flask and add 100 mL of ethanol. Reflux the mixture for 30 minutes at 60°C. Filter the solution and evaporate to dryness using rotary evaporator. Weigh about 25 mg of dried extract and dissolve in 10 mL of methanol. Filter the mixture solution through a 0.45 μm syringe filter and inject the filtrate into the HPLC column. |
| Standard solution | Dissolve 5 mg of curcumin standard [CAS no.: 458-37-7, Sigma Aldrich] in 5 mL of methanol to produce 1.0 mg/mL solution. |
| Chromatographic system |
Detector: UV 425 nm |
| Mobile Phase (Isocratic mode) | Acetonitrile-orthophosphoric acid (0.1%) in water (60 : 40 v/v). Run time (min): 15 |
| System suitability requirement |
Perform at least five replicate injections of the curcumin standard (1.0 mg/mL). The requirements of the system suitability parameters are as follow:
|
| Acceptance criteria |
|
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 8% |
| Acid-insoluble ash | Not more than 1% |
| Loss on Drying |
| Not more than 12% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 18% |
| Cold method | Not less than 14% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 16% |
| Cold method | Not less than 10% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Water extract of C. longa rhizome has been found to contain polysaccharides (e.g. ukonans A-D) [ 10 , 11 ].
Methanol extract of C. longa rhizome has been found to contain sesquiterpenes (e.g. germacron-13-al, 4-hydroxybisabola-2,10-dien-9-one, 4-methoxy-5-hydroxy-bisabola-2,10-dien-9-one, 2,5-dihydroxybisabola-3,10-dien, procurcumadiol, curcumenone, dehydrocurdione, 4S‘,5S-germacrone-4,5-epoxide, curcumenol, isoprocurcumenol, zedoarondiol, procurcumenol, epiprocurcumenol, bisabola-3,10-dien-2-one, bisacumol, bisacurone, α-turmerone and 4,5-dihydroxybisabola-2,10-dien), diarylpentanoids (e.g. 1,5-bis (4-hydroxy-3-methoxyphenyl)-penta-(1E,4E)-1,4-dien-3-one and 1-(4-hydroxy-3-methoxyphenyl)-5-(4-hydroxyphenyl)-penta-(1E,4E)-1,4-dien-3-one), diarylheptanoids (e.g. curcumin, demethoxycurcumin, bisdemethoxycurcumin and 5′-methoxycurcumin) and curcumin analogues(e.g. bis-(ρ-hydroxycinnamoyl)-methane, feroloyl, 4-hydroxycinnamoyl-methane and 4-hydroxycinnamoyl-(feruloyl)-methane) [ 12 , 13 , 14 , 15 , 16 , 17 , 18 ].
Acetone extract of C. longa rhizome had diarylheptanoids (e.g. 1-(4-hydroxy-3-methoxyphenyl)-7-(3,4-hydroxyphenyl)-1,6-heptadiene-3,5-dione and 1,7-bis(4-hydroxyphenyl)-1,4,6-heptatrien-3-one) [ 19 ]
Chloroform extract of C. longa rhizome has been found to contain sesquiterpene ketones (e.g. turmeronol A and B) [ 20 ]. Ether extract of C. longa rhizome has been found to contain curcuminoids (e.g. feruloyl-ρ-coumaroylmethane, di-ρ-coumaroylmethane and di-feruloylmethane) [ 21 ].
Essential oil of C. longa rhizome has been found to contain monoterpenoids (e.g. ρ-cymene, myrcene, α-thujene, d-3-carene, α-phellandrene, β-phellandrene, α-pinene, β-pinene, trans-ocimene, terpinolene, thymol, geranyl acetate, α-terpineol, carvacrol, 1,8-cineol, ρ-cymen-8-ol, car-2-ene, limonene, cis-ocimene, iso-artemisia ketone, borneol, terpineol, α-terpinene, isoborneol, β-ocimene, cis-carveol, geraniol, neral, linalool, camphor, carvone and ρ-methylacetophenone), sesquiterpenoids (e.g. β-farnesene, ar-turmerone, turmerone, α-turmerone, curcuphenol, α-humulene, 1-epi-cubenol, cubenol, 14-hydroxy-α-humulene, nerolidol, curzerene, curzerenone, epi-curzerenone and humulene epoxide II), bisabolane-type sesquiterpenoids(e.g. β-bisabolene, g-bisabolene, 6S,7R-bisabolene, ar-curcumene, β-curcumene, g-curcumene, g-curcumene-15-al, β-sesquiphellandrene, β-tumerone, ar-turmerol, dehydrocurcumene, zingiberene, sesquiphellandrene, dihydro-ar-turmerone, β-bisabolene-12-ol, dehydrozingerone, β-bisabolol, α-bisabolol, bisabolone, (E)-α-atlantone, (Z)-g-atlantone, (E)-g-atlantone and (Z)-α-atlantone), guaiane-type sesquiterpenoids (e.g. curcumenol and α-curcumenol), elemane-type sesquiterpenoids (e.g. β-elemene, δ-elemene and g-elemene), germacrane-type sesquiterpenoids (e.g. germacrene-D, geramacrene-B and germacrone), bergamotene-type sesquiterpenoid (e.g. α–cis-bergamotene), caryophyllane-type sesquiterpenoids (e.g. caryophyllene oxide, β-caryophyllene, caryophyllene and 14-hydroxy-9-epi-(E)-caryophyllene), sesquisabinane-type sesquiterpenoids (e.g. cis-sesquisabinene hydrate and trans-sesquisabinene hydrate), selinene-type sesquiterpenoid (e.g. α-selinene), and others (e.g. g-terpene, geranyl isovalerate, (Z)-dihydromyrcene-1,6-diol, 10-epi-g-eudesmol, g-eudesmol, hinesol, β-eudesmol, zerumbone, α-amyl cinnamyl acetate, g-eudesmol acetate, α-eudesmol acetate, 8-cedren-14-ol-acetate, ρ-cyamenene, sabinene, 1-hexen-3-ol, trans–p-menth-2-en-1-ol, teroinen-4-ol, myrtenol, undecanone, thymol acetate, α-longefoline, α-cadinene, guaiene, α-patchouline, α-muurolene, elimicin, geranyl butyrate, virdiflorol, T-cadinol, linalyl 2-methyl butyrate, curdione, geranyl hexanoat, furanodienone, n-heptyl salicylate, 2-octanol, myrtenal, cis-sabinol, sabinyl acetate, tetradecane and cinnamyl cinnamate) [ 14 , 16 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
The rhizome is traditionally used as stomachic, stimulant, carminative, hematic or styptic in all kinds of hemorrhages and a remedy for a certain type of jaundice and other liver troubles. It is applied externally to minor wounds and certain skin eruptions, also as a maturative. Its decoction relieves burning sensation of eye disease. The rhizome is considered to be very good for irregular mestruation, promoting circulation, dissolving blood clots. It is prescribed as a remedy for abdominal, chest and back pains, diarrhea, rheumatism, anti-spasmodic in diarrhoea and dysentery, and to relieve cough and tuberculosis [ 32 , 33 ].
Biological and pharmacological activities supported by experimental data
Anti-oxidant activity
Two phenolic compounds (1,5-bis(4-hydroxy-3-methoxyphenyl)-(1E,4E)-1,4-pentadien-3-one and 1-(4-hydroxy-3-methoxyphenyl)-5-(4-hydroxyphenyl)-(1E,4E)-1,4-pentadien-3-one) isolated from methanol extract of C. longa rhizome inhibited autoxidation of linoleic acid in the ethanol-water system using thiocyanate method [ 13 ].
Methanol extract and essential oil of C. longa rhizome inhibited copper-mediated oxidation of human low-density lipoprotein (LDL) with IC50 values of 0.31 and 7.8 μg/mL, respectively compared to probucol (IC50 0.30 μg/mL). Curcumin, demethoxycurcumin and bisdemethoxycurcumin isolated from methanol extract showed anti-oxidant activity with IC50 values ranging from 0.15 to 0.33 μmol/L while the major components of essential oil (ar-turmerone and zerumbone) showed IC50 values of 10.18 and 24.90 μmol/L, respectively, compared to probucol (IC50 0.57 μmol/L) [ 14 ].
Curcumin, monodemethoxycurcumin and bisdemethoxycurcumin (100 μg/mL) isolated from ethyl acetate extract of C. longa rhizome showed anti-oxidant activity with 58, 40 and 22% inhibition of liposome peroxidation, respectively, compared to tert-butylhydroquinone (1.66 µg/mL: 90% inhibition), butylated hydroxyanisole (BHA) (1.80 µg/mL: 91% inhibition), butylated hydroxytoluene (BHT) (2.20 µg/mL: 81%) and vitamin E (4.31 µg/mL: 7%) in model liposome oxidation using fluorescence spectroscopy [ 15 ].
Curcumin, demethoxycurcumin and bisdemethoxycurcumin isolated from ethyl acetate extract of C. longa rhizome showed 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity with inhibition concentration at 50% growth (IC50) of 2.8, 39.2 and 308.7 μM, respectively, compared to ascorbic acid (IC50 22.5 μM) and resveratrol (IC50 25.0 μM) [ 34 ].
Antimicrobial activity
Essential oil from C. longa rhizome containing 49.1% ar-tumerone inhibited Staphylococcus aureus with minimum inhibitory concentration (MIC) of 1.95 μL/L, Candida albicans (MIC 5.5 μL/L) and Aspergillus niger (MIC 6.7 μL/L) [ 23 ]
Antifungal activity
Ethanol extract of C. longa rhizome inhibited the growth of Trichophyton longifusus (65% inhibition) compared to miconazole (MIC 70 µg/mL) using agar tube dilution method [ 35 ].
Antivenom activity
Ar-turmerone isolated from C. longa rhizome showed potent antivenom against snakebite based on the neutralisation of the hemorrhagic activity and the lethal effect of venom in mice. Ar-turmerone (30-70 µg) and Bothrops jararaca venom (10 µg) were administered intracutaneously into shaved back skin of mice and showed to be capable of abolishing the hemorraghic activity of B. jararaca venom in the profound layer of the corium, above the muscular layer compared to negative control. Lethal activity was determined by intraperitoneal administration of ar-turmerone (30–70 µg) and Crotalus durissus terrificus venom (12.5 µg) to Swiss albino mice (20-22 g). After for 48 hours, about 70% of lethal activity was observed compared to negative control. The results suggest that ar-turmerone may act as an enzymatic inhibitor of venom enzymes with proteolytic and hemorrhagic activities or it may possess a direct antitoxin action or chemical inactivation [ 36 ].
Anti-inflammatory activity
Phytochemical compounds (5-bis(4-hydroxy-3-methoxyphenyl)-(1E,4E)-1,4-pentadien-3-one, curcumin, demethoxycurcumin, bisdemethoxycurcumin and 5’-methoxycurcumin) isolated from methanol extract of C. longa rhizome (0.6 μmol in acetone) was applied topically to the inner part of the left and right ear of male Jcl:ICR mice (aged six weeks) after 30 minutes induction of mouse ear edema using 12-O-tetradecanoylphorbol-13-acetate (TPA) (2 μg in acetone). After 6.5 hour, the compounds were found to significantly (p < 0.05) inhibited edema (52-80%) of mouse ear compared to vehicle (acetone) as the negative control [ 13 ].
Phytochemical compounds (curcumin, monodemethoxycurcumin and bisdemethoxycurcumin) (125 μg/mL) inhibited cyclo-oxygenase-1 (COX-1) enzyme from ram seminal vesicles in vitro in the range of 32.0 to 39.2% compared to aspirin (180 μg/mL; 41.13%), ibuprofen (2.06 μg/mL; 44.26%) and naproxen (2.52 μg/mL; 51.96%) and COX-2 enzyme preparation from insect cell lysate in vitro in the range of 58.9 to 89.7% compared to aspirin (180 μg/mL; 40%), ibuprofen (2.06 μg/mL; 34.26%) and naproxen (2.52 μg/mL; 29.96%) [ 15 ].
A review of anti-inflammatory activity of curcumin from C. longa rhizome revealed that it inhibited phospholipases A2, C and D, lipooxygenase, COX-2, leukotrienes, thromboxane, prostaglandins, nitric oxide, collagenase, elastase, hyaluronidase, monocyte chemoattractant protein-1, interferon-inducible protein, tumor necrosis factor, and interleukin-12 as well as platelet-aggregating factor and platelet aggregation [ 37 ].
Anti-arthritic activity
Crude hexane extract and refined essential oil from hexane extract of C. longa rhizome (56 mg equivalents of refined essential oil/kg/day) were administered intraperitoneally to female Lewis rats (110 g) with streptococcal cell wall (SCW)-induced arthritis. The treatment began 4 days prior to SCW injection and continued daily until 10 days after SCW injection, at which time dosing frequency decreased to 3-5 days per week. Joint swelling was dramatically (p < 0.05) inhibited (90-100% inhibition) compared to vehicle control. However, only moderate anti-arthritic activity was observed with oral administration of crude hexane extract (normalized to 560 mg refined essential oil/kg/day) with 21% inhibition compared to vehicle control [ 38 ].
Crude hexane extract of C. longa rhizome was also administered intraperitoneally (56 mg equivalents of refined essential oil/kg/day) or orally (normalized to 560 mg refined essential oil/kg/day) to female Lewis rats (110 g) with SCW-induced arthritis 3 days after SCW injection and continued daily until 10 days after SCW injection, at which time dosing frequency decreased to 3-5 days per week. Joint swelling was significantly (p < 0.05) inhibited (64% inhibition) compared to vehicle control. However, only moderate anti-arthritic activity was observed with oral administration of crude hexane extract (normalized to 560 mg refined essential oil/kg/d) with 21% inhibition compared to vehicle control [ 38 ].
Anticancer activity
Acetone-methanol (9:11) extract of C. longa rhizome (5%) and curcumin isolated from C. longa rhizome (0.8%) were topically applied to male Swiss albino mice (18-22 g) five minutes before 7,12-dimethylbenz(α)anthracene (DMBA) as the initiator to induce skin carcinogenesis followed by application of croton oil as the promoter of skin carcinogenesis two weeks later. The extract and curcumin significantly (p < 0.05) decreased mean number of papillomas (extract: 1.00/mouse and curcumin: 1.10/mouse) compared to DMBA-treated control (2.37/mouse). In another experiment, the extract and curcumin were applied twice weekly after two weeks of DMBA application and during the four weeks after croton oil application. Mean number of papillomas produced was much less (extract: 0.55/mouse and curcumin: 0.66/mouse) compared to DMBA-treated control (2.87/mouse). For both experiments, the papillomas appeared only at week 10 in extract- and curcumin-treated mice compared to DMBA-treated control (week eight). The study indicated that both extract and curcumin gave the best effects during the skin carcinogenesis promotion period [ 39 ].
Curcumin isolated from C. longa rhizome (2 mg) was administered subcutaneously to 20-methylcholanthrene-induced sarcomas of male Swiss albino mice (18-22 g). After 15 weeks, only 50% of curcumin-treated mice had invasive sarcomas compared to 20-methylcholanthrene-treated group (100%) [ 39 ].
Protection against testicular oxidative damage
Curcumin isolated from C. longa rhizome (200 mg/kg/day) was administered orally to male Wistar rats (180 ± 11 g) for seven days followed by daily administration of both curcumin and testicular damage inducer, di-n-butylphthalate (DBP), for the next nine days. The phytochemical compound significantly (p < 0.05) increased final body weight of animals (243 g) and serum testosterone (2.28 nmol/L) compared to DBP-treated rats (216 g and 1.41 nmol/L, respectively). Curcumin increased activities of catalase (1.50 units/mg protein), glutathione (179 μg/g tissue) and glucose-6-phosphate dehydrogenase (0.55 nmol/min/mg protein) in damage-induced testes compared to DBP-treated rats (1.10 units/mg protein, 98 μg/g tissue, 0.35 nmol/min/mg protein, respectively); but decreased the gamma-glutamyl transferase (10.11 U/L) and malondialdehyde (35.12 μmol/g tissue) activities compared DBP-treated rats (14.35 U/L and 71.74 μmol/g tissue, respectively). The compound also increased gamma-glutamyl transferase (18.97 U/L) activity and sperm motility (78%) but decreased malondialdehyde (0.24 μmol/g tissue) activity and total sperm abnormalities (2.60%) in rat spermatozoa compared to DBP-treated rats (13.44 U/L, 28% and 5.05%, respectively). Histopathologic observation revealed normal structural and functional seminiferous tubules of curcumin-treated rats. The results suggest chemoprotective effect of curcumin against DBP-testicular oxidative damage [ 40 ].
Anti-artherosclerotic effect
Hydroalcoholic extract of C. longa rhizome (1.6 and 3.2 mg/kg BW) administered orally to artherosclerosis-induced male New Zealand rabbits (3000–3200 g) significantly (p < 0.05) reduced low-density lipoprotein (LDL) peroxidation compared to 2,2’-azobis(2-amidinopropane) dihydrochloride (AAPH)-induced rabbits (1.6 mg/kg BW: 6.38 nmol/mg, AAPH-induced: 15.60 nmol/mg; 3.2 mg/kg BW: 11.01 nmol/mg, AAPH-induced: 21.49 nmol/mg). Total plasma cholesterol was also significantly (p < 0.05) reduced (1.6 mg/kg BW: 1495 (mg/dL); 3.2 mg/kg BW: 1489 mg/dL) compared to the artherosclerosis-induced rabbits (2589 mg/dL) [ 41 ]
Hydroalcoholic extract of C. longa rhizome (1.6 mg/kg BW) administered orally to New Zealand White rabbits fed with high-cholesterol diet for 10-30 days had significantly (p < 0.05) lower plasma lipid peroxide (at 10, 20 and 30 days) and lower α-tocopherol and coenzyme Q10 levels (at 20 and 30 days) than the control group. Histological analysis also showed less fatty streak lesion of the thoracic and abdominal aorta of extract-treated rabbits compared to control [ 42 ].
Antidiabetic activity
Three extracts of C. longa rhizomes, i.e. ethanol extract (E-ext; containing curcuminoids and sesquiterpenoids) (0.2 and 1.0 g/100 g diet), hexane extract (H-ext; containing sesquiterpenoids) (0.1 and 0.5 g/ 100 g diet) and ethanol extract from hexane-extraction residue (HE-ext; containing curcuminoids) (0.1 and 0.5 g/ 100 g diet), were administered to type 2 diabetic KK-Ay female mice (aged six weeks old) for four weeks. The results revealed that E-ext (0.2 and 1.0 g), H-ext (0.5 g) and HE-ext (0.5 g) significantly reduced blood glucose levels [ 43 ]
In an in vivo study, two groups of female genetically type 2 diabetic KK-Ay/Ta mice (6 weeks old) consumed a basal diet (control group) and a diet with ethanol extract of C. longa rhizome (0.2 0r 1.0 g/100g diet) (treated group) and water ad libitum for 4 weeks. The treated group significantly (p < 0.05) reduced the elevated blood glucose in diabetic mice. In an in vitro study using human subcutaneous preadipocytes, ethanol extract of C. longa rhizome significantly (p < 0.05) and dose-dependently (2-20 μg/mL) stimulated human adipocyte differentiation compared to a negative (medium) control and a positive (rosiglitazone, 0.44 μg/mL) control. The extract (5 and 10 μg/mL) also showed human peroxisome proliferator-activated receptor (PPAR)-γ ligand-binding activity in a GAL4-PPAR-γ chimera assay more potently than that of a positive control, troglitazone (0.22 and 0.44 μg/mL, respectively). The extract was found to contain curcumin, demethoxycurcumin, bisdemethoxycurcumin and ar-tumerone. These compounds significantly (p < 0.05) activated (PPAR)-γ ligand-binding activity at a concentration of 5 μg/mL [ 44 ].
Hepatoprotective effect
Aqueous and methanol (75%) extracts of C. longa rhizome (10 mg/mL) inhibited the aflatoxin (AFB1) production by 99% and 86%, respectively. In another study, C. longa rhizome (50 mg/day) and curcumin (10 mg/day) given to separate duckling groups (40-50 g) with AFB1-induced hepatotoxicity for 14 days increased weight gain, i.e. 42.5 and 47.5 (p < 0.01) g for the respective groups, compared to AFB1 (35.5 g) and normal control (61.25 g) groups. Liver glutamic-pyruvic transaminase (GPT) and alkaline phosphatase (ALP) were decreased after treatment with C. longa rhizome (GPT: 1.7 μmoles of pyruvate formed/mg protein; ALP: 0.08 μmoles of pyruvate formed/mg protein) and curcumin (GPT: 1.6 μmoles of pyruvate formed/mg protein; ALP: 0.07 μmoles of pyruvate formed/mg protein) compared to compared to AFB1 (GPT: 2.3 μmoles of pyruvate formed/mg protein; ALP: 0.24 μmoles of pyruvate formed/mg protein) and normal control (GPT: 1.8 μmoles of pyruvate formed/mg protein; ALP: 0.27 μmoles of pyruvate formed/mg protein) groups. Histopathologically, ducklings with AFB1-induced hepatotoxicity had extensive fatty changes, granular degeneration, necrosis and bile duct hyperplasia. Curcumin-treated group showed almost complete reversal of necrosis and fatty changes. Whereas in animals treated with C. longa rhizome, necrosis was found to be almost reversal with moderate fatty changes. Both treated groups showed there was no significant reversal of bile duct hyperplasia compared to the AFB1-induced groups [ 45 ]
Curcumin, demethoxycurcumin and bisdemethoxycurcumin isolated from ethyl acetate extract of C. longa rhizome showed hepatoprotective activity on tacrine-induced cytotoxicity in human HepG2 liver cells with effective concentration (EC50)values of 86.9, 70.7 and 50.2 μM, respectively, compared to silybin (EC50 = 69.0 μM) and silychristin (EC50 = 82.7 μM) [ 34 ]
Anti-ulcer activity
Ethanol (80%) extract of C. longa rhizome (100 mg/kg BW) significantly (p < 0.01) reduced gastric acid secretion and protected against the formation of gastric mucosal lesions after 1 hour of single oral dose administration to pylorus-ligated stomach of male Sprague Dawley rats (200-250 g) comparable to the positive control, ranitidine (5 mg/kg BW). Histopathological examination showed protection against lesion of the gastric mucosal layer as effective as ranitidine (5 mg/kg BW). The extract was found to specifically inhibit gastric acid secretion by blocking H2 histamine receptors in a competitive manner. This is evidenced that the ethanol extract and ethyl acetate fraction of C. longa rhizome ethanol extract significantly blocked dimaprit (H2 histamine receptor agonist)-induced cAMP production with IC50 of 30.00 and 6.0 μg/mL, respectively, but had no effect on the elevation of cAMP levels triggered by isoproterenol-induced β2-adrenoceptor activation in U937 cells. The ethyl acetate fraction (0.01 mg/mL) blocked the binding of [3H]-tiotidine to membrane receptors on HL-60 cells more potently than the ethanol extract (0.1 mg/mL) [ 46 ].
Ethanol (96%) extract of C. longa rhizome(500 mg/kg BW) administered by oral gavage to inbred Wistar albino rats (200-230 g) significantly (p < 0.01) decreased the total acid output (229 ± 72) and ulcer index (0.33 ± 0.21) after 6 hour of pyloric ligation compared to the pylorus-ligated control group receiving 5 mL/kg normal saline vehicle (total acid output = 606 ± 52; ulcer index = 1.00 ± 0.36). The extract also produced significant anti-ulcerogenic activity in rats subjected to induction of gastro and duodenal ulcers by hypothermic-restraint stress (extract lesion score = 17.2 ± 2.4, p < 0.05 compared to hypothermic-restraint stress-induced control = 26.5 ± 2.3), indomethacin (extract lesion score = 23.2 ± 3.1, p < 0.001 compared to indomethacin-induced control = 35.2 ± 21.6) and reserpine (extract lesion score = 18.0 ± 3.1, p < 0.001 compared to reserpine-induced control = 37.0 ± 2.0). However, the extract did not give statistically significant effect on cysteamine-induced duodenal ulcers. The extract significantly (p < 0.001) increased gastric wall mucus (385 ± 31 μg Alcian blue/g wet glandular tissue) compared to hypothermic-resistant stress-induced control (196 ± 9 μg Alcian blue/g wet glandular tissue). Treatment of the extract also gave significant (p < 0.001) protective effect on gastric lesions caused by cystodestructive agents including 80% ethanol (extract ulcer index = 4.00 ± 0.40 compared to ethanol-induced control = 6.83 ± 0.40), 0.6 M HCl (extract ulcer index = 1.50 ± 0.50 compared to HCl-induced control = 6.83 ± 0.79), 25% NaCl (extract ulcer index = 1.66 ± 0.42 compared to NaCl-induced control = 6.850 ± 0.76) and 0.2 M NaOH (extract ulcer index = 2.00 ± 0.36 compared to NaOH-induced control = 6.55 ± 0.80). The extract was also found to restore the non-protein sulfhydryl (NP-SH) content in the glandular stomachs of rats stimulated by 80% ethanol with values of 2.89 ± 0.23, 1.42 ± 0.06 and 2.27 ± 0.12 μmol/g tissue for the respective rat groups receiving extract, 80% ethanol and extract with 80% ethanol [ 47 ]
Wound healing activity
Fresh C. longa paste (15% w/w, in petroleum jelly) applied to rabbits (1.5-2 kg) for 14 days showed significantly (p < 0.01) higher mean tensile strength (78.00) and wound contraction size (5.78%) than the control group (mean tensile strength = 15.00; wound contraction size = 62.71%). C. longa treated group had significantly (p < 0.01) higher mean values of wound index (0.67) compared to control group (2.33). C. longa treated group showed significantly (p < 0.01) lower inflammation response in terms of neovascularization (mean value = 2.35), fibroplasias (0.83), epithelialization (2.67), and formation of collagen (3.33), elastin (2.67) and reticulin (1.33) compared to control group (1.10, 2.50, 1.28, 1.28, 1.00 and 0.33, respectively) [ 48 ]
Insecticidal and repellent activity
Ar-tumerone isolated from of hexane extract of C. longa rhizome was evaluated for insecticidal and repellent effects on maize weevil Sitophilus zeamais (Coleoptera: Curculionidae) and fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). The S. zeamais died (100%) after six days of contact with ar-turmerone at 1% (m/m), while the S. frugiperda showed a 58% mortality rate after ingestion of the compound at 1% (m/v). The width of head capsule, body length and body weight of surviving S. frugiperda caterpillars exposed to ar-turmerone were significantly (p < 0.0001) reduced to 0.99 mm, 3.20 mg and 5.90 mm, respectively, compared to the normal control caterpillars (2.5 mm, 52.2 mg and 14.60 mm respectively). Mean number of S. frugiperda caterpillars hatched after treatment with ar-turmerone (1%) was 0.28 (Day 0; p < 0.01)), 0.6 (Day 1) and 0.8 (Day 2; p < 0.01), compared to untreated control (12.6, 3.6 and 10.6 for the respective days) [ 50 ].
Rhizome powder of C. longa (1 g) was tested on stored cowpea (Vigna unguiculata)seeds to observe its toxicity to cowpea seed bruchid (Callosobruchus maculatus F. (Coleoptera: Bruchidae)). The insect mortality were assessed at 7, 14, 21 and 28 days after treatment. Mean insect mortality of C. longa rhizome powder was 52% after 28 days of treatment
[ 51 ].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute toxicity
Ethanol extract of C. longa rhizome (0.5, 1.0, and 3 g/kg BW) was administered orally to mice and observation was made after 24 hours. No significant mortality, weight gain and vital organ weights of mice were observed. However, there was a significant fall in the WBC and RBC levels compared to the control group. The gain in weights of sexual organs and increased sperm motility and sperm counts were observed, but it did not show any spermatotoxic effects. [52]
Crude hexane extract and refined essential oil from hexane extract of C. longa rhizome were administered intraperitoneally to female Lewis rats (110 g) with streptococcal cell wall (SCW)-induced arthritis. The treatment began 4 days prior to, or 3 days after, SCW injection and continued daily until 10 days after SCW injection, at which time dosing frequency decreased to 3-5 days per week. Observation was made for 28 days after SCW injection. A dose of ≥ 28 mg equivalents of refined essential oil/kg/day resulted in 20% (crude) and 36% (refined) mortality rates after 2 weeks of treatment. However, no mortality was observed after one month of daily oral administration of crude extract or refined essential oil (normalized to 560 mg refined essential oil/kg/day) following the same regime as the intraperitoneal administration [ 38 ].
Oral single dose acute toxicity study on female Sprague Dawley rats (aged between 8 and 12 weeks old) using aqueous extract of C. longa rhizome showed no toxicity effect on the parameters observed, including behaviours, body weight, food, and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. Half lethal dose (LD50) is more than 2,000 mg/kg body weight [ 60 ]
Chronic tocixity
Ethanol extract of C. longa rhizome (100 mg/kg/day) was administered orally to mice and observation was made after 90 days. No significant mortality, weight gain and spermatotoxic effect were observed. However, the treatment induced significant changes in heart and lungs weights, and fall in the WBC and RBC levels compared to the controls. The gain in weights of sexual organs and increased sperm motility and sperm counts were observed, but it did not show any spermatotoxic effects [ 52 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Clinical trials report few adverse reactions (e.g. ulcer). Cases of contact dermatitis and anaphylaxis are rare. Theoretically, susceptible individuals may have increased risk of kidney stone formation [ 53 , 54 , 55 ].
C. longa rhizome may increase the risk of bleeding or potentiate the effects of warfarin or other blood-thinning medications [ 54 ].
C. longa rhizome has been reported to have emmenagogue and abortifacient effects, thus should be used with caution in pregnancy [ 56 ]
DOSAGE
1.5-3 g of powdered C. longa rhizome is usually recommended for dyspeptic/digestive disorders [ 9 , 57 ]
C. longa rhizome is taken in doses of 5-30 g daily for acute problems or 3-10 g daily for chronic problems in inflammatory conditions. [ 57 , 58 ]
400-600 mg of curcumin or 8-60 g of fresh C. longa rhizome three times daily is recommended for arthritis [ 57 , 59 ].
Other dosage recommendations are:-
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- Malaysian Herbal Monograph. Vol. 1. Rhizoma Curcumae Longae, 1999:17-20.
- The Plant List 2010. http://www.theplantlist.org/tpl/record/kew-235249 (26th October 2012).
- Ravindran PN, Babu KN & Sivaraman K. Turmeric The Genus Curcuma. Medicinal and Aromatic Plants – Industrial Profiles. Boca Raton: Taylor & Francis Group, 2007.
- Multilingual Multiscript Plant Name Database. Sorting Curcuma names. [Internet]. Plant Names. [assessed on 19th June 2015]. Available from: http://www.plantnames.unimelb.edu.au/Sorting/Curcuma.html#longa
- Velayudhan KC, Muralidharan VK, Amalraj VA, Gautam PL, Mandal S & Kumar D. Curcuma Genetic Resources. Kerala: National Bureau of Plant Genetic Resources, Regional Station, Trichur, 1999.
- Standard of ASEAN Herbal Medicine, Vol. 1. Jakarta: ASEAN Countries, 1993.
- The Indian Pharmaceutical Codex, Vol. I. Indigenous drugs. New Delhi: Council of Scientific & Industrial Research, 1953.
- Youngken HW. Textbook of pharmacognosy, 6th ed. Philadelphia: Blakiston, 1950.
- WHO. “Rhizoma Curcumae Longae” in WHO on Selected Medicinal Plants. Geneva: WHO, 1999.
- Gonda R, Tomoda M, Shimizu N & Kanari M. Characterization of polysaccharides having activities on the reticuloendothelial system from the rhizome of Curcuma longa. Chemical and Pharmaceutical Bulletin. 1990;38(2): 482-486.
- Gonda R, Takeda K, Shimizu N & Tomoda M. Characterization of neutral polysaccharides having activities on the reticuloendothelial system from the rhizome of Curcuma longa. Chemical and Pharmaceutical Bulletin. 1992;40(1): 185-188.
- Oshiro M, Kuriyanagi M & Ueno A. Structures of sesquiterpenes from Curcuma longa. Phytochemistry. 1990;29(7):2201-2205.
- Masuda T, Jitoe A, Isobe J, Nakatani N & Yonemori S. Anti-oxidative and anti-inflammatory curcumin-related phenolics from rhizomes of Curcuma domestica. Phytochemistry. 1993;32(6):1557-1560.
- Jantan I, Saputri FC, Qaisar MN & Buang F. Correlation between chemical composition of Curcuma domestica and Curcuma xanthorrhiza and their antioxidant effect on human low-density lipoprotein oxidation. Evidence-Based Complementary and Alternative Medicine 2012:1-10.
- Ramsewak RS, DeWitt DL & Nair MG. Cytotoxicity, antioxidant and anti-inflammatory activities of curcumins I-III from Curcuma longa. Phytomedicine 2000;7(4):303-308.
- Shiyou L, Wei Y, Guangrui D, Ping W, Peiying Y & Bharat A. Chemical composition and product quality control of turmeric (Curcuma longa L.). Pharmaceutical Crops 2011;2:28-54.
- Toda S, Miyase T, Arichi H, Tanizawa H & Takino Y. Natural antioxidants. III. Antioxidative components isolated from rhizome of Curcuma longa L. Chemical Pharmaceutical Bulletin. 1985;33(4):1725-1728.
- Rao S, Basu T, N & Siddiqui HH. Anti-inflammatory activity of curcumin analogues. Indian Journal of Medical Research. 1982;75:574-578.
- Nakayama R, Tamura Y, Yamanaka H, Kikuzaki H & Nakatani N. Two curcuminoid pigments from Curcuma domestica. Phytochemistry. 1993;33(2):501-502.
- Imai S, Morikiyo M, Furihata K, Hayakawa Y & Seto H. Turmeronol A and turmeronol B, new inhibitors of soybean lipoxygenase. Agricultural and Biological Chemistry. 1990;54(9):2367-2371.
- Matthes HWD, Luu B & Ourisson G. Chemistry and biochemistry of Chinese drugs. Part VI. Cytotoxic components of Zingiber zerumbet, Curcumazedoaria and C.domestica. Phytochemistry 1980;19:2643-2650.
- Usman LA, Hamid AA, George OC, Ameen OM, Muhammad NO, Zubair MF & Lawal A. Chemical composition of rhizome essential oil of Curcuma longa L. growing in north central Nigeria. World Journal of Chemistry. 2009;4(2):178-181.
- Singh K, Sankar B, Rajesh S, Sahoo K, Subudhi E & Nayak S. Chemical Composition of turmeric oil (Curcuma longa L. cv. Roma) and its antimicrobial activity against eye infecting pathogens. Journal of Essential Oil Research. 2012;23(6):11-18.
- Leela NK, Tava A, Shafi PM, John SP & Chempakam B. Chemical composition of essential oils of turmeric (Curcuma longa L.). Acta Pharmaceutica 2002;52:137-141.
- Chane-Ming J, Vera R, Chalchat JC & Cabassu P. Chemical composition of essential oils from rhizomes, leaves and flowers of Curcuma longa L. from Reunion Island, Journal of Essential Oil Research. 2002;14(4):249-251.
- Chatterjee S, Variyar PS, Gholap AS, Padwal-Desai SR & Bongirwar DR. Effect of γ-irradiation on the volatile oil constituents of turmeric (Curcuma longa). Food Research International. 2000;33:103-106.
- Raina VK, Srivastana SK, Jain N, Ahmad A, Syamasundar KV & Aggarwal KK. Essential oil composition of Curcuma longa L. cv. Roma from the plains of northern India. Flavour and Fragrance Journal. 2002;17:99-102.
- Raina VK, Srivastava SK & Syamsundar KV. Rhizome and leaf oil composition of Curcuma longa from the lower Himalayan Region of Northern India, Journal of Essential Oil Research. 2005;17(5): 556-559.
- Uehara SI, Yasuda I & Itokawa H. Comparison of the commercial turmeric and its cultivated plant by their constituents. Shoyakugaku Zasshi 1992;46(1): 55-61.
- Jantan I, Ahmad AS, Ali NAM, Ahmad AR & Ibrahim H. Chemical composition of the rhizome oils of four Curcuma species from Malaysia. Journal of Essential Oil Research 1999;11(6):719-723.
- Braga MEM, Leal PF, Carvalho JE & Meireles MAA. Comparison of yield, composition and antioxidant activity of tumeric (Curcuma longa L.) extracts obtained using various techniques. Journal of Agriculture and Food Chemistry. 2003;51:6604-6611.
- Burkill IH. A Dictionary of the Economics Products of the Malay Peninsular. Kuala Lumpur: Ministry of Agriculture and Cooperative. 1966;Vol. 2.
- Perry LM & Metzger J. Medicinal Plants of East and Southeast Asia, Massachusetts: MIT Press. 1985;439.
- Song EK, Cho H, Kim JS, An NH, Kim JA, Lee SH & Kim YC. Diarylheptanoids with free radical scavenging and hepatoprotective activity in vitro from Curcuma longa. Planta Medica, 2001;67(9):876-877.
- Khattak S, Saeed-ur-Rehman, H US, Ahmad W & Ahmad M. Biological effects of indigenous medicinal plants Curcuma longa and Alpinia galanga. Fitoterapia. 2005;76(2):254-257.
- Ferreira LAF, Henriques OB, Anreoni AAS, Vital GRF, Camos MMC, Habermehl GG & Moraes DVLG. Antivenom and biological effect of ar-turmerone isolated from Curcuma longa (Zingiberaceae). Toxicon 1992;30:1211-1218.
- Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of turmeric (Curcuma longa). The Journal of Alternative and Complementary Medicine 2003;9(1):161-168.
- Funk JL, Frye JB, Oyarzo JN, Zhang H & Timmermann BN. Anti-arthritic effects and toxicity of the essential oils of turmeric (Curcuma longa L). Journal of Agricultural and Food Chemistry. 2010;58(2):842-849.
- Farombi EO, Abarikwu SO, Adedara IA & Oyeyemi MO. Curcumin and kolaviron ameliorate di-n-butylphthalate-induced testicular damage in rats. Basic Clinical Pharmacolology & Toxicology. 2007;100(1):43-48.
- Soudamini KK & Kuttan R. Inhibition of chemical carcinogenesis by curcumin. Journal of Ethnopharmacology. 1989;27(1-2):227-233.
- Ramírez-Tortosa MC, Mesa MD, Aguilera MC, Quiles JL, Baro´ L, Ramirez-Tortosa CL, Martinez-Victoria E & Gil Á. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis. 1999;147:371-378.
- Quiles JL, Mesa MD, Ramírez-Tortosa CL, Aguilera CM, Battino M, Gil A, Ramírez-Tortosa MC. Curcuma longa extract supplementation reduces oxidative stress and attenuates aortic fatty streak development in rabbits. Arteriosclerosis, Thrombosis and Vascular Biology. 2002;22:1225-1231.
- Nishiyama T, Mae T, Kishida H, Tsukagawa M, Mimaki Y, Kuroda M, Sashida Y, Takahashi K, Kawada T, Nakagawa K & Kitahara M. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) suppress an increase in blood glucose level in type 2 diabetic KK-Ay mice. Journal of Agricultural and Food Chemistry 2005;53(4):959-963.
- Kuroda M, Mimaki Y, Nishiyama T, Mae T, Kishida H, Tsukagawa M, Takahashi K, Kawada T, Nakagawa K & Kitahara M. Hypoglycemic effects of turmeric (Curcuma longa L. rhizomes) on genetically diabetic KK-Ay mice. Biological and Pharmaceutical Bulletin 2005;28(5):937-939.
- Soni KB, Rajan A & Kuttan R. Reversal of aflatoxin induced liver damage by turmeric and curcumin. Cancer Letters 1992;66(2):115-121.
- Kim DC, Kim SH, Choi BH, Baek NI, Kim D, Kim MJ & Kim KT. Curcuma longa extract protect against gastric ulcers by blocking H2 histamine receptors. Biological and Pharmaceutical Bulletin 2005;28(12):2220-2224.
- Rafatullah S, Tariq M, Al-Yahya MA, Mossa JS & Ageel AM. 1990. Evaluation of turmeric (Curcuma longa) for gastric and duodenal antiulcer activity in rats. Journal of Ethnopharmacology 1990;29(1):25-34.
- Kundu S, Biswas TK, Das P, Kumar S, & De DK. Turmeric (Curcuma longa) rhizome paste and honey show similar wound healing potential: A preclinical study in rabbits. The International Journal of Lower Extremity Wounds 2005;4:205-213.
- Yu ZF, Kong LD & Chen Y. Antidepressant activity of aqueous extracts of Curcuma longa in mice. Journal of Ethnopharmacology. 2002;83(1-2):161-165.
- Tavares WDS, Freitas SDS, Grazziotti GH, Parente LML, Liao LM & Zanuncio JC. Ar-turmerone from Curcuma longa (Zingiberaceae) rhizomes and effects on Sitophilus zeamais (Coleoptera: Curculionidae) and Spodoptera frugiperda. Lepidoptera: Noctuidae. Industrial Crops and Products. 2013;46:158-164.
- Asawalam EF & Dioka UJ. Evaluation of toxicity of Dennitia tripetala Baker f. and Curcuma longa L. rhizomes against cowpea seed bruchid, Callosobruchus maculatus (f.) Coleoptera: Bruchidae. Agricultural Science Research Journal. 2012;2(6):308-311.
- Qureshi S, Shah AH & Ageel AM. Toxicity studies on Alpinia galanga and Curcuma longa. Planta Medica. 1992;58(2):124-127.
- Gupta B, Kulshrestha VK, Srivastava RK & Prasad DN. Mechanisms of curcumin induced gastric ulcer in rats. Journal of Medical Research. 1980;71:806-814.
- Brinker FJ. Herb Contraindications and Drug Interactions: With Appendices Addressing Specific Conditions and Medicines, 2nd ed, OR: Eclectic Medical Publications, Sandy. 1998.
- Sandeep KG, Amit L, Vinay J, Siddharta G & Anuj JK. Phytochemistry of Curcuma longa – An overview. Journal of Pharmaceutical and Biomedical Sciences. 2010;4(4):1-9.
- Ernst E. Herbal medicinal products during pregnancy: are they safe. British Journal of Obstetrics and Gyneacology. 2002;109(3):227-235.
- European Medicines Agency Evaluation of Medicines for Human Use. Committee On Herbal Medicinal Products (HMPC). Assessment Report On Curcuma longa L. Rhizoma. London, 12 November 2009. (http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2010/02/WC500070700.pdf).
- Yarnell E & Abascal K. Herbs for curbing inflammation. Alternative and Complementary Therapies. 2006;12(1):22-8.
- Fetrow CW & Avila JR. Professional’s Handbook of Complementary and Alternative Medicine. Springhouse, PA: Springhouse; 1999.
- Teh BP, Norazila Z, Nor Liyana MY, Wan Abdul Hakim WL, Wan Mohammad Adham Afiq WZ, Hussin M. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2015. Report no.: HMRC 11-045/01/CL/RH/B