Sambung Nyawa Leaves
Gynura procumbens (Lour.) Merr
Asteraceae
DEFINITION
Sambung nyawa leaves consist of dried leaves of G. procumbens (Lour.) Merr. (Asteraceae).
SYNONYM
Cacalia procumbens Lour., C. sarmentosa Blume; Gynura cavaleriei H. Léveillé; G. emeiensis Z. Y. Zhu; G. sarmentosa (Blume) Candolle.
VERNACULAR NAMES
Leaves of the Gods, googoolipid, mollucan spinach (English); dewa raja, akar sebiak, kacham akar (Malay); bai bing cao, man san qi cao, lam fei yip, jian feng wei (Chinese)
CHARACTER
| Colour | Fleshy green leaves and shoot system |
| Odour | Odourless |
| Taste | Slightly pungent, flavourless, mild raw taste |
IDENTIFICATION
Plant Morphology
Herbs, annual evergreen shrub, scandent, twining vine, smooth except for the peduncles. Stems fleshy, procumbent, brownish or purple striate, glabrous or pubescent when young, branched. Leaves bright to darker green in colour,rather smooth to touch,stalked (uppermost ones stalkless), ovate-elliptic or lanceolate, 3.5-8 cm long, and 0.8-3.5 cm wide, with somewhat entire or toothed margins, leaf petiole 5-15 mm, glabrous, blade abaxially purplish, adaxially green, ovate, ovate-oblong, or elliptic, 3-8 × 1.5-3.5 cm, both surfaces glabrous, rarely sparsely pubescent, lateral veins 5-7-paired, curved, veinlets inconspicuous, base rounded-obtuse or cuneately attenuate into petiole, margin entire or repand-dentate, apex acute or acuminate; upper stem leaves and leaves on synflorescence branches reduced, lanceolate or linear-lanceolate, sessile or subsessile. Capitula 3-5 in each corymb, in terminal or axillary corymbs; peduncles long, slender, often with 1-3 linear bracts, sparsely shortly pubescent or glabrous; bracts involucral, involucres campanulate or funnelform, smooth, 15-17 × 5-10 mm, bracteoles at base 5 or 6, linear; phyllaries (9 or) 11-13, becoming purplish, oblong-lanceolate, 15-17 × ca. 1.5 mm, glabrous, 1-3-veined, margin narrowly scarious, apically acuminate; flowering heads panicled, narrow, yellow, and 1-1.5 cm long. Florets 20-30; corolla orange, 12-15 mm, with slender 8-10 mm tube and dilated limb, lobes ovate-lanceolate, apically acute; anthersobtuse at base, appendages triangular; style branch tips conical; papillose. Achenes brown, very small, smooth, with very close and slender ribs, cylindric, 4-6 mm, glabrous, 10-ribbed; pappus white, silky [ 1 , 2 ].
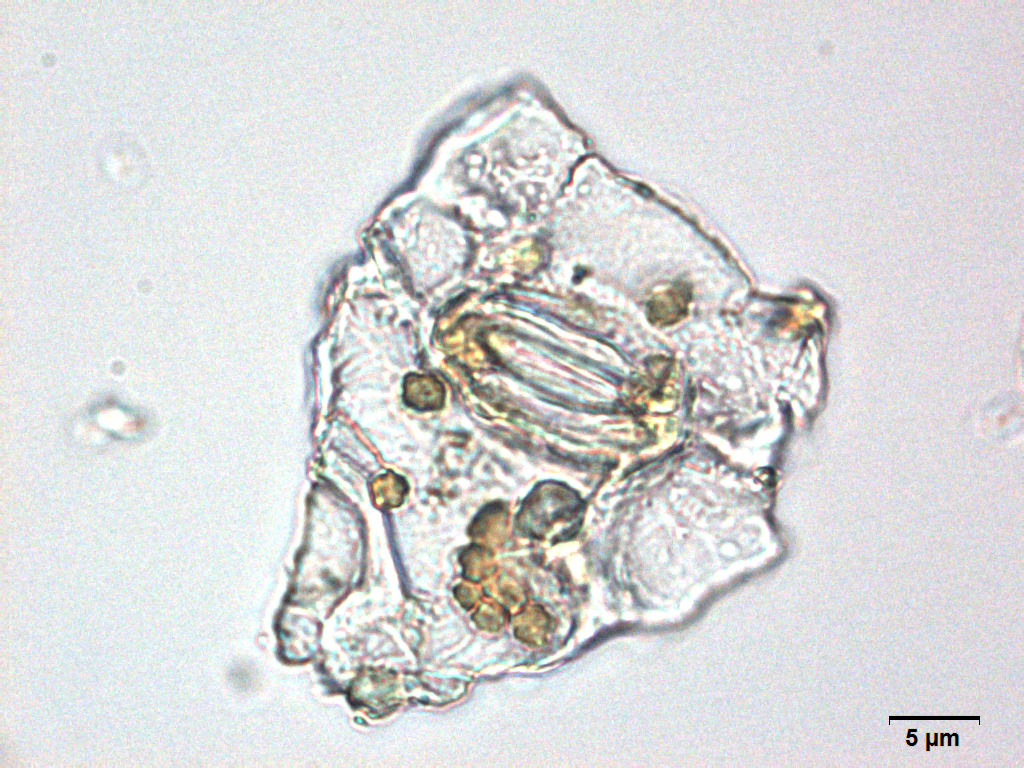
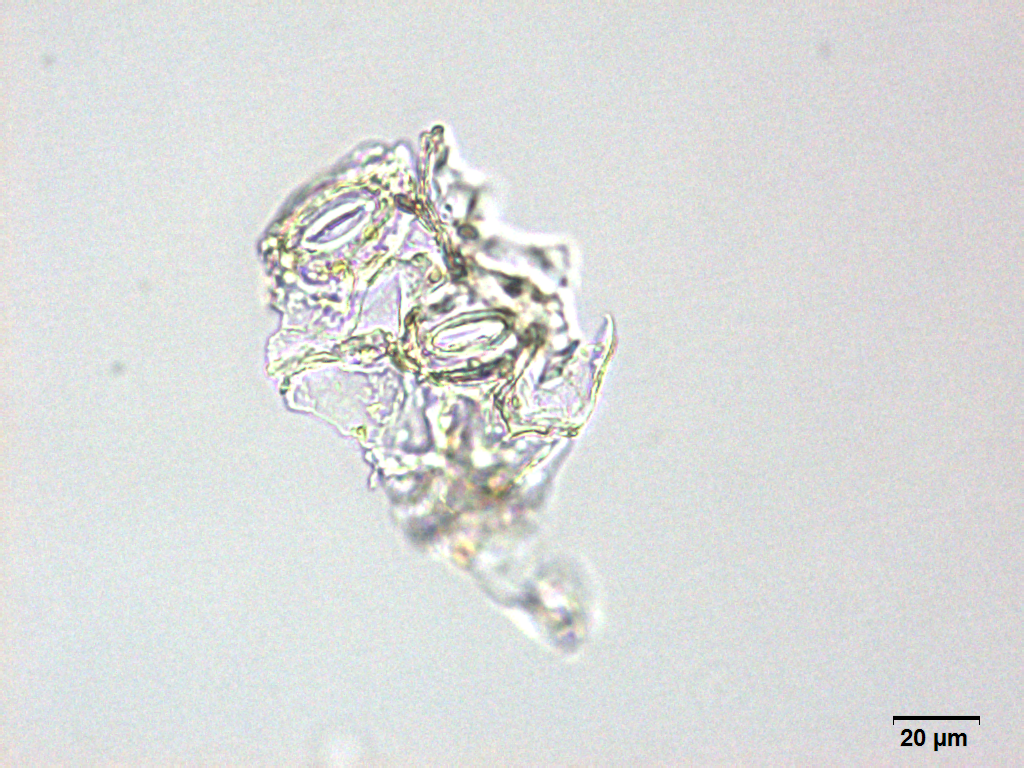
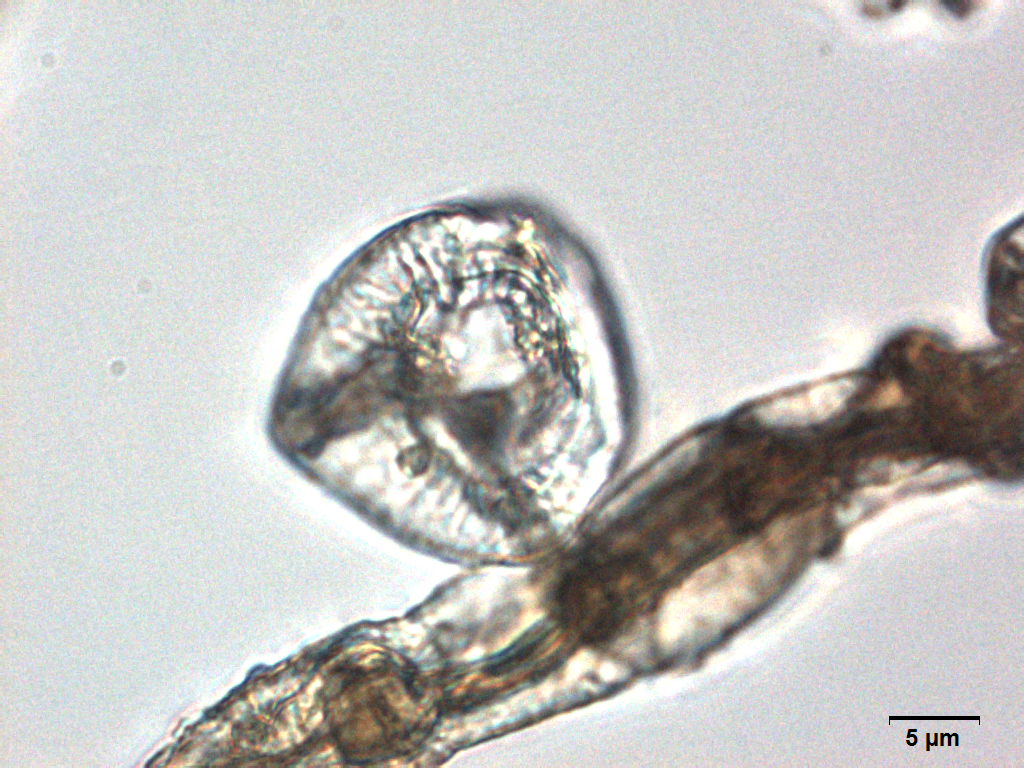
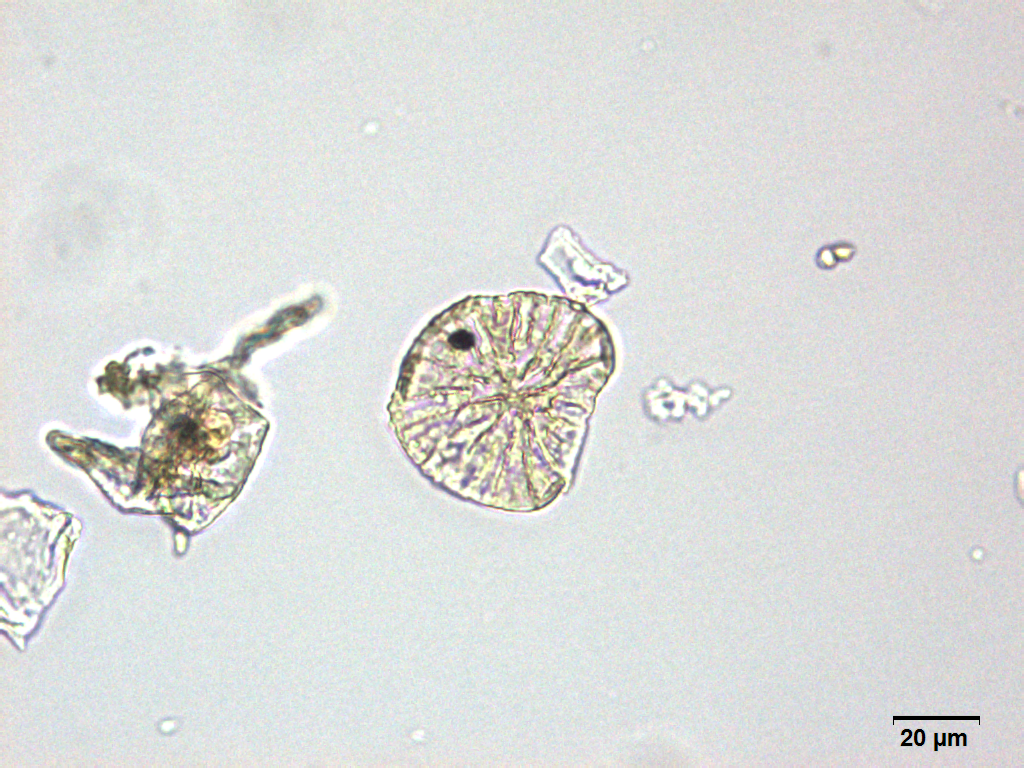
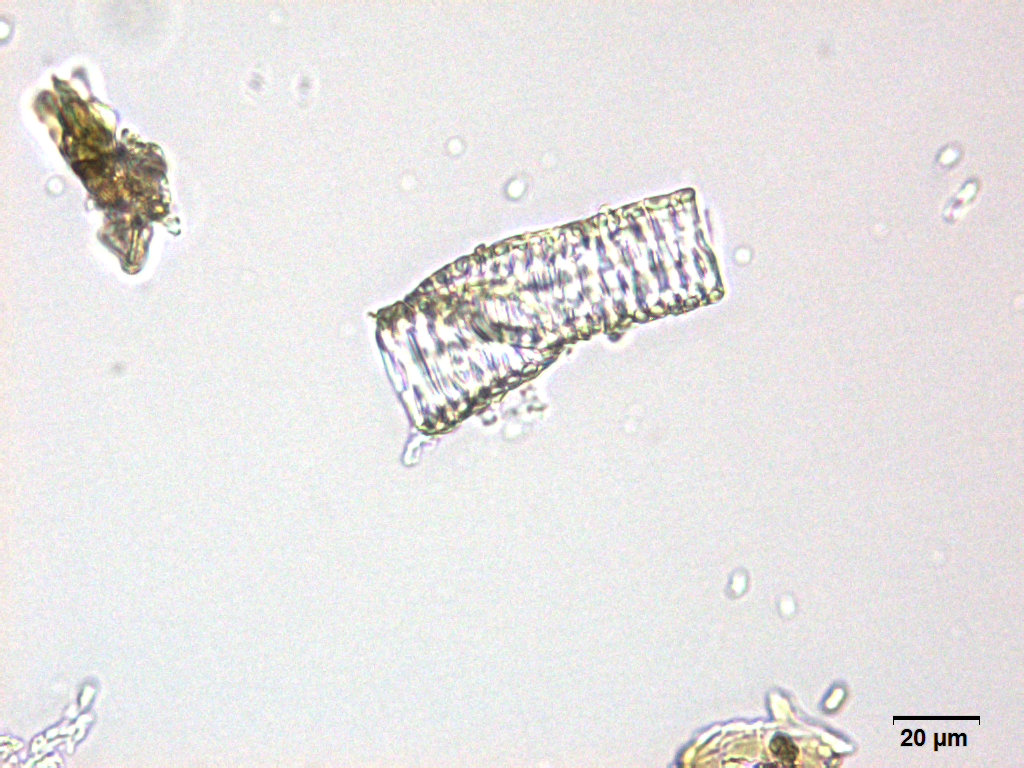
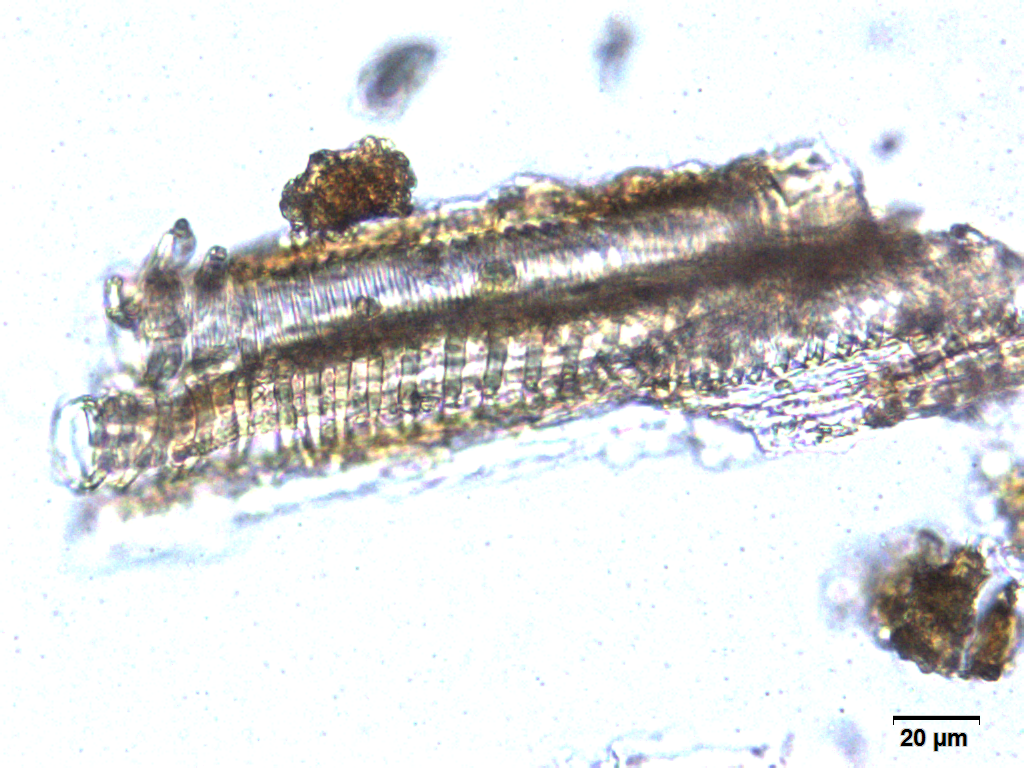
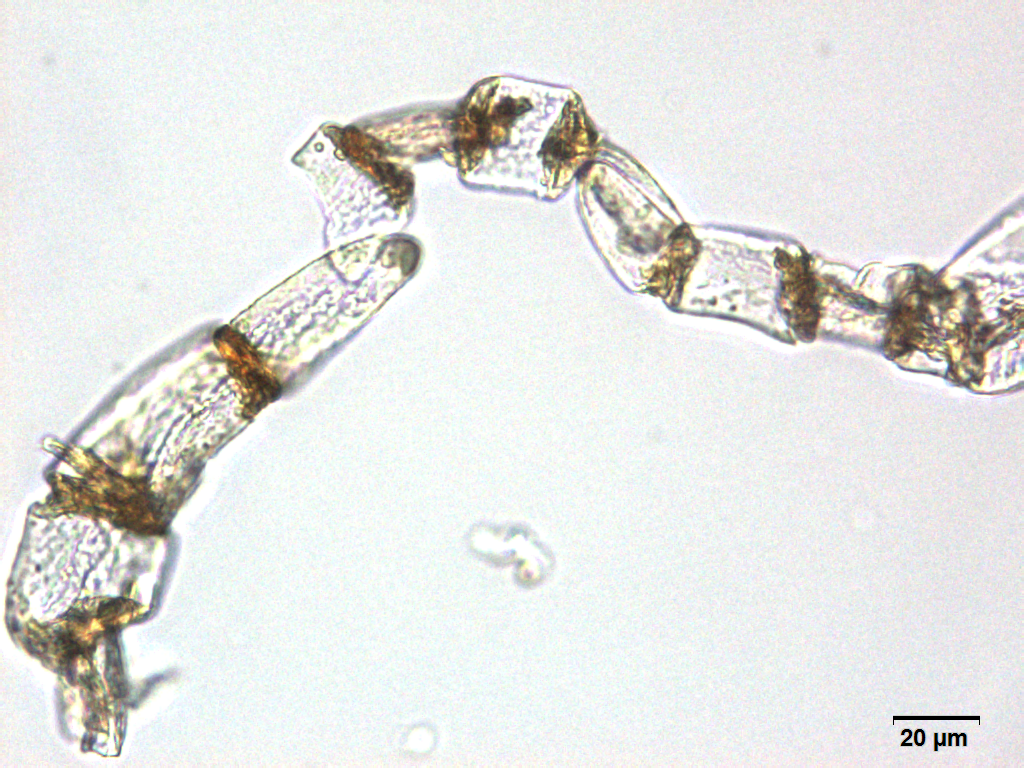
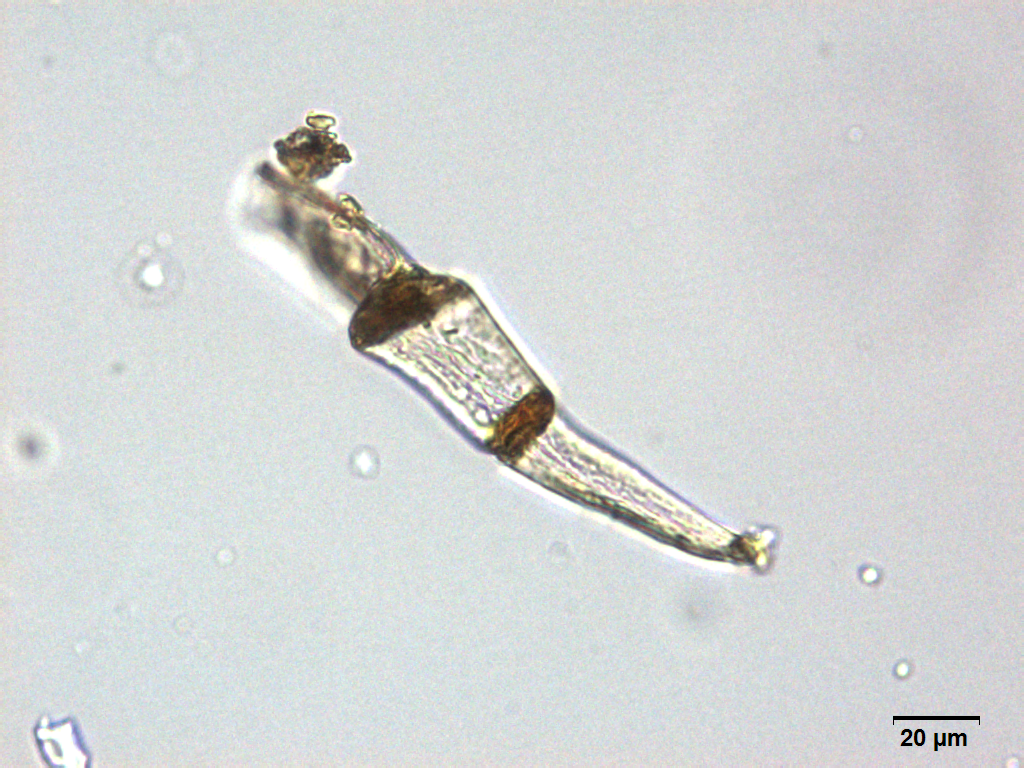
Microscopy
Microscopic characters of G. procumbens leaves powder consist of stomata attached to epidermis cells, parenchyma cells, simple multicellular trichome, spiral vessel cells, pitted vassel cells and fibre cell.
Colour Tests
Observed colour of solution after treatment with various reagents:
| H2SO4 (conc.) | Green |
| HCl (conc.) | Green |
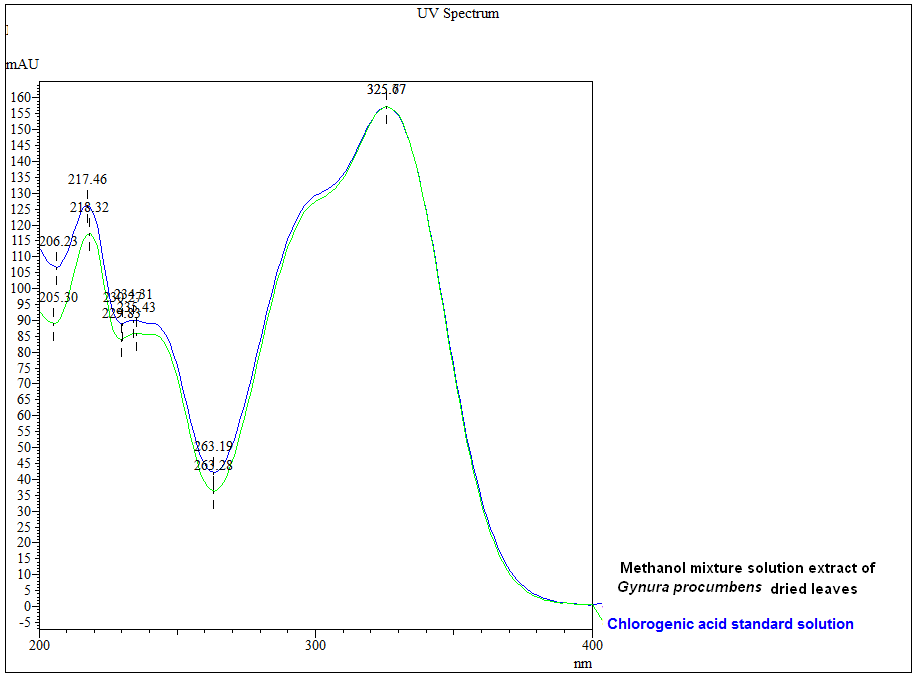
Thin Layer Chromatography (TLC)
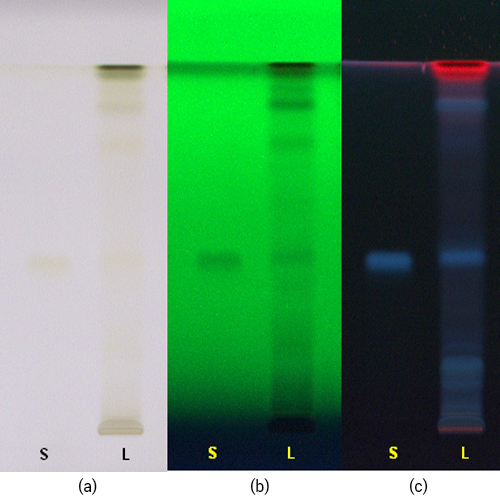
Figure 3 : TLC profiles of chlorogenic acid (S), methanol mixture solution extract of G. procumbens dried leaves powder (L) observed under (a) UV at visible light, (b) UV at 254 nm and (c) UV at 366 nm.
| Test Solutions | Weigh about 1.0 g of G. procumbens dried leaves powder in a 50 mL screw-capped conical flask and add 10 mL methanol mixture solution [methanol: hydrochloric acid (conc.); (10:0.1) v/v]. Sonicate the mixture for 15 min and filter. Use the filtrate as test solution. |
| Standard solution | Dissolve 5.0 mg of chlorogenic acid standard in 10 mL methanol to produce 500 µg/mL solution. |
| Stationary Phase | HPTLC Glass Silica Gel 60 F254, 10 x 10 cm. |
| Mobile phase | Ethyl acetate : Formic acid : Glacial acetic acid : Water; (25:3:3:5) (v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
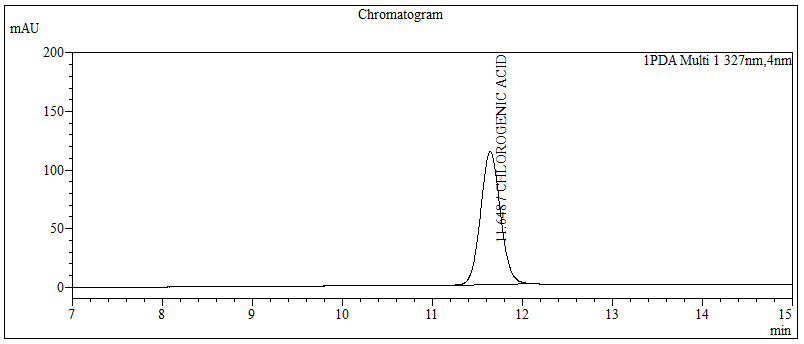
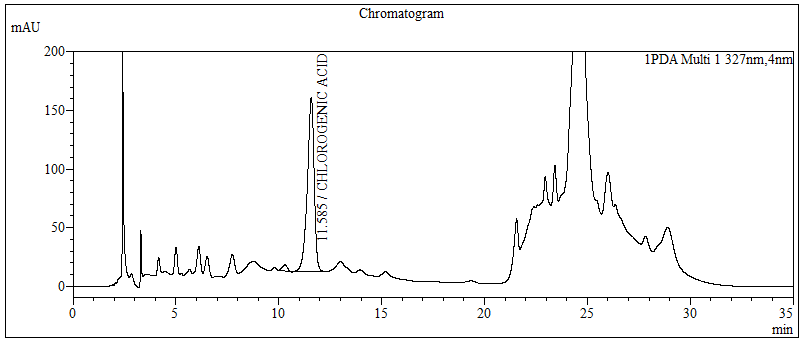
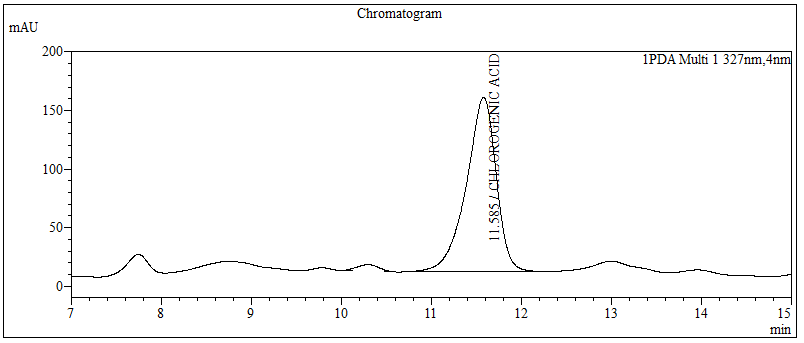
High Performance Liquid Chromatography (HPLC)
| Test solution | Extract about 1.0 g of the G. procumbens dried leaves powder with 10 mL methanol mixture solution [methanol : hydrochloric acid (conc.); (10:0.1) v/v]. Sonicate the solution for 30 min. Filter the solution through a 0.45 µm syringe filter and inject the filtrate into the HPLC column. | |||||||||||||||||||||
| Standard solution | Dissolve 5.0 mg of chlorogenic acid standard in 10 mL of methanol to produce 500 µg/mL solution. | |||||||||||||||||||||
| Chromatographic system |
Detector: UV 327 nm Column: C18 (5 µm, 4.6 mm I.D x 150 mm); Phenomenex Luna C18 (2) is recommended Column Oven temperature: 40°C Flow rate: 0.7 mL/min Injection volume: 1 µL (standard solution) 10 µL (Test solution) |
|||||||||||||||||||||
| Mobile Phase (Isocratic mode) |
|
|||||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the standard solutions (500 µg/mL). The requirements of the system suitability parameters are as follow:
|
|||||||||||||||||||||
| Acceptance criteria |
|
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 18% |
| Acid-insoluble ash | Not more than 1% |
| Loss on Drying |
| Not more than 10% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 30% |
| Cold method | Not less than 24% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 9% |
| Cold method | Not less than 7% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
n-hexane extract of the leaves had stigmasterol; whereas the ethanol (95%) extract had flavonoids (e.g. quercetin-3-O-rutinoside, isobioquercetin and kaempferol-3-O-rutinoside)
[ 3 , 4 ].
Methanol extract of the leaves has been found to contain flavonoids (e.g. kaempferol-3-O-rutinoside, kaempferol-3-O-glucoside (astragalin), a sesquiterpenoid (e.g. muurol-4-ene-1β,3β,10β-triol), sesquiterpene glycosides (e.g. muurol-4-ene-1β,3β,10β-triol 3-O-β-D-glucopyranoside and muurol-4-ene-1β,3β,15-triol 3-O-β-D-glucopyranoside) and chlorogenic acid. n-butanol fraction of the leaf methanol extract had flavonoids (e.g. kaempferol 3-O-glucoside, kaempferol 3-O-rhamnosyl (1→6) glucoside, quercetin 3-O-rhamnosyl (1→2) galactoside and quercetin 3-O-rhamnosyl (1→6) glucoside) [ 5 , 6 , 7 , 8 ].
The leaves were also found to have β-sitosterol-3-O-gentiobioside, indole-3-carboxylic acid, grasshopper ketone, β-sitosterol, drummondone A, drummondone B and n-dotriacontanol [ 9 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally, G. procumbens isused for kidney problem.The plant is also used as decoction to treat dysentery [ 10 ]. Leaves of the plant can be eaten to treat diabetes and hypertension [ 11 ].
Biological and pharmacological activities supported by experimental data
Antiplasmodial activity
Ethanol (95% v/v) of G. procumbens leaves inhibited the growth of chloroquine-sensitive strains of Plasmodium falciparum 3D7 (IC50 = 42.23 ± 7.19 µg/mL) and Plasmodium berghei NK65 (IC50 = 14.38 ± 7.53 µg/mL) using modified parasite lactate dehydrogenase method. The extract when administered intraperitoneally for four days to P. berghei NK65-infected male ICR mice (aged seven weeks old) showed significant (p < 0.05) inhibition of parasitemia (44.97–84.73%) compared to chloroquine control (100% inhibition) [ 12 ].
Aqueous extracts of G. procumbens leaves inhibited the growth of chloroquine-sensitive strains of P. falciparum 3D7 (IC50 = 25.69 ± 4.34 µg/mL) and P. berghei NK65 (IC50 = 12.4 ± 6.02 µg/mL) using modified parasite lactate dehydrogenase method. The extract when administered intraperitoneally for four days to P. berghei NK65-infected male ICR mice (aged seven weeks old) showed significant (p < 0.05) inhibition of parasitemia (50.42% – 93.06%) compared to chloroquine control (100% inhibition) [ 12 ].
Anti-oxidant activity
Ethanol extract of G. procumbens leaves showed anti-oxidant activity with hydroxyl scavenging activity (effective concentration at 50% of cell growth (EC50) = 1.63 mg/mL), inhibition of lipid peroxidation activity (EC50 = 2.75 mg/mL) compared to malondialdehyde and iron chelating activity (EC50 = 2.17 mg/mL) compared to EDTA control [ 13 ].
Ethanol (95% v/v) extract of G. procumbens leaves showed anti-oxidant activity with 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of (EC50 = 13.7 ± 0.417 mg/mL) compared to vitamin C (EC50 8.31 ± 0.327 mg/mL) [ 14 ].
Anti-ulcer activity
Ethanol (95% v/v) extracts of G. procumbens leaves (50-400 mg/kg) was administered orally as single dose to adult male SpragueDawley rats (200-225 g/body weight) one hour prior to gastric ulcer induction using ethanol. A significant decrease in gastric ulcer areas (p < 0.05) with absence of submucosal edema and leucocytes infiltration were observed histologically compared to ethanol-induced control group [ 15 ].
Anticancer activity
Protein fraction SN-F11/12 from G. procumbens leaves inhibited the growth of MDA-MB-231 breast cancer cells (concentration of proteins in the extract that inhibited 50% of cell growth (EC50)= 3.8 μg/mL) using lactate dehydrogenase cytotoxicity assay. The protein fraction was also decreased the expression of proliferation markers Ki67 (p < 0.05) and proliferating cell nuclear antigen (PCNA) (p < 0.05) after 36 hours of incubation of the cancer cells with the protein fraction compared to untreated cancer cells. The invasion marker chemokine (C-C motif) ligand 2 (CCL2) (p < 0.05) was decreased after 48 hours incubation compared to untreated cancer cells [ 16 ].
Ethanol (80% v/v) extract of G. procumbens leaves (3.5 g/kg) was administered orally (twice weekly at week ten until week 36) to 4NQO-induced (three times weekly at week one until week eight) tongue carcinogenesis male Sprague Dawley rats (aged four weeks old) (Group 3). Another group of 4NQO-induced (three times weekly at week one until week eight) tongue carcinogenesis male Sprague Dawley rats were given similar extract (twice weekly from week zero until week ten) concomitantly (Group 2). At 36th week, 25% of squamous cell carcinoma (SCC) incidence was observed in group 3 compared to 33.3% SCC incidence in group 2 [ 17 ].
Ethanol (80% v/v) extract of G. procumbens leaves (3.5 g/kg) was administered orally (twice weekly at week 18 until week 36) to 4NQO-induced (three times weekly at week one until week 17) tongue carcinogenesis male Sprague Dawley rats (aged four weeks old) (Group 6). Another group of 4NQO-induced (three times weekly at week 1 until week 17) tongue carcinogenesis male Sprague Dawley rats were given similar extract (twice weekly from week 0 until week 18) concomitantly (Group 5). At 36th week, 83.3% of squamous cell carcinoma (SCC) incidence was observed in group 6 compared to 100% SCC incidence in group 5 [ 17 ].
Ethyl acetate fraction (EAF) from the ethanol extract of G. procumbens leaves inhibited the growth of MCF-7 breast cancer cells (IC50 = 270 μg/ml) compared to doxorubicin (DOX, IC50 = 441 nM) and 5- fluorouracil (5- FU, IC50 405 µM) using MTT assay. The extract also inhibited the growth of T47D breast cancer cells (IC50 =64 μg/mL) compared to DOX (IC50 = 56 nM) and 5- FU (IC50 = 222 µM) using the same assay. EAF (0-150 μg/mL) in combination with DOX or 5-FU also enhanced the growth inhibitory effect on MCF and T47D cells (combination index (CI) < 0.8) using similar assay. EAF (100 and 200 μg/mL) as single or in combination with DOX or 5-FU induced cell cycle arrest at G1 phase on both cells. Chromatin condensation and membrane shrinkage were observed morphologically on MCF-7. EAF (0-75 μg/mL) decreased the antibodies’ protein expressions (mSin3A and α-tubulin) in both cells [ 18 ].
EAF inhibited the growth of WiDr colon cancer cells (IC50 = 125 μg/mL) compared to 5- fluorouracil (5-FU, IC50 = 848 µM) and Cisplatin (Cisp, IC50 = 43 µM) using MTT assay. EAF (100 μg/mL) in combination with 5-FU enhanced the growth inhibitory effect on WiDr cells (CI 0.2-1.1) but showed antagonism effect when combined with cisplatin. EAF (100 and 200 μg/mL) as single treatment induced cell cycle arrest at G1 phase but when combined with 5-FU induced cell cycle arrest at both G1 and S phases on WiDr cells. Chromatin condensation and membrane shrinkage were also observed morphologically in treated cells [ 19 ].
Ethanol (96% v/v) extract of G. procumbens leaves (300 mg/kg/day) was administered orally for five weeks to male Sprague Dawley rats (aged 40 days old). 7, 12-dimethylbenz(a)antracene (DMBA) was given concomitantly once a week from second to forth week to induce liver carcinogenesis. A significant (p < 0.05) antiproliferative activity was observed in the liver of treated rats compared to the liver of DMBA treated control group [ 20 ].
Antidiabetic activity
Aqueous and ethanol (95% v/v) extracts of G. procumbens leaves (100 mg/kg/day) were administered orally to streptozotocin-induced diabetic male Sprague Dawley rats (aged 6 weeks old) for 42 days. In comparison to distilled water-treated diabetic control group, there was a significant (p < 0.05) decrease in fasting blood glucose level (19.5%, 56.8% respectively) and HbA1c level (12.8%, 13.7% respectively) were observed compared to distilled water-treated diabetic control group. A significant increase in liver hexokinase (17.7%, 36.6% respectively) and liver phosphofructokinase (ethanol extract: 90.2%) activity but decrease (p < 0.05, p < 0.01 respectively) in liver fructose-1, 6-biphosphatase activity (19.5%, 28.6% respectively) were observed compared to distilled water-treated diabetic control group. No change in plasma insulin concentration was observed in normal-treated and diabetic-treated rats [ 21 ].
Hexane, ethyl acetate and n-butanol fractions from the ethanol (95% v/v) extract of G. procumbens leaves (250 mg/kg/day) was administered orally to streptozotocin-induced diabetic male Sprague Dawley rats (aged 6 weeks old) for 14 days. These fractions showed significant (p < 0.05) decrease in blood glucose level (8.5 – 13.8 mmol/L) compared to diabetic-untreated control group (27.7 mmol/L). An increased in liver glycogen content (15-40 mg/g tissue, p<0.05) but no significant insulin secretion (0.144 – 0.159 ng/mL) were observed compared to diabetic-untreated control group (liver glycogen content <10 mg/g tissue, insulin secretion = 0.145 ng/mL). Western blot analysis showed the expression of glycogen synthase kinase 3β (Ser-9) in the liver of treated diabetic rats [ 22 ].
Aqueous and ethanol (0, 25, 50, 75 and 95% v/v) extract of G. procumbens leaves (1000 mg/kg) was administered orally as a single daily dose to streptozotocin-induced diabetic Sprague Dawley rats. After 14 days, each concentration of single extract significantly (p < 0.05) decreased the blood glucose level (8.4-13.2%) compared to diabetic control (28.4%). Aqueous and ethanol extract (25% v/v) at single daily dose also significantly (p < 0.05) decreased blood glucose level (19.30-22.62%) at 15 to 120 minutes post glucose administration in comparison to diabetic control group (21.37-23.62%). However, without prior glucose administration, only ethanol extract (25% v/v) showed significant (p < 0.05) decrease in blood glucose level (10.9-14.4%) at two to seven hours of treatment compared to diabetic control group (22.9-23.8%) [ 23 ]
Aqueous extract of G. procumbens leaves (500 and 1000 mg/kg) was administered orally to streptozotocin-induced diabetic male Sprague Dawley rats twice a day for 14 days before glucose administration. A significant (p < 0.05) decrease in serum glucose level (<15 mmol/L) from 15 to 120 minutes after glucose administration was observed in treated rats compared to saline treated group (>17 mmol/L) [ 24 ].
Aqueous extract of G. procumbens leaves (1000 mg/kg) was administered orally to streptozotocin-induced diabetic male Sprague Dawley rats twice a day for 14 days. A significant (p < 0.05) decrease in fasting blood glucose (<15 mmol/L) and body weight (167.4 g) but no significant changes in plasma insulin (2.15 ng/mL) were observed on day 14 in comparison to diabetic control group (>17 mmol/L; 189.0 g; 2.11 ng/mL) [ 24 ].
Aqueous extract of G. procumbens leaves (1000 mg/kg) was administered orally as a single dose to streptozotocin-induced diabetic male and female Sprague Dawley rats. A significant (p <0.05) decrease in fasting blood glucose level was observed throughout seven hour after extract administration (22 – 30%) but no significant insulin secretion were observed compared to saline-treated diabetic control group [ 25 ].
Ethanol (95% v/v) extract of G. procumbens leaves (50-300 mg/kg) was administered orally as single dose to streptozotocin-induced diabetic male Sprague Dawley rats (aged 7 weeks old) before glucose administration. After two hours, a significant (p < 0.05) decrease in serum glucose level (maximum 15.8% at 150 mg/kg extract) was observed compared to distilled water- treated diabetic control groups [ 26 ].
Aqueous extract of G. procumbens leaves extract (1 mg/mL) showed significant increase of glucose uptake (3.77 ± 0.35 mg/g tissue weight) in rat’s isolated abdominal muscle in the absence of insulin (p < 0.05) compared to insulin-absence control group (1.76 ± 0.35 mg/g tissue weight) while significantly higher glucose uptake (5.77 ± 0.32 mg/ g tissue weight) was showed in the presence of insulin (p < 0.001) compared to insulin-presence control group (3.06 ± 0.41 mg/ g tissue weight) [ 24 ].
Antihyperlipidaemic activity
Ethanol (95% v/v) extract of G. procumbens leaves (150 mg/kg/day) was administered orally to streptozotocin-induced diabetic male Sprague Dawley rats (aged 7 weeks old) twice daily for seven days. A significant (p < 0.05) decrease in serum total cholesterol (13.9%) and triglycerides level (26.7%) was observed in comparison to distilled water treated groups (total cholesterol: 5.2% increment, triglycerides: 72.9% increment) [ 26 ].
Fertility activity
Aqueous extracts of G. procumbens leaves (100 mg/kg/day) was administered orally to normal and streptozocin-induced diabetic male Sprague Dawley rats (aged two months old) for 14 days. Diabetic rats showed a significant (p < 0.05) decrease in blood glucose level (56.5%) and sperm mortality (38%) but increase (p < 0.05) in sperm counts (20%) and lactate dehydrogenase (LDH) activities (42%) in comparison to diabetic control group. Meanwhile, normal rats showed significant increase (p < 0.05) only in LDH activities (25%) but no significant changes was observed in sperm counts, sperm mortality and blood glucose level compared to normal control group [ 27 ].
Antihypertensive activity
Aqueous extract of G. procumbens leaves (500 mg/kg/day) administered orally to spontaneously hypertensive male rats (aged 10 weeks old) for four weeks significantly (p < 0.05) decreased systolic blood pressure, lactate dehydrogenase and creatine phosphate kinase and significantly (p < 0.001) increased nitric oxide in comparison to distilled water treated group [ 28 ].
Cardiovascular activity
Butanol fraction (BU) from the ethanol (96% v/v) extract of G. procumbens leaves (2.5-20 mg/kg) was administered intravenously to adult male albino Sprague Dawley rats for 10 minutes. The dose of 10 and 20 mg/kg significantly (p < 0.05) decreased the heart rate. The mean arterial pressure (MAP) was significantly (p < 0.001) decreased (effective dose at 50% of growth (ED50) = 4.77 mg/kg of BU) [ 29 ].
BU (10-6-10-1g/mL) significantly (p < 0.05) inhibited phenylephrine-induced tonic contraction in isolated endothelium-intact with negative logarithm of inhibition concentration at 50% of growth (pIC50) at 2.46 ± 0.04 g/mL and endothelium-denuded (pIC50= 2.38 ± 0.03 g/mL) rat’s aortic rings [ 29 ].
BU (10-6-10-1g/mL) inhibited potassium chloride (KCl)–induced tonic contraction in isolated endothelium-intact (pIC50 = 2.11 ± 0.06 g/mL) and endothelium-denuded (pIC50 = 2.19 ± 0.07 g/mL) rat’s aortic rings [ 29 ].
Pre-treatment with BU (2.5 x 10-3and 5 x 10-3g/mL) for 30 minutes significantly (p< 0.05) inhibited both phenylephrine and KCl-induced vasocontraction in isolated endothelium-intact and endothelium-denuded rat’s aortic ring compared to control [ 29 ].
Pre-treatment with BU (2.5 x 10-3and 5 x 10-3g/mL) significantly (p < 0.05) inhibited calcium-induced vasocontraction in the presence of either phenylephrine or high potassium in isolated endothelium-intact and endothelium-denuded rat’s aortic ring compared to control [ 29 ].
The n-butanol sub-fractions F2 and F3 (1 mg/mL each) from the methanol extract of G. procumbens leaves significantly (p < 0.001) inhibited isoprenaline-induced contraction level in isolated rat’s left and right atrium compared to contraction baseline [ 30 ].
Pre-treatment with aqueous fraction (FA-1) from the ethanol (96% v/v) extract of G. procumbens leaves (1.0 x 10-3 g/mL) significantly (p < 0.001) inhibited angiotensin I or angiotensin II-induced contraction level in isolated endothelium-intact rat’s aortic ring compared to control [ 31 ].
Pre-treatment with FA-1 (1.0 x 10-4 g/mL and 1.0 x 10-3 g/mL) significantly (p< 0.05) increased relaxation effect of bradykinin on phenylephrine-induced contraction level in isolated rat’s aortic ring compared to control [ 31 ].
Aqueous extract of G. procumbens leaves (1 and 2 mg/mL) significantly (p < 0.05) showed vasorelaxant effect on phenylephrine-induced contraction in isolated rat’s aortic ring compared to control. The extract (0.25-1 mg/mL) significantly (p < 0.05) decreased isoprenaline-induced beats per minute in isolated rat’s right atrium compared to control. The extract (1 and 2 mg/mL) also significantly (p < 0.05) decreased the strength of isoprenaline-induced cardiac muscle contraction in isolated rat’s left atrium compared to control [ 32 ]
Immunostimulating activity
Aqueous extract of G. procumbens leaves (1-100 μg/mL) significantly (p < 0.05) increased lymphocyte proliferation 1.34-2.03 stimulation index (S.I) in human peripheral blood mononuclear cells compared to control (1.00 S.I) [ 33 ].
Wound healing activity
Ethanol (95% v/v) extract of G. procumbens leaves (100 and 200 mg/mL/day) was applied topically twice a day on the dorsal neck excision wound of adult male Sprague Dawley rats. After 14 days, the histology showed less scarring tissue at wound closure with fewer inflammatory cells observed compared to placebo group [ 34 ]
Antiphotoaging activity
Ethanol extract of G. procumbens leaves (20 µg/mL) inhibited 70% of matrix metalloproteinase-1 (MMP-1) with expression level of 135.3 ng/mL compared to control (0% inhibition with expression level of 456.8 ng/mL) and inhibited 73% of MMP-9 with expression level of 289.6 ng/mL compared to control (0% inhibition with expression level of 1064.6 ng/mL) in UV-B irradiated human dermal fibroblasts cells. The extract (50 µg/mL) also inhibited radical oxygen species production level (36%) in UV-B irradiated human HaCaT keratinocytes cells compared to control (<36%) [ 4 ]
Clinical studies
Information and data not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute toxicity
Ethanol (95% v/v) extract of G. procumbens leaves (2000 and 5000 mg/kg) was administered orally as single dose to female and male Sprague Dawley rats (aged 6-8 weeks old). After 14 days, there was no toxicity observed (6, 25). The extract (1000 and 5000 mg/kg) when administered orally as single dose to male BALB/c mice (aged 8 weeks old) also showed no toxicity effect after seven days (LD50 value > 5000 mg/kg) [ 26 ]
Oral single dose acute toxicity study on female Sprague Dawley rats (aged between 8 to 12 weeks old) using aqueous mixture of powdered G. procumbens leaves showed no toxic effect on the parameters observed which includes behaviors, body weight, food and water intakes. All rats were observed for 14 days and no death was found throughout the study period. No-observed-adverse-effect level (NOAEL) is more than 2,000 mg/kg body weight [ 35 ].
Methanol extract of G. procumbens leaves (1250-5000 mg/kg) was administered orally as a single dose to male and female Sprague Dawley rats (aged 8 weeks old). After 14 days, there was no toxicity effect observed (LD50 value > 5000 mg/kg) [ 5 ].
Sub-chronic toxicity
Methanol extract of G. procumbens leaves (125-500 mg/kg/day) administered orally to male and female Sprague Dawley rats (aged 8 weeks old) for duration of 90 days and showed no toxic effect (no-observed-adverse-effect-level (NOAEL) = 500 mg/(kgday) [ 5 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- Burkill HM. The useful plants of Tropical West Africa, vol 1 [Internet]. 1985 [10 February 2014]. Available from: http://www.aluka.org/action/showMetadata?doi=10.5555/AL.AP.UPWTA.1_944&pgs=
- Flora of China, vol. 20-21. p.538-541.
- Mustafizur-Rahman AFM, Al-Asad MS. Chemical and biological investigations of the leaves of Gynura procumbens. International Journal of Biosciences. 2013;3(4):36-43.
- Kim J, Lee CW, Kim EK, Lee SJ, Park NH, Kim HS, KimHK, Char K, Jang YP, Kim JW. Inhibition effect of Gynura procumbens extract on UV-B-induced matrix-metalloproteinase expression in human dermal fibroblasts. Journal of Ethnopharmacology. 2011;137(1):427-433.
- Rosidah, Yam MF, Sadikun A, Ahmad M, Akowuah GA, Asmawi MZ. Toxicology evaluation of standardized methanol extract of Gynura procumbens. Journal of Ethnopharmacology. 2009;123(2):244-249.
- Zhang Y, Jiang K, Zhai YM, Tan JJ, Meng DL, Guo SL, Qu SJ, Tan CH. Sesquiterpenoids and their glycosides from Gynura procumbens. Helvetica Chimica Acta. 2014;97:369-374.
- Rosidah, Yam MF, Sadikun A, Asmawi MZ. Antioxidant potential of Gynura procumbens. Pharmaceutical Biology. 2008;46:616-625.
- Akowuah GA, Sadikun A, Mariam A. Flavonoid identification and hypoglycaemic studies of the butanol fraction from Gynura procumbens. Pharmaceutical Biology. 2002;40(6):405-410.
- Zhang Y, Jiang K, Yang LJ, Tan JJ, Meng DL, Tan CH. Isolation and identification of chemical constituents from leaves of Gynura procumbens (Lour.) Merr. Journal of Shenyang Pharmaceutical University. 2012;29(5):337-339.
- Burkill I. A dictionary of the economic products of the malay peninsula. Kuala Lumpur: Ministry of Agriculture Malaysia. 1935;p.1122.
- Ong HC, Rosnani MZ, Milow P. Traditional knowledge of medicinal plants among the Malay villagers in Kampung Mak Kemas, Terengganu, Malaysia. Ethno Med. 2011;5(3):175-185.
- Vejanan V, Latip J, Chin LP, Embi N, Sidek HM. In vitro and in vivo antiplasmodial activities of Gynura procumbens. Sains Malaysiana. 2012;41(12):1535–1542.
- Puangpronpitag D, Chaichanadee S, Naowaratwattana W, Sittiwet C, Thammasarn K, Luerang A, Kaewseejan N. Evaluation of nutritional value and antioxidative properties of the medicnal plant Gynura procumbens extract. Asian Journal of Plant Sciences. 2010;9(3):146-151.
- Maw SS, Mon MM, Oo ZK. Study on antioxidant and antitumor activities of some herbal extracts. World Academy of Science, Engineering and Technology. 2011;51:450-455.
- Mahmood AA, Mariod AA, Al-Bayaty F, Abdel-Wahab SI. Anti-ulcerogenic activity of Gynura procumbens leaf extract against experimentally-induced gastric lesions in rats. Journal of Medicinal Plants Research. 2010;4(8):685-691.
- Hew CS, Khoo BY, Gam LH. The anti-cancer property of proteins extracted from Gynura procumbens (Lour.) Merr. PLoSONE. 2013;8(7):e68524.
- Agustina D, Wasito, Haryana SM, Supartinah A. Anticarcinogenesis effect of Gynura procumbens (Lour) Merr on tongue carcinogenesis in 4NQO-induced rat. Indian Journal of Dental Research. 2006;39(3):126–132.
- Nurulita NA, Meiyanto E, Sugiyanto, Matsuda E, Kawaichi M. Gynura procumbens modulates the microtubules integrity and enhances distinct mechanism on doxorubicin and 5-flurouracil-induced breast cancer cell death. Oriental Pharmacy and Experimental Medicine. 2012.
- Nurulita NA, Meiyanto E, Sugiyanto, Matsuda E, Kawaichi M. The ethyl acetate fraction of Gynura procumbens sensitizes widr colon cancer cell line against 5-fluorouracil but shows antagonism with cisplatin. International Journal of Phytomedicine. 2011;3:392-405.
- Nisa F, Hermawan A, Murwanti R, Meiyanto E. Antiproliferative effect of Gynura procumbens (Lour.) Merr. leaves etanolic extract on 7,12 di methylbenz(a)antra cene induced male rat liver. Advanced Pharmaceutical Bulletin. 2012;2(1):99-106.
- Lee HW, Hakim P, Rabu A, Sani HA. Antidiabetic effect of Gynura procumbens leaves extracts involve modulation of hepatic carbohydrate metabolism in streptozotocin-induced diabetic rats. Journal of Medicinal Plants Research. 2012;6(5):796-812.
- June CC, Wen LH, Sani HA, Latip J, Gansau JA, Chin LP, Embi N, Hasidah MS. Hypoglycemic effects of Gynura procumbens fractions on streptozotocin-induced diabetic rats involved phosphorylation of GSK3β (ser-9) in liver. Sains Malaysiana. 2012;41(8):969–975.
- Algariri K, Meng KY, Atangwho IJ, Asmawi MZ, Sadikun A, Murugaiyah V, Norhayati I. Hypoglycemic and anti-hyperglycemic study of Gynura procumbens leaf extracts. Asian Pacific Journal of Tropical Biomedicine. 2012;3(5):974-981.
- Hassan Z, Yam MF, Ahmad M, Yusof AP. Antidiabetic properties and mechanism of action of Gynura procumbens water extract in streptozotocin-induced diabetic rats. Molecules. 2010;15(12):9008-9023.
- Hassan Z, M. Yusof MAAP, Naidu SR, Kumar GS, Umachigi SP. Hypoglycaemic effects of aqueous extract of Gynura procumbens. Pharmacologyonline. 2008;1:30-50.
- Zhang X, Tan B. Effects of an ethanolic extract of Gynura procumbens on serum glucose, cholesterol and triglyceride levels in normal and streptozotocin-induced diabetic rats. Singapore Medical Journal. 2000;41(1):9-13.
- Hakim P, Sani HA, Noor MM. Effects of Gynura procumbens extract and glibenclamide on sperm quality and specific activity of testicular lactate dehydrogenase in streptozotocin-induced diabetic rats. Malaysian Journal of Biochemistry and Molecular Biology. 2008;16(2):10-14.
- Kim MJ, Lee HJ, Wiryowidago S, Kim HK. Antihypertensive effects of Gynura procumbens extract in spontaneously hypertensive rats. Journal of Medicinal Food. 2006;9(4):587–590.
- Hoe SZ, Lee CN, Mok SL, Kamaruddin MY, Lam SK. Gynura procumbens Merr. decreases blood pressure in rats by vasodilatation via inhibition of calcium channels. Clinics. 2011;66(1):143-150.
- Abrika OSS, Yam MF, Asmawi MZ, Sadikun A, Dieng H, Hussain EA. Effects of extracts and fractions of Gynura procumbens on rat atrial contraction. Journal of Acupuncture and Meridian Studies. 2013;6(4):199-207.
- Poh TF, Ng HK, Hoe SZ, Lam SK. Gynura procumbens causes vasodilation by inhibiting angiotensin II and enhancing bradykinin actions. Journal of Cardiovascular Pharmacology. 2013;61:378–384.
- Kaur N, Awadh AI, Ali RB, Sadikun A, Sattar MZBA, Asmawi MZB. Cardio-vascular activity of Gynura procumbens Merr. leaf extracts. International Journal of Pharmaceutical Sciences and Research. 2012;3(5):1401-1405.
- Sriwanthana B, Treesangsri W, Boriboontrakul B, Niumsakul S, Chavalittumrong P. In vitro effects of Thai medicinal plants on human lymphocyte activity. Songklanakarin Journal of Science and Technology. 2007;29:17-28.
- Zahra AA, Kadir FA, Mahmood AA, hadi AAA, Suzy SM, Sabri SZ, Latif II, Ketuly KA. Acute toxicity study and wound healing potential of Gynura procumbens leaf extract in rats. Journal of Medicinal Plants Research. 2011;5(12):2551-2558.
- Teh B, Hamzah N, Rosli S, Yahaya M, Zakiah I, Murizal Z. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health, 2012. Report No.: HMRC 11-045/01/GP/L/J.