MAS COTEK LEAF
Ficus deltoidea var. deltoidea Jack
Moraceae
DEFINITION
Mas cotek leaf consists of dried leaves of F. deltoidea var. deltoidea Jack .
SYNONYM
None.
VERNACULAR NAMES
Mas cotek, serapat angin, telinga beruk, pokok barito, pokok raja ubat, secotek emas (Malay); mistletoe fig, mistletoe rubber plant (English) [ 1 , 2 ].
CHARACTER
The dried leaves are brown in colour, odourless and have slight taste.
IDENTIFICATION
Plant Morphology
The leaves of F. deltoidea are deltoid with a distinctive forked midrib, leaf length is from 3.0-5.0 cm and width from 3.0-4.0 cm, light green leaf and leathery; upper side shows rusty golden spots and the lower side yellowish green with black spots; leaf base is cuneate and apex shape is rounded or truncate; petiole length 0.7-1.3 cm [ 3 ].
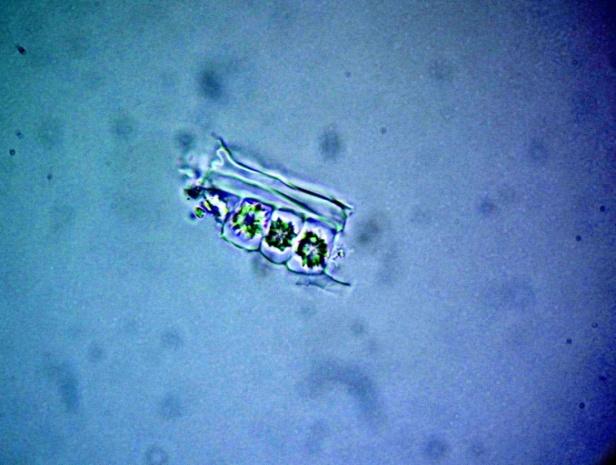
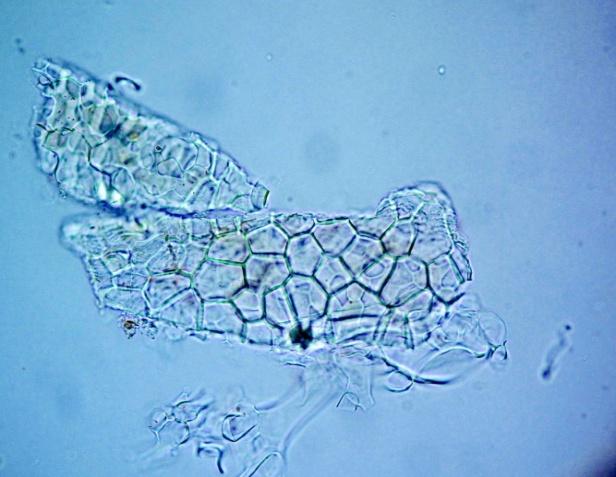
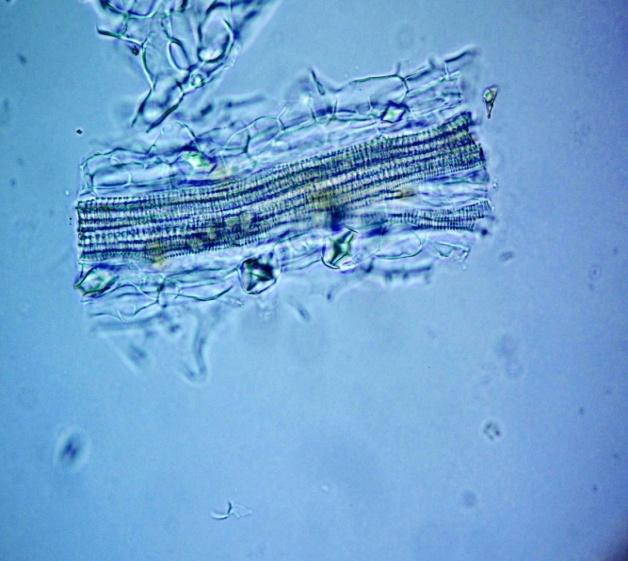
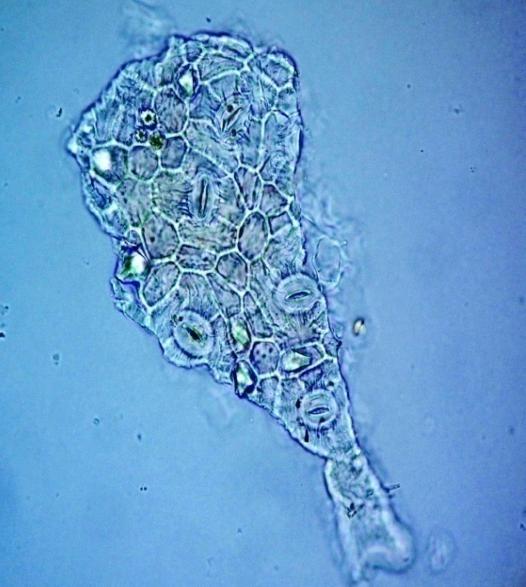
Microscopy
The leaves powder has fairly abundant stomata. It also has scelereid which occur singly elongated, rectangular in outline.Vessel also is abundance. Parenchyma cell can be easily identified.
Colour Tests
Observed colour of solution after treatment with various reagents:
| H2SO4 (conc.) | Reddish black |
| HCl (conc.) | Dark brown |
| 5% NaOH | Brown |
| 5% KOH | Brown |
| 25% NH4OH | Reddish brown |
| 5% FeCl3 | Greenish brown |
Thin Layer Chromatography (TLC)
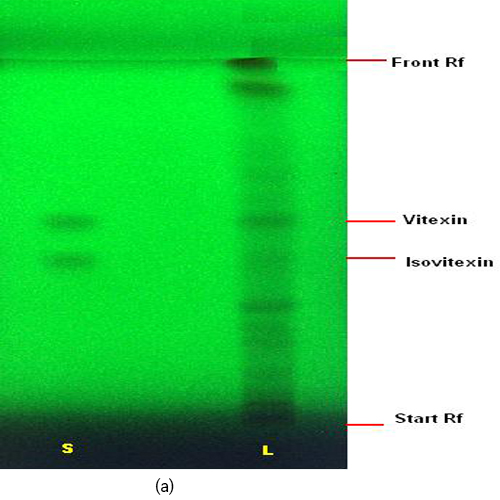
Figure 3 : TLC profiles of a mixture of isovitexin and vitexin standards (S) and methanol extract of F. deltoidea var. deltoidea leaves (L) observed under (a) UV at 254 nm
| Test Solutions | Weigh about 5.0 g of F. deltoidea var. deltoidea dried leaves powder in round bottom flask and add 50 mL methanol. Reflux the sample for 30 min and allows to cool. Filter the mixture and use the filtrate as the test solution. |
| Standard solution | Separately dissolve 2.0 mg of isovitexin and vitexin standards in 10 mL of methanol to give 200 µg/mL solutions. Pipette 1.5 mL of isovitexin and 1.5 mL of vitexin into a 10-mL volumetric flask and make up to volume with methanol to give 30 µg/mL solution. |
| Stationary Phase | HPTLC silica gel 60 F254, 5 x 10 cm |
| Mobile phase | Ethyl acetate-formic acid-glacial acetic acid-water, 10:1.1:1.1:2.6 (v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
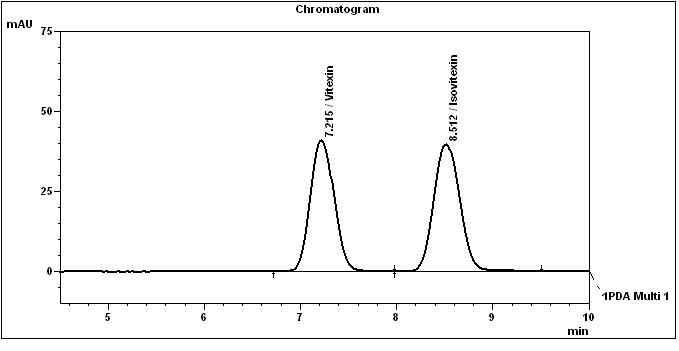
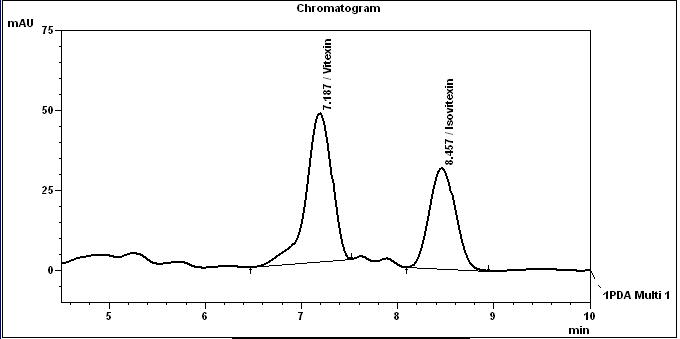
High Performance Liquid Chromatography (HPLC)
| Test solution (Ethanol Extract) |
Extract about 5.0 g of dried powder of F. deltoidea var. deltoidea dried leaves powder with 80 mL of ethanol by reflux for 30 min. Filter the mixture through a filter paper. Evaporate the filtrate to dryness using a rotary evaporator. Then, dissolve 50.0 mg of the dried extract in 10 mL of methanol. Filter the mixture solution through a 0.45 µm syringe filter and inject the filtrate into the HPLC column. |
| Reference standard | Separately dissolve 2.0 mg of isovitexin and vitexin standards in 10 mL of methanol to give 200 µg/mL solutions. Pipette 1.5 mL of isovitexin and 1.5 mL of vitexin into a 10-mL volumetric flask and make up to volume with methanol to give 30 µg/mL solution. |
| Chromatographic system |
Detector: UV 330 nm Column: C18 (5 µm, 4.6 mm I.D x 250 mm) Column oven temperature: 30 °C Flow rate: 1.0 mL/min Injection volume: 10 µL |
| Mobile Phase (Isocratic mode) |
Isocratic elution using the mobile phase described below:
|
| System suitability requirement |
Perform at least five replicate injections of the standard mixture (30 µg/mL). The requirements of the system suitability parameters are as follow:
|
| Acceptance criteria |
|
Table 1 : The Relative Retention Times (RRT) for the four characteristic peaks
| Standard | RRT |
| Vitexin (as reference) | 1.00 |
| Isovitexin | 1.18 |
Note: The RRTs provided only serve as a guidance.
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 8% |
| Acid-insoluble ash | Not more than 1% |
| Loss on Drying |
| Not more than 13% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 26% |
| Cold method | Not less than 19% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 15% |
| Cold method | Not less than 12% |
SAFETY TEST
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Aqueous and methanol extracts of the leaves have been found to contain flavonoids (e.g. vitexin, isovitexin), tannins, polysaccharides and proteins [ 4 , 5 ]. Additionally, the aqueous extract also had flavonoids (e.g. flavan-3-ol, flavone glycosides, chalcone) and others (e.g. phenylalanine, cinnamic acid, proanthocyanidins) [ 6 ]. Whereas, the methanolic extract had flavonoids (e.g. moretenol, rutin, narigenin, quercetin) [ 7 , 8 ].
Essential oil of the leaves has been found to contain monoterpenes (e.g. 6-methyl-5-heptane-2-one, mycrene, (Z)-β-ocimene, (E)-β-ocimene, cis-furanoid linalool oxide, trans-furanoid linalool oxide, linalool, limone), sesquiterpenes (e.g. dendrolasine, α-cubebene, cyclosativene, α-ylangene, α-copaene, β-bourbonene, 1,5-diepi-β-bourbonene, β-cubebene, β-elemene, α-gurjunene, α-cis-bergamotene, β-aryophyllene, α-santalene, selina-3,6-diene, cda-trans bergamotene, α-humulene, alloaromadendrene, aciphyllene, germacrene δ, β-selinene, δ-selinene, α-selinene, bicyclogermacrene, α-muurolene, germacrene A, α-amorphene, δ-cadinene, (E,E)- α-farnesene, 2-epi-α-selinene, α-cardinene, cadina-1,4-diene, germacrene β, caryophyllene oxide) and others (e.g. phenol, 2,4-bis(dimethylbenzyl)-6-t-butylphenol, cyanogen, octaethylene glycol, octaethylene glycol monododecyl ether, phthalic acid, 6,10,14-trimethyl-2-pentadecanone, carbonic acid, 1,4,7,10,13,16-hexaoxacyclooctadecane, hexagol, butanoic acid, 2,3-dihydro-1,1,3-trime-1H-indene, 2-(diethylboryl)pheny]-15-crown-5, 4-amino-2,6-dimethyl-3-pyridyl-1-adamantanecarboxylate, methyl ester hexadecanoic acid, cyclopenthyl ester 2-methoxybenzoic acid, methyl 16-methylheptadecanoate, 1-propoxy-3,3-diethyltriazene 2-oxide, heptacosane, hexagol) [ 9 , 10 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data.
The decoction of the leaf of F. deltoidea is used mainly by women as afterbirth treatment. It is believed that it helps to contract the uterine and the vaginal muscles, improve blood circulation and regain body strength as well as for treating disorders related to the menstrual cycle [ 11 , 12 ].
Biological and pharmacological activities supported by experimental data
Anti-inflammatory activity
Standardised methanol and aqueous extracts of F. deltoidea var. deltoidea leaves at concentration of 10 mg/mL were evaluated for anti-inflammatory activity using lipoxygenase (LOX), hyaluronidase (HAase) and TPA-induced oedema assay methods. Vitexin and isovitexin were used as marker for extracts standardization. The result of LOX assay using 15-soybean lipoxygenase show that both methanol and aqueous extracts from both varieties did not display anti-inflammatory activity via LOX mechanism (0-10.35% inhibition). Result for HAase assay indicated that both methanol and aqueous extracts show moderate anti-inflammatory property by inhibiting HAase at 47.05-62.95% and 48.97-51.00% inhibition activity respectively. TPA-induced ear oedema test result shows that anti-inflammatory activity of methanolic extracts (38.74-80.46%) was comparable to apigenin, nordihydroguaiaretic acid, indomethacin, which were used as control. However, aqueous extracts show very low activity in this test (8.38-22.53% inhibition). This study indicates that standardized extracts of leaves of F. deltoidea possess anti-inflammatory properties [ 13 ].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical study (Toxicology studies)
Acute toxicity
Oral single dose acute toxicity study using aqueous mixture of F. deltoidea var. deltoidea leaves on female Sprague Dawley rats (aged between 8 and 12 weeks old) showed no toxic effect on the parameters observed, which include behaviours, body weight, food and water intakes. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. Half lethal dose (LD50) is more than 2,000 mg/kg body weight [ 14 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- Bailey LH, Bailey EZ. Hortus. 3rd ed. Macmillan General Reference, NY. 1976.
- Yaacob M. Penanaman tumbuhan ubatan & beraroma. Institut Penyelidikan dan Kemajuan Pertanian Malaysia (MARDI), Kementerian Pertanian dan Industri Asas Tani, Malaysia. 2005;p.21-27.
- Mat N, Rosni NA, Rashid NZA, Haron N, Nor ZM, Nudin NFH, Yunus AG, Ali AM. Leaf morphological variations and heterophylly in Ficus deltoidea Jack (Moraceae). Sains Malaysiana. 2012;41(5):527-538.
- Abdullah Z, Hussain K, Zhari I. Rasadah MA. Anti-inflammatory activity of standardised extracts of leaves of three varieties of Ficus deltoidea. International Journal of Pharmaceutical Chemistry Research. 2009;1(3):100-105.
- Abdullah Z, Hussain K, Zhari I, Rasadah MA, Mazura P, Jamaludin F, Sahdan R. Evaluation of extracts of leaf of three Ficus deltoidea varieties for antioxidant activities and secondary metabolites. Pharmacognosy Research. 2009;1(4):216-223.
- Omar MH, Mullen W, Crozier A. Identification of proanthocyanidin dimers, and trimers, flavone C-glycosides, and antioxidants in Ficus deltoidea, a Malaysian herbal tea. Journal of Agricultural and Food Chemistry. 2011;59(4):1363-1369.
- Mohd-Lip J, Nazrul-Hisham D, Arif-Zaidi, Musa Y, Ahmad AW, Normah A, Sharizan A. Isolation and Identification of moretenol from Ficus deltoidea leaves. Journal of Tropical Agriculture and Food Science. 2009;37(2):195-201.
- Ong SL, Ling APK, Poospooragi R, Moosa S. Production of flavonoid compounds in cell cultures of Ficus deltoidea as influenced by medium composition. International Journal of Medicinal and Aromatic Plants. 2011;1(2):62-74.
- Grison-Pigé L, Hossaert-McKey M, Greeff JM, Bessiére JM. Fig volatile compounds – a first comparative study. Phytochemistry. 2002;(61):61-71.
- Lee SW, Wee W, Yong JFS, Syamsumir DF. Characterization of antioxidant, antimicrobial, anticancer property and chemical of Ficus deltoidea Jack leaf extract. Journal of Biologically Active Products from Nature. 2011;1(1):1-6.
- Burkill IH, Haniff M. Malay village medicine. Garden’s Bulletin. 1930;6(2):67-332.
- Fasihuddin BA, Din LB. Medicinal Plants used by various ethnic groups in Sabah. Paper presented at The French Malaysian-Symposium on Natural Products. Department of Chemistry, University of Malaya, Kuala Lumpur. 2002;p.85.
- Zunoliza A, Hussain K, Ismail Z, Rasadah MA. Anti-inflammatory activity of standardized extracts of leaves of three varieties of Ficus deltoidea. International Journal of Pharmaceutical and Clinical Research. 2009;1(3):100-105.
- Teh BP, Hamzah NF, Rosli SNS, Yahaya MAF, Zakiah I, Murizal Z. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2012. Report No .: HMRC 11-045/01/FDD/L/J.