Kulat susu rimau Sclerotium
Lignosus rhinocerus (Cooke) Ryvarden
Polyporaceae






Figure 1 : Lignosus rhinocerus. (a) Fungus in the wild; (b) whole fungus; (c) sclerotium (wild); (d) pileus; (e) sclerotium, top view (cultivated); (f) sclerotium, side view (cultivated). (Photos courtesy of National Pharmaceutical Regulatory Agency, 2018; NAS Agro Farm, 2018 and LIGNO Biotech Sdn. Bhd, 2018).
DEFINITION
Kulat susu rimau sclerotium consists of the powder of dried sclerotia of Lignosus rhinocerus (Polyporaceae). The types of sclerotia used to develop the monograph are laboratory-cultivated (soft) and wild (hard).
SYNONYM
Lignosus rhinocerus (Cooke) Ryvarden, Fomes rhinoceros (Cooke) Sacc, Microporus rhinoceros (Cooke) Imazeki, Polyporus rhinocerus Cooke, Polyporus sacer var. rhinocerus, Polystictus rhinoceros (Cooke) Boedjin, Scindalma rhinocerus (Cooke) Kuntze [ 1 ].
VERNACULAR NAMES
Tiger milk mushroom (English); kulat susu harimau, cendawan susu harimau (Malay); lao hu nai, hu ru ling zhi (Chinese).
CHARACTER
| Colour | Brown (fresh plant part); off-white (powder) |
| Odour | Odourless |
| Taste | Characteristic |
IDENTIFICATION
Plant Morphology
Basidiocarps annual, terrestrial, centrally stipitate, solitary, the stipe arising from a distinct sclerotium, without odour or taste when fresh, hard corky to woody hard when dry. Pileus more or less circular, up to 13 cm in diameter, and 2 mm thick at the centre. Pileal surface light brown to dark brown, glabrous, concentrically zonate; margin sharp, wrinkled, dark brown. Pore surface cream to cream-buff when dry, pores round, 6–8 per mm (94.17 μm–140.94 μm), dissepiments thin, entire. Context cream, up to 2 mm thick. Stipe single, muddy brown, central, up to 16 cm long, up to 0.8 cm at top near pileal and up to 1.2 cm at bottom near sclerotium; context from stipe cream, soft corky. Sclerotium irregular, elongated, up to 9.8 cm long, 4.1 cm wide, wrinkled, the surface dirty brown to fawn-brown, the context cream, loose or soft. Sclereids common in sclerotium, variable in shaped from globose, pear-shaped, ellipsoid to irregular, thick-walled, with a rather narrow lumen, 16–37 x 17–53 μm. Generative hyphae infrequent; skeleton hyphae dominant, often branched. Hyphal system trimitic; generative hyphae bearing clamp connections [ 2 ].
Macroscopy




Figure 2 : Macroscopic characters of L. rhinoceros. (a) Pores (magnification 1000x); (b) hyphae from the cap pileipellis (magnification 1000x); (c) hyphae from the cap trama (magnification 1000x); (d) hyphae from the sclerotium (magnification 1000x). [Scale bars: a = 50 µm; b, c, d, = 20 µm]
Microscopy
Powdered material consists of hyaline, rounded and minutely granulose basidiospores, interwoven, hyaline skeleton hyphae and hyaline and infrequently branched hyphae [ 3 ].








Figure 3 : Microscopic characters of L. rhinocerus sclerotia powder of 0.355 mm size. (a) skeletal hyphae (magnification 40x); (b) generative hyphae (magnification 40x); (c) brachysclereid (red arrow) and starch grain (yellow arrow) (magnification 40x); (d) pseudoparenchyma (magnification 40x); (e) starch hilum (magnification 40x); (f) starch (yellow arrow) and brachysclereid (red arrow) under polarized (magnification 40x); (g) basidiospore (magnification 60x); (h) rhizomorphys (magnification 40x). [Scale bars: a, c, d, e, f = 50 µm; b = 20 µm] (Photos courtesy of Universiti Kebangsaan Malaysia, 2018)
Chemical Tests
Observed colour of solution after treatment with reagent:
| Test for the presence of triterpenes/steroid | Bluish green |
Thin Layer Chromatography (TLC)

Figure 4 : HPTLC profiles of Ergosterol (S) and methanol extract of L. rhinocerus dried sclerotium powder (W : wild, C : cultivated) observed under visible light and 366 nm after derivatisation with 50% v/v ethanolic sulphuric acid.
| Test solution | Weigh about 5.0 g of L. rhinocerus dried sclerotium powder into 50 mL of conical flask. Add 35 mL of methanol. Sonicate the mixture for 1 hr. Filter the mixture through filter paper and evaporate the filtrate to dryness. Reconstitute the residue with 2 mL of methanol and use this for test. |
| Standard solution | Dissolve ergosterol standard [CAS no. 57-87-4] in methanol to produce concentration 0.5 mg/mL. [Sonicate 10 min at room temperature before use]. |
| Stationary phase | HPTLC Glass Silica Gel 60 F254, 10 x 10 cm |
| Mobile phase | Acetone : hexane; (3 : 7) (v/v) |
| Application |
|
| Development distance | 80 mm; manual chamber |
| Drying | Air drying |
| Detection |
|
High Performance Liquid Chromatography (HPLC)
| Test solution | Weigh about 5.0 g of L. rhinocerus dried sclerotium powder into a 50 mL conical flask and add 30 mL of methanol and sonicate the mixture for 1 hr. Filter the mixture through a filter paper and use the filtrate as test solution. |
| Standard solution | Dissolve ergosterol (CAS no: 57-87-4 ) in methanol to produce 1.0 mg/mL standard solution. |
| Chromatographic system |
Detector: 280 nm Column: C18 column (5.0 µm, 4.6 mm I.D x 250 mm) Column oven temperature: 25°C Flow rate: 1.0 mL/min Injection volume: 10 µL |
| Mobile phase (isocratic mode) | Water : methanol (HPLC grade); (2 : 98) (v/v) |
| Run time | 35 min |
| System suitability requirements |
Perform at least five replicate injections of the standard solutions (1.0 mg/mL). The requirements of the system suitability parameters are as follow:
|
| Acceptance criteria |
|

(a)

(b)

(c)
Figure 5 : Whole HPLC chromatogram of (a) ergosterol standard solution (1.0 mg/mL) at tr = 18.783 min, (b) methanol extract of L. rhinocerus dried sclerotium powder (wild) showing peak corresponding to ergosterol standard solution at tr = 18.677 min, methanol extract of L. rhinocerus dried sclerotium powder (cultivated) showing peak corresponding to ergosterol standard solution at tr = 18.582 min.

(a)

(b)

(c)
Figure 6 : HPLC chromatogram highlighting the eluting region ergosterol in (a) ergosterol standard solution (1.0 mg/mL) at tr = 18.783 min, (b) methanol extract of L. rhinocerus dried sclerotium powder (wild) showing peak corresponding to ergosterol standard solution at tr = 18.677 min, (c) methanol extract of L. rhinocerus dried sclerotium powder (wild) showing peak corresponding to ergosterol standard solution at tr = 18.582 min.
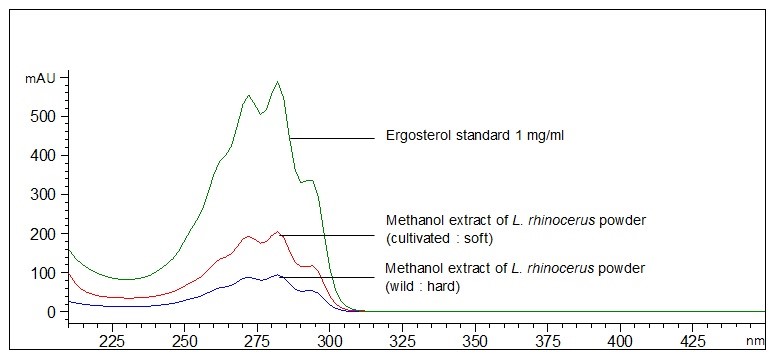
Figure 7 : UV spectrum of ergosterol standard (1.0 mg/mL) overlaid with methanol extract of L. rhinocerus sclerotium powder (wild: hard, and cultivated: soft).
PURITY TESTS
The purity tests, except foreign matter test are based on L. rhinocerus dried sclerotium powder of 0.355 mm particle size.
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 5% |
| Acid-insoluble ash | Not more than 2% |
| Water soluble ash | Not less than 1% |
| Loss on Drying |
| Not more than 15% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 3% |
| Cold method | Not less than 1% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 0.5% |
| Cold method | Not less than 0.1% |
SAFETY TESTS
The safety tests are based on L. rhinocerus dried sclerotium powder of 0.355 mm particle size.
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Water extract of L. rhinocerus dried tuber (sclerotium) was found to contain fat, protein, carbohydrate, flavonoid and b-D-glucan [ 18 ].
Methanol extract of L. rhinocerus dried sclerotium was found to contain esters (e.g. methyl linolelaidate, methyl palmitate, methyl dodecanoate), fatty acids (e.g. palmitic acid, tetradecanoic acid, linoleic acid, lauric acid), sterols (e.g. ergosterol, beta-sitosterol) [ 4 , 5 ].
Ethyl acetate ether extract of L. rhinocerus dried sclerotium was found to contain fatty acids (e.g. palmitic acid, linoleic acid), sterols (e.g. ergosterol, ergost-5,8(14)-dien-3-ol) [5].
Diethyl ether extract of L. rhinocerus dried sclerotium was found to contain esters (e.g. methyl linolelaidate, methyl lignocerate), fatty acids (e.g. palmitic acid, tetradecanoic acid, oleic acid), sterols (e.g. ergosterol, ergost-5,8(14)-dien-3-ol) [ 5 ].
Petroleum ether extract of L. rhinocerus dried sclerotium was found to contain esters (e.g. methyl linolelaidate, methyl palmitate, methyl dodecanoate, methyl heptadecanoate, methyl octadecanoate), fatty acids (e.g. palmitic acid, tetradecanoic acid, linoleic acid, pentadecanoic acid, palmitoleic acid), sterols (e.g. ergosterol, ergost-5,8(14)-dien-3-ol, 3,5-cyclo-6,8(14),22-ergostatriene, stigmast-4-en-3-one ) [ 5 ].
Hexane extract of L. rhinocerus dried sclerotium was found to contain fatty acids (e.g. palmitic acid, tetradecanoic acid, linoleic acid), sterols (e.g. beta-sitosterol, (22E)-ergosta-4,6,8(14),22-tetraen-3-one) [ 5 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally, Lignosus spp has been used for coughs and colds [ 6 , 7 ].
Biological and pharmacological activities supported by experimental data
Antioxidant activity
Hot and cold water extract of wild type L. rhinocerus (1000 µg/mL) exhibited superoxide anion radical scavenging activities with Trolox equivalent antioxidant capacity (TEAC) of 11.23 ± 0.12 mmol TE/g and 9.09 ± 0.04 mmol TE/g compared to quercetin (11.43 ± 0.10 mmol TE/g) and rutin (9.62 ± 0.07 mmol TE/g) using cell-based superoxide anion radical scavenging assay [ 13 ].
Cold water extract of wild type L. rhinocerus (1000 µg/mL) exhibited superoxide anion radical scavenging activities with TEAC of 9.90 ± 0.09 mmol TE/g compared to rutin (9.62 ± 0.07 mmol TE/g) using cell-based superoxide anion radical scavenging assay [ 13 ].
Antimicrobial activity
Aqueous extract of wild type L. rhinocerus dried sclerotia (300 µg/disc) significantly (p < 0.01) inhibited the growth of Staphylococcus aureus with minimum inhibitory concentration (MIC) of 17.67 ± 0.33 mm compared to amoxicillin (MIC = 16.00 ± 0.00 mm) using disc diffusion method [ 12 ].
Neurite-outgrowth stimulatory effect acitivity
Aqueous extract of cultivated type cultivated type L. rhinocerus freeze-dried sclerotia (20 µg/mL) significantly (p < 0.01) promoted the neurite outgrowth in mouse neuroblastoma-2a (N2a) cells by 38.1 ± 2.6% for a duration of 48 hours compared to nerve growth factor (NGF) (26.4 ± 3.6%) using neurite outgrowth assay [ 11 ].
Anti-inflammatory activity
Cold water extract of cultivated type L. rhinocerus freeze-dried sclerotia (200 mg/kg) showed higher percentage inhibition of oedema volume in Sprague-Dawley rats (2nd hr : 83%; 3rd : 88%; 4th hr : 88% and 5th hr : 89%) as compared to indomethacin (10mg/kg) control group (2nd hr : 69%; 3rd hr : 69%; 4th hr : 59% and 5th hr : 57%) [ 8 ].
Immunomodulatory activity
Polysaccharides isolated from hot water extract of wild type L. rhinocerus dried sclerotial powder (20 mg/kg) given intraperitoneally to male and female athymic BALC/c nude mice (7 – 8 weeks old) for 10 consecutive days significantly increased (p < 0.05) spleen weight (0.135 0.01 g) compared to phosphate buffered saline-control (0.109 0.009 g) [ 9 ].
Polysaccharides isolated from hot water extract of wild type L. rhinocerus dried sclerotial powder (20 mg/kg) given intraperitoneally to male and female athymic BALC/c nude mice (7 – 8 weeks old) for 10 consecutive days significantly increased (p < 0.05) T-helper cell (CD3+/CD4+) population (37.5%) compared to phosphate buffered saline control group (6.74%) [ 9 ].
Polysaccharide fractions (LRP-4) of wild type L. rhinoceros sclerotia (20 mg/kg) administered intraperitoneally once daily to cyclophosphamide-treated immunosuppressive special pathogen free (SPF) Kunming mice (female, 18 – 22 g body weight) for 8 days produced higher spleen index (3.91 ± 0.40 mg/g) compared to untreated immunosuppression model control (2.97 ± 0.44 mg/g) and lentinan saline solution-control (3.85 ± 0.49 mg/g) [ 10 ].
Polysaccharide fractions (LRP-2) of wild type L. rhinoceros sclerotia (20 mg/kg) administered intraperitoneally once daily to cyclophosphamide-treated immunosuppressive SPF Kunming mice (female, 18 – 22 g body weight) for 8 days produced higher level of interleukin-10 (944.70 ± 41.36 pg/mL) compared to untreated immunosuppression model control (872.17 ± 22.32 pg/mL) and lentinan saline solution-control (895.89 ± 9.64 pg/mL) ELISA kit [ 10 ].
Polysaccharide fractions (LRP-2) of wild type L. rhinoceros sclerotia (20 mg/kg) administered intraperitoneally once daily to cyclophosphamide-treated immunosuppressive SPF Kunming mice (female, 18 – 22 g body weight) for 8 days produced higher level of tumour necrosis factor-alpha (997.00 ± 39.32 pg/mL) compared to untreated immunosuppression model control (821.25 ± 29.63 pg/mL) and lentinan saline solution-control (980.50 ± 8.38 pg/mL) ELISA kit [ 10 ].
Polysaccharide fractions (LRP-1) of wild type L. rhinoceros sclerotia (20 mg/kg) administered intraperitoneally once daily to cyclophosphamide-treated immunosuppressive SPF Kunming mice (female, 18 – 22 g body weight) for 8 days produced higher level of interferon alpha (880.24 ± 25.93 pg/mL) compared to untreated immunosuppression model control (749.64 ± 15.12 pg/mL) and lentinan saline solution-control (881.04 ± 21.20 pg/mL) ELISA kit [ 10 ].
Antidiabetic activity
Cultivated type L. rhinoceros powder in water (100 and 250 mg/kg) respectively administered orally to streptozotocin-induced diabetic male Sprague Dawley rats twice daily for 2 months significantly (p < 0.05) showed higher glutathione activity (1.319 ± 0.276 nmol/g and 1.500 ± 0.720 nmol/g) in liver tissues compared to metformin (1.309 ± 0.492 nmol/g)
[ 18 ].
Cultivated type L. rhinoceros powder in water (100 and 250 mg/kg) respectively administered orally to streptozotocin-induced diabetic male Sprague Dawley rats twice daily for 2 months significantly (p < 0.05) showed higher superoxide dismutase (SOD) activity (0.1439 ± 0.0317 nmol/g and 0.1306 ± 0.0224 nmol/g) in liver tissues compared to metformin (0.1423 ± 0.0376 nmol/g) [ 18 ].
Cultivated type L. rhinoceros powder in water (250 and 500 mg/kg) respectively administered orally to streptozotocin-induced diabetic male Sprague Dawley rats twice daily for 2 months significantly (p < 0.05) showed higher catalase activity (0.0917 ± 0.0255 nmol/g and 0.0829 ± 0.0205 nmol/g) in liver tissues compared to metformin (0.0876 ± 0.0148 nmol/g) [ 18 ].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
28-days sub-acute toxicity study
The water extract of L. rhinocerus dried sclerotial powder (1000 mg/kg for both wild and cultivated type) administered orally once a day to Sprague Dawley rats (female and male, 5 weeks old) for 28 days showed no effect on the growth rate and weight, hematological and histological aspects (lung, kidney, heart, spleen and liver) and clinical biochemistry of the rats. As the highest tested dose of 1000 mg/kg was not associated with any toxicity concern, the NOAEL dose is higher than 1000 mg/kg. [ 16 ].
180-days toxicity study
Water extract of L. rhinocerus dried sclerotia (250, 500 and 1000 mg/kg) administered orally once a day to Sprague Dawley rats (female and male, 5 weeks old) for 180 days showed no adverse effect on the general clinical observations, body weight, hematology, clinical biochemistry, urinalysis, absolute organ weight as well as relative organ weight, nor induced histological changes in the organs. The no-observed-adverse-effect level (NOAEL) was found to be more than 1000 mg/kg. [ 14 ].
Genotoxicity study
Water extract of L. rhinocerus dried sclerotia (250, 500 and 1000 mg/kg) did not cause gene mutations by base pair changes or frameshifts in the genome of Salmonella typhimurium strains TA 98, TA 100, TA 1535, TA 1537 and Escherichia coli WP2 using Ames test in the presence and absence of metabolic activation (S9) [ 14 ].
Teratogenicity
Water extract of L. rhinocerus dried sclerotial powder (100 mg/kg) administered orally once a day to Sprague Dawley rats (female, 5 weeks old) for 7 to 8 weeks. There was no teratogenicity effect observed in extract-treated group in relation to the number, body weight, day of eyes opened, righting reflex, ambulation and external malformations of the offspring [ 14 ].
Acute oral toxicity
Oral single dose acute toxicity study on female Sprague Dawley rats (aged between 8 and 12 weeks old) using aqueous extract of L. rhinocerous sclerotium showed no toxic effect on the parameters observed, including behaviours, body weight, food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. Half lethal dose (LD50) is 2,000 mg/kg body weight [ 19 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- Catalogue of Life: Lignosus rhinocerus (Cooke) Ryvarden, 1972 [Internet]. Catalogueoflife.org. 2017 [cited 13 December 2017]. Available from: http://www.catalogueoflife.org/col/details/species/id/2b14c3168a84cba4f2c631b995d69f26
- Sanusi M, Mansor P. Technical report for species identification of Lignosus specimens. Kepong, Selangor: Forest Research Institute Malaysia (FRIM); 2017.
- Abdul Rahman M. Microscopy report for Lignosus rhinocerus. Universiti Kebangsaan Malaysia; 2018.
- Mohamad Zainoor N, Wainwright M, Rostron C, Brandt S. Distribution of ergosterol in different parts of Lignosus rhinocerus, J Pharm Pharmacol. 2007: 59: A59-A60
- Johnathan M, Nurul AA, Ezumi MF, Gan SH. Gas chromatography mass spectrometry analysis of volatile compounds from Lignosus rhinocerus (tiger milk mushroom). Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2016 (July-August); vol 7 (issue 4): 5-16.
- Lee S. and Chang Y. (2004). Utilisation of macrofungi species in Malaysia.Fungal Diversity, 15, pp.15-22.
- Burkill IH. A dictionary of the economic product of the Malay Peninsula, Vol.1. London; Published on behalf of the governments of the Straits settlements and Federated Malay states by the Crown agents for the colonies. 1935; p. 223-229.
- Lee S, Tan N, Fung S, Sim S, Tan C, Ng S. Anti-inflammatory effect of the sclerotium of Lignosus rhinocerotis (Cooke) Ryvarden, the tiger milk mushroom. BMC Complementary and Alternative Medicine. 2014;14(1).
- Wong K, Lai C, Cheung P. Immunomodulatory activities of mushroom sclerotial polysaccharides. Food Hydrocolloids. 2011:25(2): 150-158.
- Hu T, Huang Q, Wong K, Yang H. Structure, molecular conformation and immunomodulatory activity of four polysaccharide fractions from Lignosus rhinocerotis International Journal of Biological Macromolecules. 2017;94:423-430
- Phan C, David P, Naidu M, Wong K, Sabaratnam V. Neurite outgrowth stimulatory effects of culinary-medicinal mushrooms and their toxicity assessment using differentiating Neuro-2a and embryonic fibroblast BALB/3T3. BMC Complementary and Alternative Medicine. 2013:13(1).
- Mohanarji S, Dharmalingam S, Kalusalingam A. Screening of Lignosus rhinocerus extracts as antimicrobial agents against selected human pathogens. Journal of Pharmaceutical and Biomedical Sciences. 2012;18(11).
- Yap Y, Nget H, Yee Fung S, Aziz A, Tan C, Szu T. Nutrient composition, antioxidant properties and anti-proliferative activity of Lignosus rhinocerus Cooke sclerotium. JSciFoodAgric. 2013;93: 2945-295.
- Lee S, Enchang F, Tan N, Fung S, Pailoor J. Preclinical toxicological evaluations of the sclerotium of Lignosus rhinocerus (Cooke), the Tiger Milk mushroom. Journal of Ethnopharmacology. 2013;147(1):157-163.
- Jamil N, Rashid N, Hamid M, Rahmad N, Al-Obaidi J. Comparative nutritional and mycochemical contents, biological activities and LC/MS screening of tuber from new recipe cultivation technique with wild type tuber of tiger’s milk mushroom of species Lignosus rhinocerus. World Journal of Microbiology and Biotechnology [Internet]. 2017 [cited 18 May 2018];34(1). Available from: https://www.researchgate.net/publication/321488529_Comparative_nutritional_and_mycochemical_contents_biological_activities_and_LCMS_screening_of_tuber_from_new_recipe_cultivation_technique_with_wild_type_tuber_of_tiger’s_milk_mushroom_of_species_Ligno
- Lee S, Tan N, Fung S, Pailoor J, Sim S. Evaluation of the sub-acute toxicity of the sclerotium of Lignosus rhinocerus (Cooke), the Tiger Milk mushroom. Journal of Ethnopharmacology. 2011;138(1):192-200.
- Lau B, Adullah N, Aminudin N. Chemical composition of the tiger’s milk mushroom, Lignosus rhinocerotis (Cooke) ryvarden, from different developmental stages. Journal of Agricultural and Food Chemistry. 2013; 61(20):4890-97
- Nyam K, Chow C, Tan C, Ng S. Antidiabetic properties of the tiger’s milk medicinal mushroom, Lignosus rhinoceroties (Agaricomecetes), in streptozotocin-induced diabetic rats. International Journal of Medicinal Mushrooms. 2017;19(7):607-61
- Teh BP, Jumriani AA, Noorashikin AH, Lalitha Suganthi S. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2019. Report no: NON-GLP/2019/09/01.

