Serai Wangi Aerial
Cymbopogon nardus (L.) Rendle
Poaceae
DEFINITION
Serai wangi aerial consist of the powder of dried leaves and stem of Cymbopogon nardus (Poaceae).
SYNONYM
Andropogonconfertiflorus Steud., Andropogongrandis Nees ex Steud., Andropogonhamulatus Nees ex Steud., Andropogonkhasianus (Hack.) Munro ex Duthie, Andropogonnardus L., Andropogonnardus var. confertiflorus (Steud.) Stapf ex Bor, Andropogonnardus subsp. grandis (Nees) Hack., Andropogonnardus var. luridus Hook.f., Andropogonnardus subsp. nilagiricus Hack., Andropogonnardus var. prolixus Stapf, Andropogonnardus var. Validus Stapf, Andropogonpseudohirtus Steud., Andropogonthwaitesii Hook.f., Cymbopogonafronardus Stapf, Cymbopogonclaessensii Robyns, Cymbopogonconfertiflorus (Steud.) Stapf, Cymbopogonnardus var. confertiflorus (Steud.) Bor, Cymbopogonnardus var. luridus (Hook.f.) N.Rama Rao, Cymbopogonnardus var. nardus, Cymbopogonnardus subsp. nilgiricus Hack., Cymbopogonprolixus (Stapf) E.Phillips, Cymbopogonthwaitesii (Hook.f.) Willis, Cymbopogonvalidus (Stapf) Stapf ex Burtt Davy, Cymbopogonvalidus var. lysocladus Stapf, Cymbopogon virgatus Stapf ex Bor, Sorghum nardus (L.) Kuntze [ 1 ].
VERNACULAR NAMES
Blue citronella grass, ceylon citronella, citronella, citronella grass, citronella oil grass, false citronella, mana grass, nard grass, new citronella grass (English); sĕreh wangi, serai wangi (Malay); ya xiang mao (Chinese); kamachipillu (Tamil) [ 2 , 3 , 4 ].
CHARACTER
| Colour | Brownish-green |
| Odour | Aromatic |
| Taste | Characteristic |
IDENTIFICATION
Plant Morphology
C. nardus is a perennial and caespitose. Basal sheaths persistent and investing base of culm; culms tufted, robust, 75–300 cm tall, 1–2 cm in diameter. Leaf sheaths reddish purple at base, smooth, glabrous; leaf blades dark green or dark brown when dry, 30–100 cm long, 1–2 cm wide, glabrous, abaxial surface scabrid, adaxial surface smooth, base narrow, apex long acuminate; ligule an eciliate membrane 3–9 mm long. Synflorescence compound, linear, 15–60 cm long, dense; inflorescence composed of racemes, 1–1.5 cm long, racemed-bases flattened, subequal, subtended by a spatheole; spatheole elliptic, 1–2.5 cm long, reddish brown; spikelet sessile, oblong-lanceolate, 3–4.5 mm long, 1–1.2 mm wide, base obtuse, inserted; pedicelled spikelet 3.5–7 mm; lower glume flat or slightly concave, reddish brown or purplish upward, sharply 2-keeled; upper glume lanceolate, 1-keeled; keels narrowly winged, obscurely 0–3-veined between keels; upper lemma linear, entire or slightly 2-lobed, mucronate or very shortly awned; palea absent or minute.[ 4 , 5 ].
Microscopy
Powdered material consists of parenchyma cells; anomocytic type of stomata attached to epidermis; a group of vessels, annular, pitted and spiral vessel; elongated fibre and fragment of sclerenchyma cells; simple unicellular, and grandular trichomes with multicellular stalks and multicellular globular heads found isolated; macrosclereid; calcium oxalate in the form of druses.













Figure 2 : Microscopic characters of C. nardus aerial powder of 0.355 mm size. (a) Parenchyma cells (magnification 50x); (b–c) stomata (arrow) (magnification 50x);(d) a group of vessels (magnification 50x); (e) fragment of annular vessel (magnification 50x); (f) pitted vessels (magnification 50x); (g) spiral vessels (magnification 50x); (h) fibre (magnification 50x); (i) sclerenchyma (arrow) (magnification 50x); (j) simple unicellular trichome (arrow) (magnification 50x); (k) glandular trichome (magnification 50x); (l) macrosclereid (arrow) (magnification 50x); (m) druses calcium oxalate crystal (magnification 50x). [Scale bars: a–m = 20 µm]
Thin Layer Chromatography (TLC)

Figure 3 : TLC chromatogram of geraniol (S) and essential oil of C. nardus dried aerial (L) observed under visible light after derivatisation
| Test solution | Weigh about 150 g of C. nardus dried aerial powder of 0.355 mm size in 5 L of round bottom flask. Add 1.8 L of water. Distill for 4 hr using Clevenger apparatus. Discard the water in Clevenger and collect the oil in 10 mL of volumetric flask. Dissolve the oil in acetonitrile. The mixture was then vortex for 10 s. Use the solution as test solution. |
| Standard solution | Dissolve geraniol standard [CAS no.: 48798] in acetonitrile to produce a standard concentration of 1.0 mg/mL. Vortex standard for 10 s before use. |
| Stationary phase | HPTLC Silica gel 60 F254, 10 x 10 cm |
| Mobile phase | Toluene : ethyl acetate; (36 : 5) (v/v) |
| Application | (a) Geraniol standard solution (S); 4 µL, as a band (b) Essential oil of nardus dried aerial powder (L); 4 µL, as a band |
| Development distance | 8 cm |
| Drying | Air drying |
| Detection | Visible light after derivatisation with vanillin-sulphuric acid |
High Performance Liquid Chromatography (HPLC)
| Test solution | Weigh about 150 g of C. nardus dried aerial powder of 0.355 mm size in 5 L round bottom flask. Add 1.8 L of water. Distill for 4 hr using Clevenger apparatus. Discard the water in Clevenger and collect the oil in 10 mL of volumetric flask. Dissolve the oil in acetonitrile to produce a concentration of 0.5 mg/mL. Vortex the mixture for 10 sec and use as test solution. | ||||||||||||||||||
| Standard solution | Dissolve geraniol standard [CAS no.: 48798] in acetonitrile to produce a standard concentration 0.5 mg/mL. | ||||||||||||||||||
| Chromatographic system |
Detector: UV 200 nmColumn: C18 (3.5 µm, 4.6 mm I.D x 150 mm) (Zorbax SB-C18 if necessary) Column oven temperature: 25˚C Flow rate: 1 mL/min Injection volume: 10 µL |
||||||||||||||||||
| Mobile Phase (gradient mode) |
|
||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the standard solutions (0.5 mg/mL). The requirements of the system suitability parameters are as follow:
|
||||||||||||||||||
| Acceptance criteria |
|
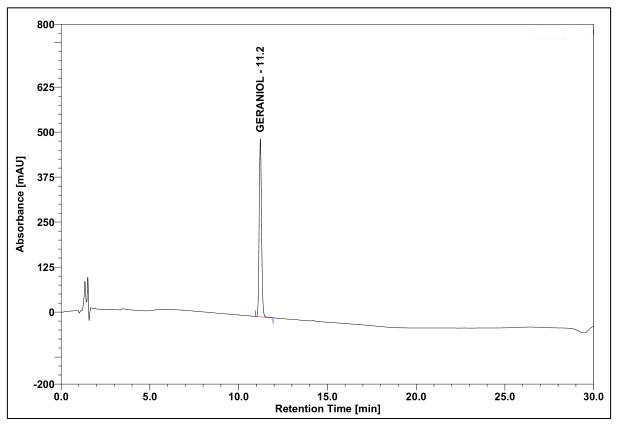
(a)
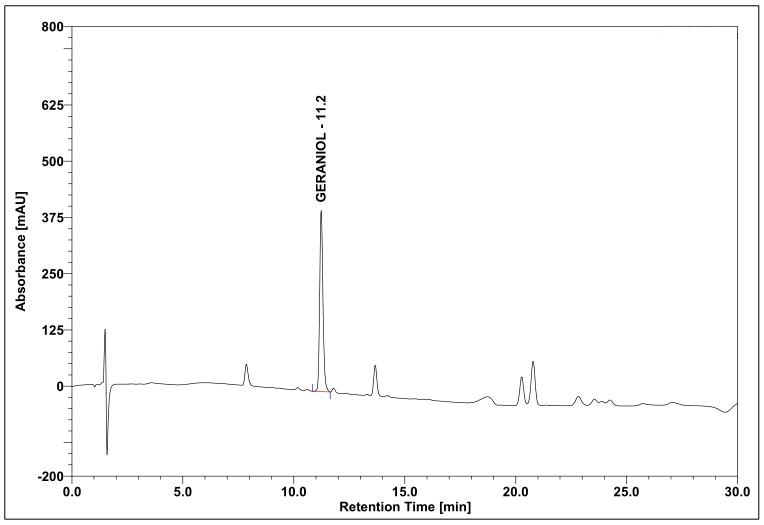
(b)
Figure 4 : Whole HPLC chromatogram of (a) geraniol standard solution (0.5 mg/mL) at tr= 11.2 min and (b) essential oil of C. nardus dried aerial powder showing peak corresponding to geraniol standard solution at tr= 11.2 min.
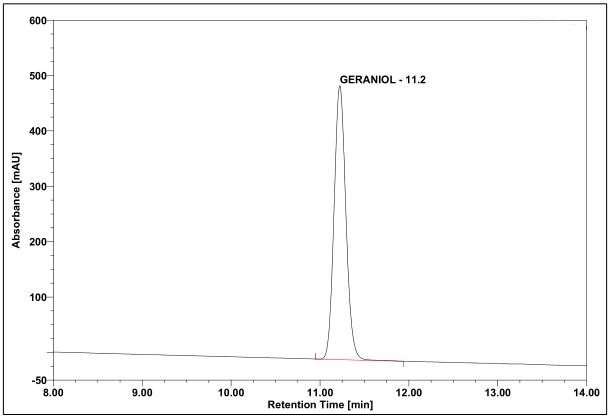
(a)

(b)
Figure 5 : HPLC chromatogram highlighting the elution region of (a) geraniol standard solution (0.5 mg/mL) at tr = 11.2 min and (b) essential oil of C. nardus dried aerial powder showing peak corresponding to geraniol standard solution at tr = 11.2
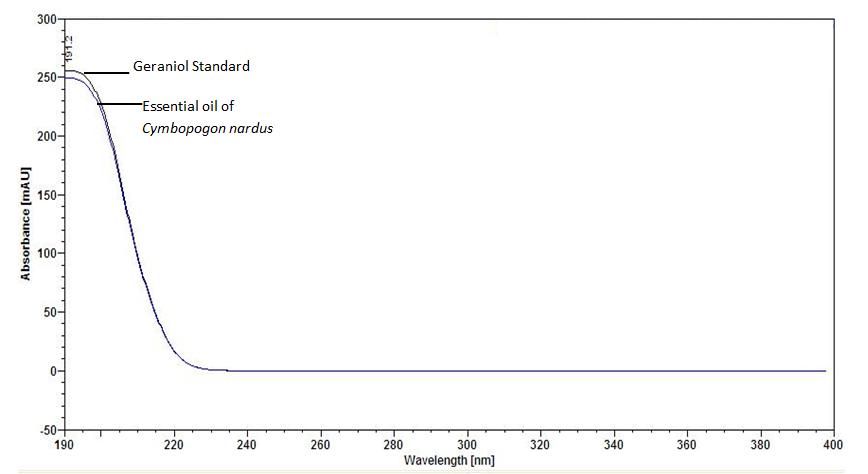
Figure 6 : UV spectrum of geraniol standard solution (0.5 mg/mL) and essential oil of C. nardus dried aerial powder.
PURITY TESTS
The purity tests, except foreign matter test are based on C. nardus dried aerial powder of 0.355 mm particle size.
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 5% |
| Acid-insoluble ash | Not more than 2% |
| Water soluble ash | Not less than 1% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 11% |
| Cold method | Not less than 6% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 6% |
| Cold method | Not less than 3% |
SAFETY TESTS
The safety tests are based on C. nardus dried aerial powder of 0.355 mm particle size.
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Essential oil of C. nardus leaves was found to contain sesquiterpenoids (e.g. α-eudesmol, α-bergamotene, β-elemene, β-eudesmol, β-myrcene, myrcene, α-cadinol, epi-γ-cadinol, trans-cadinol, α-copaene, beta-copaene, α-cadinene, α-farnesene, α-humulene, α-muurolene, α-muurolol, epi-γ-muurolol, α-pinene oxide, β-caryophyllene, β-cubebene, γ-cadinene, γ-eudesmol, δ-cadinene, (Z,E)-farnesol, (Z,Z)-farnesol, 14-hydroxy-a-humulene, bicyclogermacrene, caryophyllene, caryophyllene oxide, eudesmol, germacrene, ledol, limonene, linalool, longipinanol), terpenes (e.g. α-terpineol, , β-linalool, β-citronellal, β-selinene, bergamal, β-citronellol, (Z)-citral, alloaromadendrene, eugenol, geranial, geranyl acetate, geranyl formate, geranylisovalerate, geraniol, germacrene-D, (E)-β-ocimene, cis-ocemene, trans-ocemene, citral, citronellal, citronellol, 3,7-dimethyl-2,6-octadienal, 3,7-dimethyl-1,6-octadien-3-ol, camphene, citronellol acetate, , citronellyl acetate, citronellyl butyrate, citronellyl tiglate, cis-carveol, cis-carvone oxide, cubenol, D-limonene, isopulegol, endo-borneol, neral, nerol, pinocarvone, sabinene, seychellene), ketones (e.g. 6-methyl-5-hepten-2-one, 6-methylhept-5-en-2-one, 4-nonanone, methyl-5-pent-2-one), aldehydes (e.g. cis-4-decenal, decanal, melonal, n-octanal), esters (e.g. isobornyl acetate, octadecanoic acid methyl ester) and organic acids (e.g. 3,7-dimethyl-6-octenoic acid, neric acid) [ 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 ].
Essential oil of C. nardus dried aerial was found to contain sesquiterpenoids (e.g. α-caryophyllene, β-carophyllene, limonene, γ-cadinene) and terpenes (e.g. citronellal, citronellol, eugenol, geraniol, geranial, germacrene-D, neral) [ 14 , 15 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Volatile oil of C. nardus is traditionally used in a small dose to comfort the stomach and aid digestion. It is also used to stimulate or increase menstrual flow. This plant is also traditionally used as an infusion or decoction to maintain general health [ 3 ].
Biological and pharmacological activities supported by experimental data
Antimicrobial activity
Essential oil of C. nardus leaves (80 µg/mL) showed antifungal activity against Candida albicans with inhibition zone (IZ) of 47 mm diameter compared to clotrimazole (20 µg/mL) (IZ = 46 mm) using disc diffusion method [ 6 ].
Essential oil of C. nardus leaves (80 µg/mL) showed antifungal activity against Candida glabrata (IZ = 44 mm diameter) compared to clotrimazole (20 µg/mL) (IZ = 41 mm) using disc diffusion method [ 6 ].
Essential oil of C. nardus leaves (80 µg/mL) showed antifungal activity against Candida tropicalis (IZ = 43 mm diameter) compared to clotrimazole (20 µg/mL) (IZ = 44 mm) using disc diffusion method [ 6 ].
Essential oil of C. nardus aerial showed antibacterial activity against Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa) with minimum inhibitory concentration (MIC) value of 0.244 µg/mL compared to kanamycin (MIC = 31 µg/mL) using two-fold broth microdilution method [ 14 ].
Antitrypanosomal activity
Essential oil of C. nardus leaves showed antitrypanosomal activity against Trypanosoma brucei brucei strain 427 (MIC = 5.71 ± 1.40 µg/mL) compared to suramine (MIC = 0.11 ± 0.02 µg/mL) using long incubation low inoculation test method [ 7 ].
Cytotoxicity activity
Essential oil of C. nardus leaves showed low cytotoxicity against the Chinese Hamster ovary (CHO) cells and human non-cancer fibroblast cell line (WI38) with 50% inhibition concentration (IC50) value more than 50 µg/mL compared to campthotecin (IC50 = 0.74 µg/mL) using 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetra- zolium bromide (MTT) colorimetric assay [ 7 ].
Antiviral activity
Octadecanoic acid methyl ester compound isolated from methanol extract of C. nardus dried leaves (25 µg/mL) showed antiviral activity towards measles virus-infected Vero cell with 84.01% inhibition percentage compared to ribavirin (0.1 µg/mL) (91.09%) using antiviral assay [ 9 ].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute toxicity
Oral single dose acute toxicity study on female Sprague Dawley rats (aged between 7 and 12 weeks old) using aqueous extract of C. nardus leaves showed no toxic effect on the parameters observed, including behaviours, body weight, food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality but several histological lesion were found. Half lethal dose (LD50) is not more than 2,000 mg/kg body weight [ 16 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The plant list. [Internet] Cymbopogon nardus. Version 1.1; [cited on 1th September 2018]. Available from: http://www.theplantlist.org/tpl1.1/record/kew-406227
- Quattrocchi UFLS. CRC world dictionary of medicinal and poisonous plants: common names, scientific names, eponyms, synonyms, and etymology. Vol. III E-L. United States: CRC Press. 2012; p.259-260.
- Burkill IH. A dictionary of the economic product of the Malay Peninsula. Vol. 1. London: Published on behalf of the Governments of the Straits Settlements and Federated Malay States by the Crown Agents for the Colonies. 1935; p.727-728.
- Flora of China. [Internet] Cymbopogon nardus (Linnaeus) Rendle; [cited on 30th September 2018]. Available from:http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200025100
- [Internet] Invasive Species Compendium. Wallingford, UK: CAB International. [cited on 30th September 2018]. Available from: https://www.cabi.org/isc/datasheet/120396#47CFAA54-571B-4B99-AC41-808210427C38
- Raghuram K, Sanjeeb K, Bhaswati C, Suvankanta D, Kasturi K, Jinon K. Chemical composition and anti-candidiasis mediated wound healing property of Cymbopogon nardus essential oil on chronic diabetic wounds. Frontiers in Pharmacology. 2016;7(198):1-8.
- Salome K, Joanne B, Pierre A, Fernand G, Benedicta KK, Brice S, Georges A, Michel F, Mansourou M, Joelle QL. Chemical composition, cytotoxicity and in vitro antitrypanosomal and antiplasmodial activity of essential oil of four Cymbopogon species from Benin. Journal of Ethnopharmacology. 2014;151:652-659.
- Noudogbessi JP, Alitonou GA, Djenontin T, Avlessi F, Figueredo G, Chalard P, Chalchat JC, Sohounhloue DCK. Chemical compositions and physico-chemical properties of three varieties essential oil of Cymbopogon growing to the spontaneous State in Benin. Oriental Journal of Chemistry. 2013;29(1):59-67.
- Reagan Entigu AL, Samuel L, Ismail A. Isolation of antiviral compound from Cymbopogon nardus methanolic fractions. International Journal of Health and Pharmaceutical Sciences. 2013;2(2):1-7.
- De Toledo LG, Ramos MA, Spósito L, Castilho EM, Pavan FR, Lopes Éde O, Zocolo GJ, Silva FA, Soares TH, Dos Santos AG, Bauab TM, De Almeida MT. Essential oil of Cymbopogon nardus (L.) Rendle: a strategy to combat fungal infections caused by Candida International Journal of Molecular Sciences. 2016;17(1252):1-16.
- Koba K, Sanda K, Guyon C, Raynaud C, Chaumont JP, Nicod L. In vitro cytotoxic activity of Cymbopogon nardus essential oils from Togo. Bangladesh Journal of Pharmacology. 2009;4:29-34.
- Kadir R, Mohamad Ali NA, Jamil M, Soit Z, Faridz ZM. Toxicity and antitermite activity of the essential oils from Cymbopogon nardus against Coptotermes curvignathus. Wood Science Technology. 2013;47:1273-1284.
- Silva CF, Moura FC, Mendes MF, Pessoa FLP. Extraction of citronella (Cymbopogon nardus) essential oil using supercritical CO2: experimental data and mathematical modeling. Brazilian Journal of Chemical Engineering. 2011;28(2):343-350.
- Lee SW, Wendy W. Chemical composition and antimicrobial activity of Cymbopogon nardus citronella essential oil against systemic bacteria of aquatic animals. Iranian Journal of Microbiology. 2013;5(2):147-152.
- Clain E, Baranauskiene R, Kraujalis P, Sipailiene A, Mazdzierine R, Kazernaviciute R, Kalamouni CE, Venskutonis PR. Biorefining of Cymbopogon nardus from Reunion Island into essential oil and antioxidant fractions by conventional and high pressure extraction methods. Industrial Crops & Products. 2018;126:158-167.
- Elda Nurafnie IR, Nor Azlina Z, Umi Rubiah SZ, Janice CSW, Tavinraj R, Nurmaziah MS, Teh BP. Acute oral toxicity study of selected Malaysian medicinal herbs (Cymbopogon nardus leaves) on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2020. Report no.: NON-GLP/020/04/01.




