Ketum Leaves
Mitragyna speciosa (Korth.) Havil
Rubiaceae
DEFINITION
Ketum leaves consists of dried leaves of Mitragyna speciosa (Korth.) Havil. (Rubiaceae).
SYNONYM
Nauclea luzoniensis Blanco, Nauclea speciosa (Korth.) Miq., Stephegyne speciosa Korth [ 1 ].
VERNACULAR NAMES
Kratom (English); ketum, bia, biak, kutum (Malay) [ 2 ]; sinnakadambu (Tamil)
CHARACTER
| Colour | Brownish yellow (powder) |
| Odour | Slight odour |
| Taste | Bitter |
IDENTIFICATION
Plant Morphology
M. speciosa is a tree of 50-60 feet in height and without hooks. Stems erect and branching; bark is smooth and grey with pinkish inner bark. Leaves broad, edges are smooth, ovate to elliptic, simple, large, 3-20 cm x 2-12 cm; stipule lanceolate and conspicuous, 1 x 4 cm; blade is coriaceous, elliptic, ovate or obovate, slightly hairy beneath with inconspicuous tertiary nerves, pointed apex and round base and several conspicuous secondary nerves, 11-17 pairs; petiole slender, 1-4 cm long. Inflorescence in a solitary, globose and small, 0.9-1.3 cm head; bracteoles minute, 4-6 mm, pale, hairy and concealing the calyces of flower buds in the young age; calyx tiny, 2 mm; corolla salver-shaped, yellow, hairy inside the throat, and developing 5 valvate lobes. Fruits small, 2-3 cm, fruiting heads containing fruitlets, 7-9 mm x 4-5 mm. Seeds shortly winged on both ends, the lower wing shallowly bifid or notched [ 2 , 3 , 4 ].
Microscopy
Powdered material consists of fibres in a group; parenchyma cells in fragments and sometimes found druse crystals attached to it; a few fragments of vascular tissue with pitted or spiral thickened vessel; covering trichomes isolated, thick wall, simple unicellular and non-glandular hair cell [ 5 , 6 ].

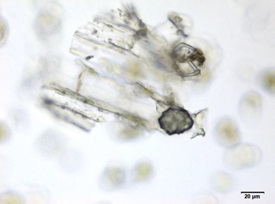

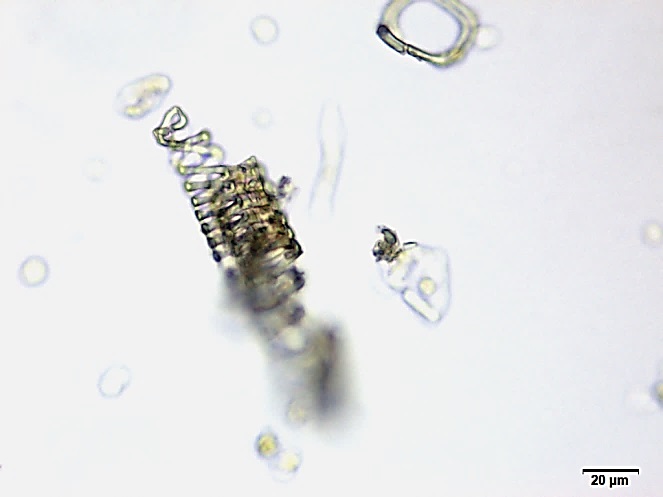
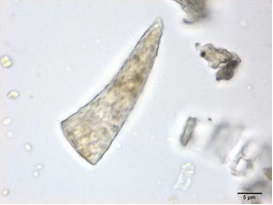
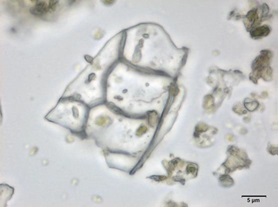
Figure 2 : Microscopic characters of M. speciosa leaves powder. (a) A group of fibres (magnification 10x); (b) parenchyma cells attached with druse (magnification 100x); (c) fragment of pitted thickened vessel (magnification 20x); (d) fragment of spirally thickened vessel (magnification 200x); (e) one-celled non-glandular trichome (magnification 100x); (f) fragment of parenchyma cells (magnification 100x). [Scale bars: a = 50 µm; b, c, d = 20 µm; e, f = 5 µm]
Colour Tests
Observed colour of solution after treatment with various reagents:
| HCl (conc.) | Green |
| NaOH (5%) | Brown |
Thin Layer Chromatography (TLC)
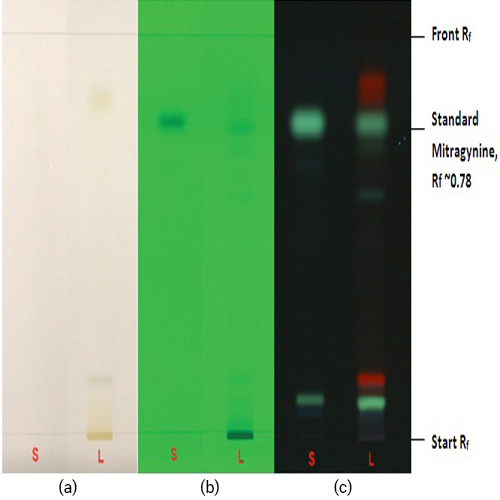
Figure 3 : TLC profile of mitragynine (S), ethanol extract of M. speciosa dried leaves powder (L) observed under (a) at visible light after derivatized, (b) UV at 254 nm before derivatization and (c) UV at 366 nm before derivatization.
| Test Solutions | Weigh about 0.5 g of M. speciosa dried leaves powder in a glass vial screw cap. Add 5 mL of ethanol and macerate the mixture for 20 minutes at room temperature. Filter the mixture with Nylon syringe filter (0.45 µm). Use the filtrate as test solution. |
| Standard solution | Prepare 1 mg of mitragynine standard [CAS no.: 4098-40-2] in 1 mL of ethanol to produce a standard concentration 1000 µg/mL. |
| Stationary Phase | HPTLC Silica gel 60 F254, 10 x 10 cm |
| Mobile phase | Toluene : ethyl acetate : methanol : 25% ammonia; (30 : 30 : 15 : 1) (v/v/v/v) |
| Application |
|
| Development distance | 7 cm |
| Drying | Air drying |
| Detection |
|
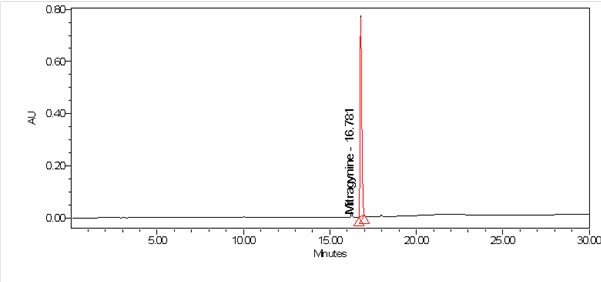
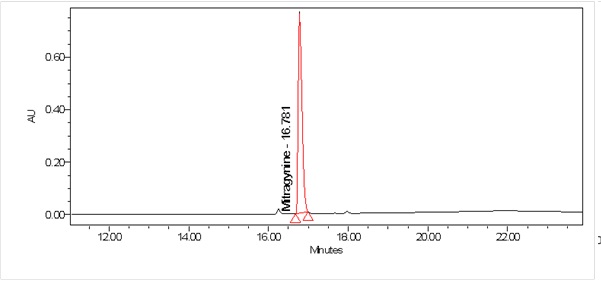
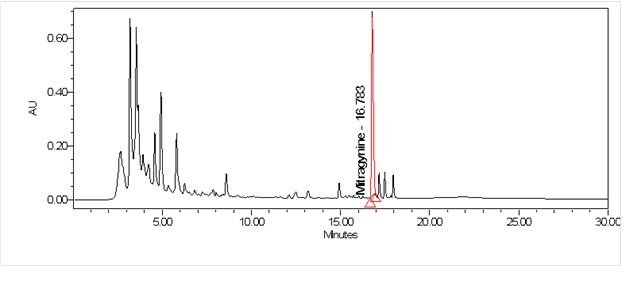
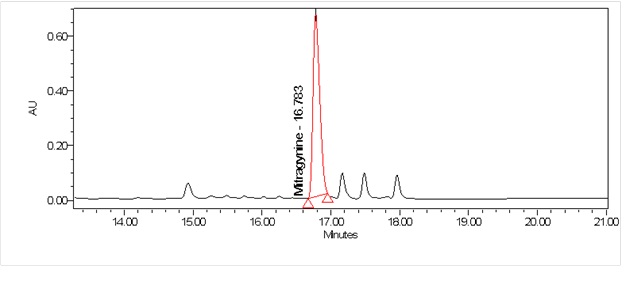
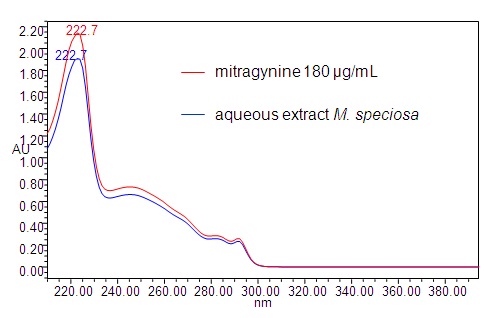
High Performance Liquid Chromatography (HPLC)
| Test solution | Weigh about 0.5 g of M. speciosa dried leaves powder in a glass vial with screw cap. Add 10 mL of water and tap the glass vial gently to dissolve the mixture. Macerate the mixture for 30 minutes at room temperature. Filter the mixture with Nylon syringe filter (0.45 µm) into a HPLC vial. Use the filtrate as test solution. | ||||||||||||||||||||||||
| Standard solution | Prepare mitragynine standard [CAS no.: 4098-40-2] in ethanol to produce a standard concentration 180 µg/mL. | ||||||||||||||||||||||||
| Chromatographic system |
Detector: PDA 246 nm Column: C18 (2.5 µm, 4.6 mm I.D x 150 mm) (preferably XBridge Ethylene-Bridged Hybrid (BEH) C18, Waters column) |
||||||||||||||||||||||||
| Mobile Phase (gradient mode) |
|
||||||||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the mitragynine standard solutions (180 µg/mL). The requirements of the system suitability parameters are as follow:
|
||||||||||||||||||||||||
| Acceptance criteria |
|
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 7% |
| Acid-insoluble ash | Not more than 1% |
| Loss on Drying |
| Not more than 10% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 24% |
| Cold method | Not less than 16% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 20% |
| Cold method | Not less than 8% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Methanol extract of the M. speciosa dried leaves have been reported to contain alkaloid (e.g. mitragynaline, 3-dehydromitagynine, 3,4,5,6-tetradehydromitragynine, mitrasulgynine, mitragynine, speciogynine, speciociliatine, paynantheine, 7α-hydroxy-7H-mitragynine, mitralactonal, mitragynaline, corynantheidinaline, mitragynalic acid, corynanthedinalic acid), phenolics [e.g. (-)epicatechin, pinoresinol] [ 7 , 8 , 9 ].
Ethyl acetate extract of the M. speciosa dried leaves have been reported to contain alkaloid [e.g. (-) -9-methoxymitralactonine, (-)-mitralactonine] [ 10 , 11 ].
Other extract of the M. speciosa dried leaves have been reported to contain corynantheidine and isocorynoxeine [ 10 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally used to apply to the wounds in pounded form while poultice of the leaves was used to expel worms for children. It is also useful in relieving fever [ 2 ].
Biological and pharmacological activities supported by experimental data
Antimicrobial activity
Methanol extract of M. speciosa leaves inhibited the growth of Salmonella typhi and Bacillus subtilis (minimal inhibition concentration (MIC) = 6.25 mg/mL) while alkaloid extract showed MIC value of 3.12 mg/mL against both microbes using broth dilution method [ 12 ].
Antioxidant activity
Methanol extract of M. speciosa leaves showed antioxidant activity with 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity (inhibitory concentration at 50% (IC50) = 37.08 ± 3.54 mg/mL) compared to butylated hydroxytoluene (4.50 ± 0.23 mg/mL) [ 12 ].
Aqueous extract of M. speciosa leaves (100 mg/kg/day) administered orally to male Sprague Dawley rats for 14 days significantly (p < 0.05) increased glutathione transferases specific activity compared to untreated group [ 13 ].
Antidiarrheal activity
Methanol extract of M. speciosa leaves (100-400 mg/kg) was administered orally as a single dose to adult Wistar rats with castor oil-induced diarrhea. After eight hours, a significant (p < 0.05) decrease in defecation frequency (0.10-1.90), total diarrheal score (1.70-3.90) and fecal weight (1.01-2.37 g) were observed compared to vehicle treated group (propylene glycol: Tween 80: distilled water (4:1:4)) (5.40 defecation frequency; 11.40 total diarrheal score; 8.52 g fecal weight) [ 14 ].
Methanol extract of M. speciosa leaves (200 mg/kg) was administered intraperitoneally to adult male Sprague Dawley rats at 30 minutes before cotton pellet was inserted subcutaneously for granuloma induction. The extract was administered intraperitoneally for following seven days. A significant (p < 0.05) inhibition of granuloma tissue growth (49.8 ± 0.003 mg) was observed compared to NaCl treated group (90.5 ± 0.010 mg) [ 15 ].
Anti-inflammatory activity
Methanol extract of M. speciosa leaves (200 mg/kg) was administered intraperitoneally as a single dose to adult male Sprague Dawley rats at 30 minutes before paw edema induction using carrageenan. A significant (p < 0.05) inhibition of paw edema (0.18 ± 0.07 mL) was observed within five hours of edema induction compared to NaCl treated group (0.48 ± 0.04 mL) [ 15 ].
Methanol extract of M. speciosa leaves (200 mg/kg) was administered intraperitoneally to adult male Sprague Dawley rats at 30 minutes before cotton pellet was inserted subcutaneously for granuloma induction. The extract was administered intraperitoneally for following seven days. A significant (p < 0.05) inhibition of granuloma tissue growth (49.8 ± 0.003 mg) was observed compared to NaCl treated group (90.5 ± 0.010 mg) [ 15 ].
Analgesic activity
7-hydroxymitragynine isolated from M. speciosa leaves inhibited electrically stimulated contraction with pD2 (−log concentration producing 50% of the drug maximum effect (-logEC50 values) in guinea pig ileum. 7-hydroxymitragynine has a higher affinity for m-opioid receptor (binding affinity (pKi)= 8.01 ± 0.03) compared to other opioid receptors (d- (6.84 ± 0.12) and k- (6.71 ± 0.11)) [ 16 ].
Mitragynine isolated from M. speciosa leaves inhibited electrically stimulated contraction (IC50 = 6.91 ± 0.04 nM) in guinea pig ileum compared to morphine (IC50 = 7.68 ± 0.11 nM). The involvement of opioid receptor in the analgesic effect of mitragynine and morphine (control) were demonstrated by the reversing effect of naloxone (10-300 nM) on electrically stimulated twitch contraction in guinea pig ileum. Mitragynine (3 and 10 mM) significantly (p < 0.05 (3 mM) and p < 0.001 (10 mM)) inhibited withdrawal contraction induced by naloxone in the ileum after five minute exposure of the ileum to morphine [ 17 ].
Antinociceptive activity
Methanol extract of M. speciosa leaves (200 mg/kg) administered intraperitoneally as a single dose to male Balb C mice at 30 minutes before the induction of abdominal constriction using acetic acid significantly (p < 0.05) inhibited writhing response (64.2 ± 11.3) compared to NaCl treated group (134.7 ± 12.3) [ 15 ].
Methanol extract of M. speciosa leaves (200 mg/kg) administered intraperitoneally as a single dose to adult male Sprague Dawley rats at 30 minutes before pain induction on the paw using formalin. A significant (p < 0.05) pain inhibition was observed in early phase (0-5 min after pain induction; 40.4 ± 4.6 s of time spent for licking) and late phase (15-60 min; 109.0 ± 14.1 s) of formalin injection compared to NaCl treated group (64.6 ± 6.5 s and 205.5 ± 12.8 s) [ 15 ].
Methanol extract of M. speciosa leaves (200 mg/kg) was administered intraperitoneally as a single dose to male Balb C mice. A significant (p < 0.05) increase in response latency time to the heat stimulation (6.68-8.40 s) was observed from 60 to 240 minutes compared to NaCl treated group (4.99-5.82 s) in hot plate test [ 15 ].
Methanol extract of M. speciosa leaves (100 mg/kg) was administered orally as a single dose to male Swiss mice. A significant (p < 0.05) increase in response latency time to the heat stimulation (16.3-18.5 s) was observed from 30 to 60 minutes compared to control group (10.4-11.7 s) in hot plate test [ 17 ].
Mitragynine isolated from alkaloid extract of M. speciosa leaves (35 mg/kg) was administered intraperitoneally as a single dose to male ICR mice. A significant (p < 0.05) increase in response latency time to the heat stimulation was observed from 30 to 120 minutes compared to saline treated group in hot plate test [ 18 ].
Mitragynine (35 mg/kg) was administered intraperitoneally to male ICR mice at 15 minutes after administration of non-selective opioid receptor antagonists (naloxone hydrochloride and naltrindole). A significant (p < 0.05) decrease in response latency time was observed compared to mitragynine treated group. Results demonstrated that the antinociceptive activity of mitragynine works through the activation of opioid receptor system [ 18 ].
Methanol (200 mg/kg), aqueous (400 mg/kg) and alkaloidal (20 mg/kg) extracts of M. speciosa leaves were administered orally as a single dose to male Sprague Dawley rats (130-180 g body weight). A significant (p < 0.05) increase in response latency time (12.98-23.63 s in hot plate test and 6.09-7.41 s in tail flick test) was observed from 15 to 30 minutes compared to vehicle control (7.36-7.44 s and 3.16-4.19 s) [ 19 ].
A combination of mitragynine (25 mg/kg) and morphine (5 mg/kg) administered intraperitoneally to male ICR mice for 9 days significantly (p < 0.05) increase in response latency time from day 1 to day 9 compared to morphine treated group in hot plate test. The combination treatment decreased the development of tolerance with significant (p < 0.05) decrease in the expression of cyclic adenosine monophosphate (cAMP) and cAMP response element binding protein compared to morphine treated group and showed significant (p < 0.05) increase in creatinine level compared to saline treated group [ 20 ].
Mitragynine isolated from M. speciosa leaves (200 mg/kg) administered orally as a single dose to male and female albino mice at 30 minutes before the induction of abdominal constriction using acetic acid significantly (p < 0.05) decreased writhing response (9.6 ± 0.6 counts) compared to gum acacia control group (17.5 ± 2.8 counts) [ 21 ].
Mitragynine isolated from M. speciosa leaves (200 mg/kg) was administered orally as a single dose to male and female albino mice. A significant (p < 0.05) antinociceptive effect were observed from 15 to 60 minutes with the percentage of mean possible analgesia at 30 minutes were 42.8 ± 4.2% in hot tail-flick and 49.0 ± 5.9% in cold tail-flick test compared to gum acacia control group [ 21 ].
7-hydroxymitragynine isolated from M. speciosa leaves (10 mg/kg) administered subcutaneously or orally to male ddY mice showed dose-dependent antinociceptive activity (maximum possible effect (MPE) = 100% at 15-30 min) in tail-flick and hot-plate test (MPE = 94% at 15 min) compared to morphine control (MPE = 60% at 45 min for tail-flick and MPE = 79% at 30 min for hot-plate test) [ 22 ].
Antidepressant activity
Mitragynine isolated from the methanol extract of M. speciosa leaves (10 mg/kg and 30 mg/kg) administered intraperitoneally as a single dose to male ICR mice. After 30 minutes, a significant (p < 0.05) decrease in the duration of immobility time and serum corticosterone level were observed compared to vehicle treated group (20% Tween-80) in force swimming test and tail suspension test [ 23 ].
Aqueous extract of M. speciosa leaves (100-500 mg/kg) was administered orally as a single dose to male albino Swiss mice. After 45 minutes, a significant (p < 0.05) decrease in the duration of immobility time was observed compared to distilled water treated group in tail suspension test [ 16 ].
Aqueous extract of M. speciosa leaves (300 mg/kg) was administered orally as a single dose to ethanol withdrawal-induced male albino Swiss mice. After 45 minutes, a significant (p < 0.05) decrease in rearing, displacement and head weaving behaviors were observed compared to ethanol withdrawal group treated with distilled water [ 16 ].
Muscle relaxation activity
Methanol extract of M. speciosa leaves (0.1-1 mg/mL) and mitragynine (0.0156 mg/mL) showed indirect muscle relaxation effect on the isolated phrenic nerve-hemidiaphragm preparation and direct muscle relaxation effect on the diaphragm skeletal muscle. The extract (10-40 mg/mL) and mitragynine (2 mg/mL) also blocked the nerve conduction, amplitude and duration of compound nerve action potential in isolated sciatic nerve preparation [ 24 ].
Appetite suppressant activity
Alkaloidal extract of M. speciosa leaves (45 and 50 mg/kg) administered intraperitoneally as a single dose to male Wistar rats. After 24 hours, a significant (p < 0.01) decrease in food intake in both groups (47.8 ± 7% and 45.0 ± 8%) and significant (p < 0.05) decrease in water consumption (81.4 ± 16% and 57.3 ± 15%) in both groups compared to saline treated group [ 25 ].
Alkaloidal extract of M. speciosa leaves (40 mg/kg/day) administered intraperitoneally to male Wistar rats for a duration of 60 days significantly (p < 0.05) decreased the food intake (18.0%), water consumption (25.6%) and less weight gain (17.6%) compared to saline treated group [ 25 ].
Cognitive activity
Methanol extract of M. speciosa leaves (1000 mg/kg) was administered orally as a single dose to adult male albino Sprague Dawley rats. A significant (p < 0.05) increase in learning ability on day 2 was observed compared to day 1 in one-way passive avoidance response test and a significant (p < 0.05) increase on the first day of training was observed compared to morphine treated groups in two-way active avoidance response test but there was no benefit on long term memory consolidation [ 26 ].
Both alkaloidal extract and mitragynine isolated from M. speciosa leaves (20-80 mg/kg) was administered orally as a single dose to male albino Swiss mice at 30 minutes before Y-maze and motor coordination test was conducted. A significant (p < 0.05) increase in the total number of arm entries and rearing frequencies (both are components of exploratory behavior), a significant (p < 0.05) decrease in self-grooming and immobility time were observed in treated rats compared to Tween 20 treated group [ 27 ].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute toxicity.
Standardised aqueous extract of M. speciosa leaves (175-2000 mg/kg) was administered orally as a single dose to male and female Sprague Dawley rats (aged between eight and 12 weeks old). The toxicity effect was observed for 14 days and showed no mortality and caused only a slight toxicity effect (signs of fatigue and sleep for male rats while only sign of fatigue for female rats at highest dose) (LD50 value > 2000 mg/kg) [ 28 ].
Ethanol extract of M. speciosa leaves was administered orally as a single dose to Sprague Dawley rats. The toxicity effect was observed for 14 days and showed no toxic effect (LD50 value > 3000 mg/kg) [ 29 ].
Methanol extract of M. speciosa leaves administered orally as a single dose to male Swiss mice showed toxic effects (LD50 = 4900 mg/kg) while alkaloidal extract also showed toxic effect with LD50 =17320 mg/kg [ 30 ].
Standardized methanol extract of M. speciosa leaves (100-1000 mg/kg) was administered orally as a single dose to adult male albino Sprague Dawley rats. The toxicity effect was observed for 14 days and showed no mortality however it caused toxicity effect at 1000 mg/kg (significant increase in blood pressure, acute severe hepatoxicity and mild nephrotoxicity) with LD50 > 1000 mg/kg [ 31 ].
Oral single dose acute toxicity study on female Sprague Dawley rats ( aged between 8 and 12 weeks old) using aqueous extract of M. speciosa leaves showed no toxic effect on the parameters observed, including behaviours, body weight, food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. Half lethal dose (LD50) is more than 2,000 mg/kg body weight [ 32 ].
Brine shrimp lethality toxicity.
M. speciosa leaves extracts showed toxicity effect on brine shrimp with lethal concentration at 50% (LC50) = 98 µL/mL (aqueous extract), 62 µL/mL (alkaloidal extract) and 44 µL/mL (mitragynine) [ 33 ].
Neurotoxicity
Ethanol extract of M. speciosa leaves (1000 and 1500 mg/kg/day) was administered orally to pregnant Sprague Dawley rats between day 8 and day 13 of gestation day significantly (p < 0.001) showed a widening of the vertebral arch in the thoracic, lumber and cervical vertebral regions of spinal cord and significantly (p < 0.001) increased the brain diameter of the fetuses [ 29 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Adverse reaction
A young man aged 25 years old demonstrated intrahepatic cholestasis after ingested two teaspoons (one teaspoon approximately 2.3 to 3.5 g, corresponding five to eight dried M. speciosa leaves) twice daily for two weeks [ 34 ].
Side effect
44-year old man with a history of alcohol dependence and anxiety disorder admitted to the hospital for the purpose of M. speciosa detoxification. He demonstrated dependence on M. speciosa with withdrawal symptoms consisting of anxiety, restlessness, tremor, sweating and craving for M. speciosa. The patient described that the detoxification from M. speciosa was harder than alcohol in the past [ 35 ].
Mitragynine isolated from M. speciosa leaves (5-15 mg/kg/day) was administered intraperitoneally for 28 days to male ICR mice significantly (p < 0.05) decreased discrimination ratio time compared to Tween 80 group in object location task and significantly (p < 0.05) decreased locomotor activity compared to amphetamine in open-field test [ 36 ].
Potential herb-drug interaction
Alkaloid extract of M. speciosa leaves inhibited the activities of human recombinant cytochrome P450 (CYP450) with the IC50 values of 0.636 µg/mL (CYP2D6) and 0.78 µg/mL (CYP3A4) and 39 µg/mL (CYP1A2). Competitive inhibition was demonstrated for CYP2D6 while non-competitive inhibition for CYP3A4, CYP1A2 and CYP2C19 [ 37 ].
Methanol extract of M. speciosa leaves inhibited the activities CYP450 enzymes with the IC50 values of 3.6 ± 0.1 µg/mL (CYP2D6) and 142.8 ± 13.8 µg/mL (CYP3A4) [ 38 ].
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The plant list. [Internet] Mitragyna speciosa. Version 1.1; [cited on 25th December 2014]. Available from: http://www.theplantlist.org/tpl/record/kew-1288052.
- Burkill IH. A dictionary of the economic products of the Malay peninsula. Vol.2. London; Published on behalf of the governments of the Straits settlements and Federated Malay states by the Crown agents for the colonies: 1935. p.1483.
- Christophe W. Medicinal plants of Southeast Asia. Prentice Hall Pearson Malaysia Sdn Bhd. 2002;p.295.
- Goh SH. Malaysian medicinal plants for treatment of cardiovascular disease. Petaling Jaya; Pelanduk Publications. 1995;p.103.
- Kowalczuk AP, Lozak A, Zjawiony JK. Comprehensive methodology for identification of kratom in police laboratories. Forensic Science International. 2013;233:238-243.
- Malaysian Herbal Monograph Committee. Malaysian Herbal Monograph. Vol. 2. Kuala Lumpur; Published on behalf of the Forest Research Institute Malaysia: 2009. p. 62-68.
- Houghton PJ, Said IM. 3-Dehydromitragynine: an alkaloid from Mitragyna speciosa. Phytochemistry. 1986; 25:2910-2912.
- Takayama H, Kurihara M, Kitajima M, Said IM, Aimi N. New indole alkaloids from the leaves of Malaysian Mitragyana speciosa. Tetrahedron. 1998;54(29):8433-8440.
- Houghton PJ, Aishah L, Said IM. Alkaloid from Mitragyana species. Phytochemistry. 1991;30:347-350.
- Takayama H, Kurihara M, Kitajima M, Said IM, Aimi N. Structure elucidation and chiral total synthesis of a new indole Alkoid, (-)-9-methoxymitralactonine, isolated from Mitragyna speciosa in Malaysia. Tetrahedron. 2000;56 :3145-3151.
- Takayama H, Kurihara M, Kitajima M, Said IM, Aimi N. Isolation and asymmetric total synthesis of a new mitragyna indole alkaloid, (-)-mitralactonine. Journal of Organic Chemistry. 1999;64:1772-1773.
- Parthasarathy S, Azizi JB, Ramanathan S, Ismail S, Sasidharan S, Said MIM, Mansor SM. Evaluation of antioxidant and antibacterial activities of aqueous, methanolic and alkaloid extracts from Mitragyna speciosa (Rubiaceae family) leaves. Molecules. 2009;14(10):3964-3974.
- Azizi J, Ismail S, Mordi MN, Ramanathan S, Said MIM, Mansor SM. In vitro and in vivo effects of three different Mitragyna speciosa Korth leaf extracts on phase II drug metabolizing enzymes—glutathione transferases (GSTs). Molecules. 2010;15:432-441.
- Chittrakarn S, Sawangjaroen K, Prasettho S, Janchawee B, Keawpradub N. Inhibitory effects of kratom leaf extract (Mitragyna speciosa Korth.) on the rat gastrointestinal tract. Journal of Ethnopharmacology. 2008;116(1):173-178.
- Shaik Mossadeq WM, Sulaiman MR, Tengku Mohamad TA, Chiong HS, Zakaria ZA, Jabit ML, Baharuldin MT, Israf DA. Anti-inflammatory and antinociceptive effects of Mitragyna speciosa Korth methanolic extract. Medical Principles and Practice : International Journal of the Kuwait University, Health Science Centre. 2009;18(5):378-84.
- Kumarnsit E, Keawpradub N, Nuankaew W. Effect of Mitragyna speciosa aqueous extract on ethanol withdrawal symptoms in mice. Fitoterapia. 2007;78(3):182-185.
- Watanabe K, Yano S, Horie S, Yamamoto LT. Inhibitory effect of mitragynine, an alkaloid with analgesic effect from thai medicinal plant Mitragyna speciosa, on electrically stimuiated contraction of isolated guinea-pig ileum through the opioid receptor. Life Sciences. 1997;60(12):933-942.
- Shamima AR, Fakurazi S, Hidayat MT, Hairuszah I, Moklas MAM, Arulselvan P. Antinociceptive action of isolated mitragynine from Mitragyna speciosa through activation of opioid receptor system. International Journal of Molecular Sciences. 2012;13:11427-11442.
- Sabetghadam A, Ramanathan S, Mansor SM. The evaluation of antinociceptive activity of alkaloid, methanolic, and aqueous extracts of Malaysian Mitragyna speciosa Korth leaves in rats. Pharmacognosy Research. 2010; 2(3): 181–185.
- Fakurazi S, Rahman SA, Hidayat MT, Ithnin H, Moklas MAM, Arulselvan P. The combination of mitragynine and morphine prevents the development of morphine tolerance in mice. Molecules. 2013;18: 666-681.
- Idid SZ, Saad LB, Yaacob H, Shahimi MM. Evaluation of analgesia induced by mitragynine, morphine and paracetamol on mice. ASEAN Review of Biodiversity and Environmental Conservation (ARBEC). May 1998 (Article IV.).
- Matsumoto K, Horie S, Ishikawa H, Takayama H, Aimi N, Ponglux D, Watanabe K. Antinociceptive effect of 7-hydroxymitragynine in mice: discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sciences. 2004;74(17):2143-2155.
- Idayu NF, Hidayat MT, Moklas MA, Sharida F, Raudzah AR, Shamima AR, Apryani E. Antidepressant-like effect of mitragynine isolated from Mitragyna speciosa Korth in mice model of depression. Phytomedicine. 2011;18(5):402-407.
- Chittrakarn S, Keawpradub N, Sawangjaroen K, Kansenalak S, Janchawee B. The neuromuscular blockade produced by pure alkaloid, mitragynine and methanol extract of kratom leaves (Mitragyna speciosa Korth.). Journal of Ethnopharmacology. 2010;129(3):344-349.
- Kumarnsit E, Keawpradub N, Nuankaew W. Acute and long-term effects of alkaloid extract of Mitragyna speciosa on food and water intake and body weight in rats. Fitoterapia. 2006;77(5):339-345.
- Senik MH, Mansor SM, J. JTK, Abdullah JMB. Effect of acute administration of Mitragyna speciosa Korth. standardized methanol extract in animal model of learning and memory. Journal of Medicinal Plants Research. 2012;6(6):1007-1014.
- Hazim AI, Mustapha M, Mansor SM. The effects on motor behaviour and short-term memory tasks in mice following an acute administration of Mitragyna speciosa alkaloid extract and mitragynine. Journal of Medicinal Plants Research. 2011;5(24):5810-5817.
- Kamal MSA, Ghazali AR, Ashikin Yahya N, Wasiman MI, Ismail Z. Acute toxicity study of standardized Mitragyna speciosa Korth aqueous extract in Sprague Dawley rats. Journal of Plant Studies. 2012;1(2):120-129.
- Muhammad BY, Abdullahi AD, Kadir SNA, Razak TBA, Ahmed QU. In-utero effects of the crude ethanolic extract of the leaves of Mitragyna speciosa on neural tube formation in rats. Asian Journal of Experimental Biological Sciences. 2010;1(2):404 -408.
- Reanmongkol W, Keawpradub N, Sawangjaroen K. Effects of the extracts from Mitragyna speciosa Korth. leaves on analgesic and behavioral activities in experimental animals. Songklanakarin Journal of Science Technology. 2007;29(Suppl 1):39-48.
- Harizal SN, Mansor SM, Hasnan J, Tharakan JK, Abdullah J. Acute toxicity study of the standardized methanolic extract of Mitragyna speciosa Korth in rodent. Journal of Ethnopharmacology. 2010;131(2):404-409.
- Norizah A, Elda Nurafnie IR, Nurul Fariza R, Izwah H, Amiruddin M, Teh BP. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute Medical for Research, Ministry of Health; 2016. Report no.: HMRC 11-045/01/MS/L/K.
- Moklas M, Raudzah AN, Hidayat MT, Sharida F, Idayu NF, Zulkhairi A, Shamima AR. A preliminary toxicity study of mitragynine, an alkaloid from Mitragyna speciosa Korth and its effects on locomotor activity in rats. Advances in Medical and Dental Sciences. 2008;2(3):56-60.
- Kapp FG, Maurer HH, Auwarter V, Winkelmann M, Hermanns-Clausen M. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa). Journal of Medical Toxicology. 2011;7(3):227-231.
- McWhirter L, Morris S. A case report of inpatient detoxification after kratom (Mitragyna speciosa) dependence. European Addiction Research. 2010;16(4):229-231.
- Apryani E, Hidayat MT, Moklas MA, Fakurazi S, Idayu NF. Effects of mitragynine from Mitragyna speciosa Korth leaves on working memory. Journal of Ethnopharmacology. 2010;129(3):357-360.
- Kong WM, Chik Z, Ramachandra M, Subramaniam U, Aziddin RE, Mohamed Z. Evaluation of the effects of Mitragyna speciosa alkaloid extract on cytochrome P450 enzymes using a high throughput assay. Molecules. 2011;16(9):7344-7356.
- Hanapi NA, Azizi J, Ismail S, MAnsor SM. Evaluation of selected Malaysian medicinal plants on etabolizing enzymes, CYP2C9, CYP2D6 and CYP3A4 activities in vitro. International Journal of Pharmacology. 2010;6(4):494-499.