Urang-aring Leaves
Eclipta prostrata (L.) L.
Asteraceae
DEFINITION
Urang-aring leaves consist of the powder of dried leaves of Eclipta prostrata (L.) L. (Asteraceae).
SYNONYM
Eclipta alba (L.) Hassk., Eclipta alba var. alba, Eclipta alba f. alba, Eclipta alba var. erecta (L.) Hassl., Eclipta alba var. erecta (L.) Miq., Eclipta alba f. longifolia Hassk., Eclipta alba var. longifolia Bettfr., Eclipta alba var. parviflora (Wall. Ex DC) Miq., Eclipta alba var. prostrata (L.) Miq., Eclipta alba f. prostrata Huber, Eclipta alba f. zippeliana (Blume) Hassk. and Eclipta alba var. zippeliana (Blume) Miq. [ 1 ]
VERNACULAR NAMES
False daisy (English); urang-aring, aring-aring, ari-aring, biu, daun dakelin, dawah, keremak jantan, keremak hutan, nigus, urang-aring, urang-uring (Malay); li chang (Chinese); karisalaankanni (Tamil) [ 2 , 3 , 4 , 5 ].
CHARACTER
| Colour | Brownish-green |
| Odour | Characteristic |
| Taste | Tasteless |
IDENTIFICATION
Plant Morphology
E. prostrata is an annual or rather short-lived perennial herb, 10–80 cm tall with prostrate or erect branches and usually numerous, small, white heads, hairy and reddish brown. Leaves are simple, oblong to lance-shaped, opposite, sessile or short-stalked, with less coarse hairs; margins entire or slightly toothed, size up to 2−6 cm long like with toothed margin and hairy. Inflorescence terminal and axillary, about 1 cm across, white or cream, on peduncles to 7 cm long. Flowers are white, small and arranged in small clusters, which resemble small aster forms. Fruit achene, warted, brown or black in colour, size up to 2−3 mm long [ 6 , 7 ].
Microscopy
Powdered material consists of staurocytic stomata found on the epidermis, sponge cells, epidermal cells, pitted and spiral vessel cells, secretory cells and four types of trichome; capitate glandular trichome, fusiform glandular trichome, ornamented simple unicellular trichome, and ornamented glandular trichome.








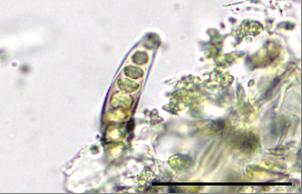



Figure 2 : Microscopic characters of E. prostrata leaves powder of 0.355 mm size. (a) Staurocytic stomata (magnification 100x); (b) sponge cells on surface view (magnification 40x); (c) sponge cells (side view) (magnification 40x); (d) epidermal cells (magnification 40x); (e) pitted vessel cells (magnification 40x); (f) spiral vessel cells (magnification 40x); (g) secretory cells (magnification 40x); (h) capitate glandular trichome (magnification 40x); (i) fusiform glandular trichome (magnification 40x); (j) capitate glandular trichome (magnification 40x); (k) ornamented simple unicellular trichome (magnification 40x); (l) ornamented glandular trichome. (magnification 40x). [Scale bars: a = 20 µm, b–l = 50 µm]
Chemical Tests
Observation of solution after treatment with various reagents:
| Test for the presence of saponins | positive (foam) |
Thin Layer Chromatography (TLC)

Figure 3 : TLC chromatogram of a wedelolactone (S) and ethanol extract of E. prostrata dried leaves powder (L) observed under (a) visible light (b) UV at 366 nm.
| Test solution | Weigh about 1 g of E. prostrata dried leaves powder of 0.355 mm size in 50 mL erlenmeyer flask. Add 10 mL of ethanol (100%). Sonicate the mixture at room temperature for 30 min. |
| Standard solution | Dissolve wedelolactone standard [CAS no.: 524-12-9] in ethanol to produce a standard concentration of 0.5 mg/mL. |
| Stationary phase | HPTLC silica gel 60 F254, 10 x 10 cm |
| Mobile phase | Toluene : acetone : formic acid; (11 : 6 : 1) (v/v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
High Performance Liquid Chromatography (HPLC)
| Test solution | Weigh about 1.0 g of E. prostrata dried leaves powder of 0.355 mm size in 50 mL erlenmeyer flask. Add 10 mL of ethanol (100%). Sonicate the mixture at room temperature for 30 min. Extract solution was filtered by using 0.22 µm nylon membrane filter and use the filtrate as test solution. |
| Standard solution | Dissolve wedelolactone standard [CAS no.: 524-12-9] in ethanol to produce a standard concentration of 0.5 mg/mL. |
| Chromatographic system | Detector: 351 nmColumn: C18 (3.5 µm, 4.6 mm I.D x 150 mm) (Waters XBridge-C18 if necessary)
Column oven temperature: 30˚C Flow rate: 0.7 mL/min Injection volume: 1 µL |
| Mobile phase (Isocratic mode) | Water + 0.2% formic acid : acetonitrile; (65 : 35) (v/v) |
| Run time | 15 min |
| System suitability requirements | Perform at least five replicate injections of the standard solutions (0.5 mg/mL). The requirements of the system suitability parameters are as follow:
|
| Acceptance criteria |
|

(a)

(b)
Figure 4 : Whole HPLC chromatogram of (a) wedelolactone standard solution (0.5 mg/mL) at tr = 4.816 min and (b) ethanol extract of E. prostrata dried leaves powder showing peak corresponding to wedelolactone standard solution at tr = 4.816 min.

(a)

(b)
Figure 5 : HPLC chromatogram highlighting the elution of wedelolactone in (a) wedelolactone standard solution (0.5 mg/mL) and (b) ethanol extract of E. prostrata dried leaves powder showing peak corresponding to wedelolactone standard solution at tr = 4.816 min.

Figure 6 : UV spectrum of wedelolactone standard solution (0.5 mg/mL) and ethanol extract of E. prostrata dried leaves powder.
PURITY TESTS
The purity tests, except foreign matter test are based on E. prostrata dried leaves powder of 0.355 mm particle size.
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 16% |
| Acid-insoluble ash | Not more than 4% |
| Water soluble ash | Not less than 3% |
| Loss on Drying |
| Not more than 8% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 26% |
| Cold method | Not less than 18% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 14% |
| Cold method | Not less than 10% |
SAFETY TESTS
The safety tests are based on E. prostrata dried leaves powder of 0.355 mm particle size.
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Methanol extracts of E. prostrata dried leaves was found to contain alkaloids (e.g. 20-epi-3-dehydroxy-3-oxo-5,6-dihydro-4,5-dehydroverazine, 20-epi-verazine, verazine, ecliptalbine, 20-epi-4β-hydroxyverazine, 4β-hydroxyverazine, 20-epi-25β-hydroxyverazine, 25β-hydroxyverazine), saponin (e.g. dasyscyphin C), fatty acid (e.g. 10-methyldodecanoic acid methyl ester; 10-octadecenoic acid methyl ester; 1,2-benzenedicarboxylic acid butyl octyl ester; oleic acid eicosyl ester), sterol (e.g. β-sitosterol; [20R] 4α-dimethyl-9,19-cyclocholestan-3-ol-7-one), 2-ethyl-2-methyl-1-tridecanol; 1-heptatriacotanol) [ 8 , 9 , 10 ].
Ethyl acetate fraction from methanol extract of E. prostrata dried leaves was found to contain coumestan (e.g. wedelolactone) [ 11 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally, the decoction of E. prostrata leaves is consumed. The leaves are also used in hair treatment and help to promote the hair growth. The boiled leaves are rubbed on the gums for toothache [ 12 ]. It is also used for diarrhoea, constipation [ 13 ] and for body heat [ 14 ]. It is also has astringent effect [ 15 ].
Biological and pharmacological activities supported by experimental data
Antimicrobial activity
Hexane extract of aerial parts of E. prostrata (100 mg) showed antibacterial effect with inhibition zone of 12.3 mm against Salmonella typhi compared to ciprofloxacin (25 µg, 19.4 mm) using agar well diffusion method [ 16 ].
Hair growth promoting activity
Petroleum ether extract of dried aerial parts of E. prostrata (5% ointment) was applied topically on shaved dorsal hair of male Wistar rats (120 – 150 g) once a day for 30 days. The extract showed a minimum time to initiate the hair growth at (5 ± 0.2 days) and completion time for hair growth at (19 ± 0.61 days) comparable to minoxidil treated group at (initiation time : 6 ± 0.41 days; completion time : 20 ± 1.08 days). The extract also showed significant (p < 0.05) increased in the percentage of hair follicles after 30 days (233.3 ± 1.2%) compared to minoxidil at (203 ± 1.1%) [ 17 ].
Petroleum ether extract of aerial parts of E. prostrata (5 mg) in vehicle mixture (propylene glycol : ethanol : dimethyl sulfoxide, 67 : 30 : 3% v/v/v) was applied topically on the backs skin of male Athymic nude BALB/c mice (aged of 7 weeks) once daily for 20 days. The extract showed significant (p < 0.001) hair growth at more than 75% of hair coverage on day 16 compared to minoxidil (25 – 50% of hair growth) [ 18 ].
Hepatoprotective effect
Aqueous extract of E. prostrata leaves (500 mg/kg) administered orally for seven days to either sex of Wistar albino rats (100 – 200 g) upon induction of hepatotoxicity with paracetamol (2 g/kg) showed significant (p < 0.05) reduction of elevated level of aspartate transaminase (AST: 97.91 ± 1.65 U/L), alanine transaminase (ALT: 60.87 ± 1.33 U/L), alkaline phosphate (ALP: 14.12 ± 0.88 U/L), lactate dehydrogenase (LDH: 115.53 ± 10.39 U/L) and gamma glutamyl transferase (γ-GT: 2.11 ± 0.05 U/L) compared to the paracetamol-induced group (AST: 265.67 ± 13.33 U/L; ALT: 166.61 ± 11.60 U/L; ALP: 45.43 ± 1.57 U/L; LDH: 195.47 ± 13.54 U/L; γ-GT: 4.85 ± 0.02 U/L) and normal control (AST: 93.26 ± 1.14 U/L; ALT: 58.49 ± 1.36 U/L; ALP: 11.62 ± 1.21 U/L; LDH: 110.30 ± 10.60 U/L; γ-GT: 2.01 ± 0.01 U/L) [ 19 ].
Ethanol (50%) extract of E. prostrata leaves (0.1 g/kg and 0.25 g/kg) administered orally to albino mice (15-30 g) daily for five consecutive days after 48 hr induction of hepatotoxicity with paracetamol (500 mg/kg solution) showed significant (p < 0.05) reduction in the elevated serum transaminase levels (41.01 ± 6.82 and 34.62 ± 3.86 IU/L) compared to paracetamol-treated group (50.31 ± 6.81 IU/L) [ 20 ].
Antidiabetic activity
Ethanol extract of E. prostrata dried leaves (200 mg/kg) administered orally to alloxan-induced Swiss albino mice (30-32 g) daily for six consecutive weeks showed significant (p < 0.01) decreased in the blood glucose level (98.0 ± 0.59 mg/dL) compared to diabetic control group (290.0 ± 1.56 mg/dL). The extract also showed significant (p < 0.001) decreased in ALT (29.5 ± 1.12 IU/L), AST (31.3 ± 1.33 IU/L) and ALP (104.23 ± 1.56 IU/L) compared to diabetic control group (AST: 80.74 ± 0.81 IU/L; AST: 83.74 ± 0.81 IU/L; ALP: 186.3 ± 1.32 IU/L) [ 21 ].
Clinical studies
Antidiabetic activity
A study involving 60 type 2 diabetic patients who were on sulfonylurea were selected for antidiabetic activity. The patients were divided into control and experimental group (30 patients each group). Experimental group were received ethanol (50%) extract of E. prostrata leaves tablets (2 g/kg and 4 g/kg) and were asked to take twice daily for a period of 90 days. Post prandial blood glucose levels in experimental group decreased from 220 mg/dL to 150 mg/dL in patients receiving 2 g/kg of extracts and 120 mg/dL in patients receiving 4 g/kg of extracts compared to control group (gluco care) from 180 mg/dL to 170 mg/dL [ 22 ].
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Information and data have not been established.
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
DOSAGE
Whole plant (3 – 6 mL in fresh juice) and 13 – 36 g in decoction [ 23 ].
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The Plant List. [Internet] Eclipta prostrata 2013 [cited on September 2018]. Available from: www.theplantlist.org
- Burkill LH. A Dictionary of the Economic Products of Malay Peninsula. Vol 1 (A-H). Kuala Lumpur: Ministry of Agriculture Malaysia. 1966. p595-597.
- Jukema J, Wulijarni-Soetjipto N, Lemmens RHMJ, Hildebrand JW.. Eclipta alba(L.) Hassk. In: Lemmens RHMJ, Wulijarni-Soetjipto N. (Edt.). Plant Resources of South-East Asia No. 3: Dye and tannin producing plants. Bogor: Prosea Foundation.1991 p133-134.
- Flora of China, li chang Eclipta prostrata (Linnaeus) Linnaeus, Mant. Pl. 2: 286. 1771.
- Encyclopedia of life. [Internet] Eclipta prostrata [cited on Oct 2018]. Available from: www.oel.org
- Holm L, Pancho JV, Herberger JP & Plucknett DL. 1979. A geographical atlas of world weeds. New York (USA): John Wiley & Sons, Inc. p.391.
- Li, H. 1978. Compositae. Flora of Taiwan 4:768-965.
- Abdel-Kader MS, Bahler BD, Malone S, Werkhoven MCM, Troon FV, David, Wisse JH, Bursuker I, Neddermann KM, Mamber AW, Kingston DG. DNA-damaging steroidal alkaloids from Eclipta alba from the Suriname rainforest. Journal of Natural Products 1998:61:1202-1208.
- Khanna VG, Kannabiran K. Non-proliferative activity of saponins isolated from the leaves of Gymnena sylvestre and Eclipta prostrata on HEPH2 cells-in vitro study. International Journal of Pharmaceutical Sciences and Research (IJPSR). 2010:1(8):38-42.
- Anand D, John Wyson W, Saravanan P, Rajarajan S. Phytochemical analysis of leaf extract of Eclipta alba (L.) Hassk by GC-MS Method. International Journal of Pharmacognosy and Phytochemical Research 2016:6(3):562-566.
- Yuliana T. Isolation and purification of wedelolactone from Eclipta alba Hassk Plant. Indonesian Journal of Applied Chemistry 2013:15(1):1-7.
- Burkill IH. Some changes in plant names. Bulletin of miscellaneous information (royal botanic gardens, Kew) Springer.1935:1935(5):316-319.
- [Internet]. 2016 Eclipta alba [cited on November 2018]. Available from: www.uses.plantnet-project.org.
- Mustapha NM, Nik Mahmood NZ, Mohd Ali NA, Haron N. Khazanah Perubatan Melayu. Tumbuhan Ubatan. Vol.I. Selangor: Institut Penyelidikan Perhutanan Malaysia. 2017:264-265.
- Indonesian Materia Medica [Internet] [cited on November 2018]. Available from: https://books.google.com.my.
- Pandey MK, Singh GN, Sharma RK, Lata S. Antibacterial activity of Eclipta alba (L.) Hassk. Journal of Applied Pharmaceutical Science. 2011:1(7):104-107.
- Roy RK, Thakur M, Dixit VK. Hair growth promoting activity of Eclipta alba in male albino rats. Archives of Dermatological Research 2008:300:357-364.
- Begum S, Lee MR, Gu LJ, Hossain J, Sung CK. Exogenous stimulation with Eclipta alba promotes hair matrix keratinocyte proliferation and downregulates TGF-β1 expression in nude mice. International Journal of Molecular Medicine. 2015:35:496-502.
- Prabu K, Kanchana N, Mohamed Sadiq A. Hepatoprotective effect of Eclipta alba on paracetamol induced liver toxicity in rats. Journal of Microbiology and Biotechnology Research 2011:1(3):75-79.
- Tabassum N, Agrawal SS. Hepatoprotective activity of Eclipta alba Against paracetamol induced hepatocellular damage in mice. Experimental Medicine. 2004:11(4):278-280.
- Nivedita, Vijay P. Antidiabetic effects of alba on alloxan-induced diabetic mice. International Journal of Pharmaceutical Sciences and Research. 2015: 308-314.
- Singh B, Saxena AK, Chandan BK, Agarwal SG, Bhatia MS and Anand KK. Hepatoprotective effect of ethanolic extract of Eclipta alba on experimental liver damage in rats and mice. Phytotheraphy Research. 1993;7:154-158.
- The Ayurvedic Pharmacopoeia of India Part – II (Formulations) Vol II). [Internet] [31 October 2018]. Available from: https://naturalingredient.org/wp/wp-content/uploads/API-II-Vol-2.pdf








