Peria katak fruit
Momordica charantia L.
Cucurbitaceae
DEFINITION
Peria katak fruits consist of dried deseeded fruits of M. charantia (Cucurbitaceae).
SYNONYM
Cucumis argyi H.Lév, Cucumis intermedius M.Roem, Momordica charantia subsp. abbreviata (Ser.) Greb, Momordica charantia f. abbreviata (Ser.) W.J.de Wilde & Duyfjes, Momordica charantia var. abbreviata Ser., Momordica charantia var. longirostrata Cogn, Momordica charantia var. muricata (Willd.) Chakrav., Momordica chinensis Spreng, Momordica elegans Salisb, Momordica indica L., Momordica muricata Willd., Momordica sinensis Spreng., Momordica thollonii Cogn, Sicyos fauriei H. Lév [ 1 , 2 ].
VERNACULAR NAMES
Bitter melon, balsam apple, bitter gourd (English); peria katak (Malay); ku gua, liang gua, ban sheng gua, jin li zhi, lai pu tao (Chinese); pavakai, karela (Tamil) [ 3 , 4 , 5 ].
CHARACTER
| Colour | Green (fresh fruit), orange (ripe fruit), green to brownish (powder) |
| Odour | Characteristic |
| Taste | Bitter |
IDENTIFICATION
Plant Morphology
M. charantia is a monoecious climber up to 5 m long, slightly pubescent, the tendril up to 20 cm long. Stem ridge, glabrous or hairy. Leaves are simple, alternate leaves 4-12 cm across, palmately 5-7 lobed, with 3-7 deeply separated lobes, stipules absent, blade broadly ovate, cordate at base. Flower unisexual, regular, yellow; calyx 5-lobed with obconic tube; petals 5; male flowers with 3 stamen, anthers coherent in center of flower; female flower with inferior, ovoid to fusiform, muricate-turbeculate ovary; stigma 3-lobed. Fruits are fleshy, oblong-cylindric, irregularly warty, coarsely ridged and bumpy-tuberculate, usually 3-11 cm, can be up to 20 cm length, 2-8 cm diameter; unripe fruit is green, ripe fruit is orange or dark yellow; hollow in cross-section with a thin layer of flesh surrounding a central seed and pith; skin is tender and edible. Seeds and pith appear white in unripe fruit, numerous, oblong, ripening to red [ 3 , 6 , 7 , 8 ].
Microscopy
Powdered material consists of fragments of spiral vessels thickening, with long or short vessels, sometimes embedded in parenchyma; prismatic calcium oxalate crystals; fragments of the parenchyma cells in surface view, with rounded cells and triangular spaces; abundant starch granules, simple, oblong, oval or irregular, found in agglomerated, and may be free or included in parenchymatous cells; fragment of fibres, isolated or sometimes found in group; isolated oil globules; isolated sub-rectangular sclereid and slightly thickened walls [ 7 , 8 ].
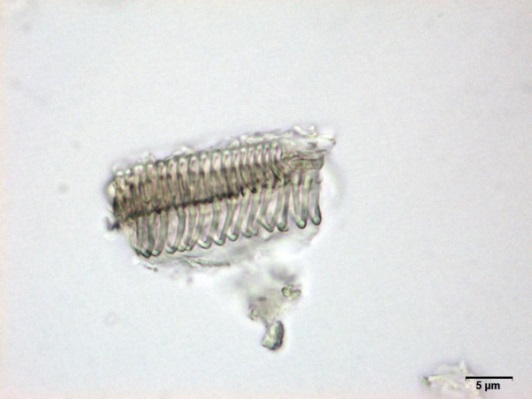
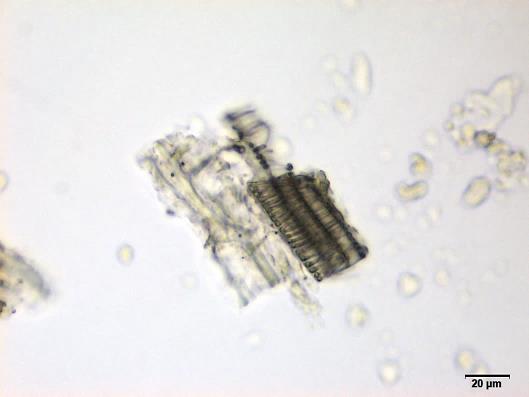
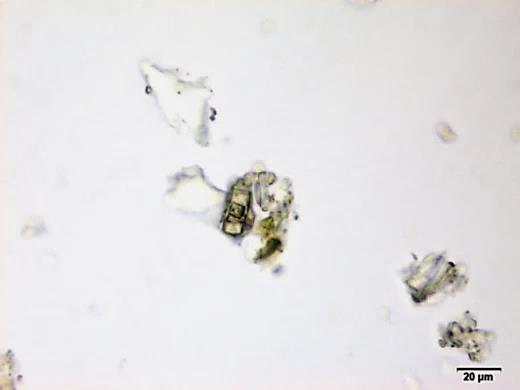
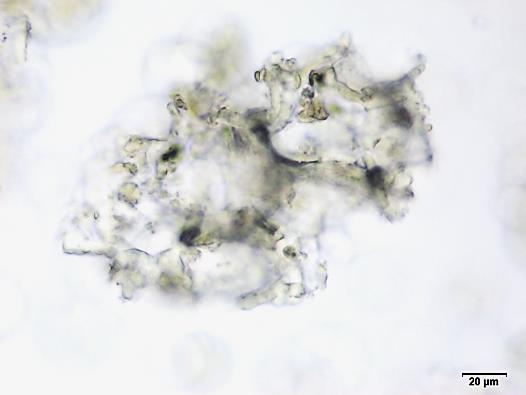
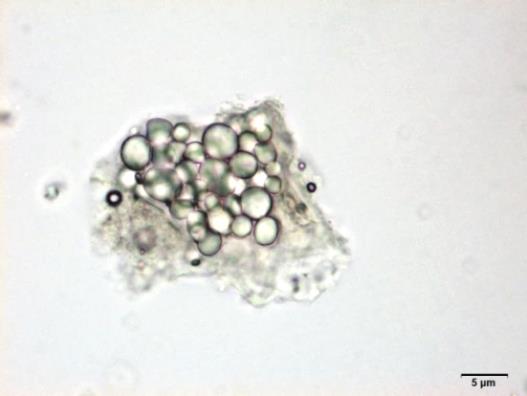
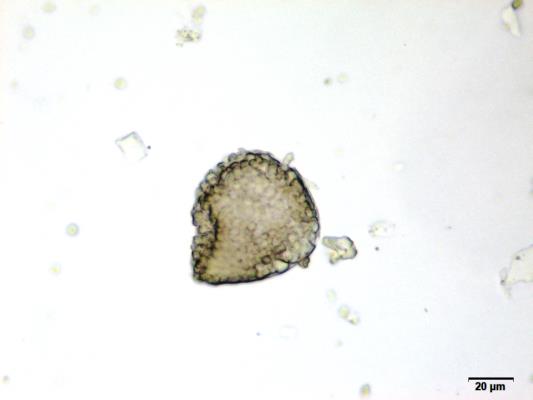
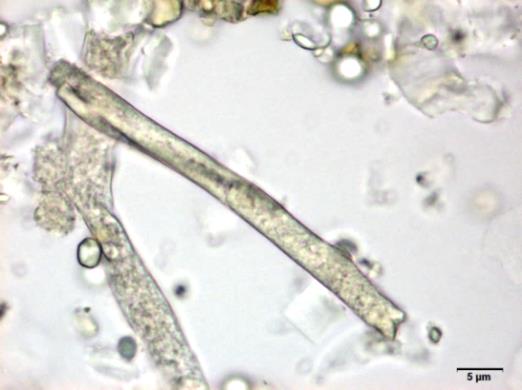
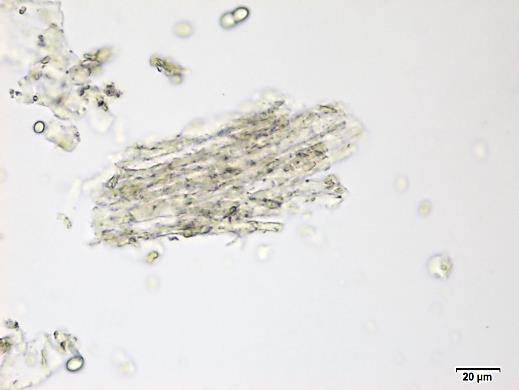
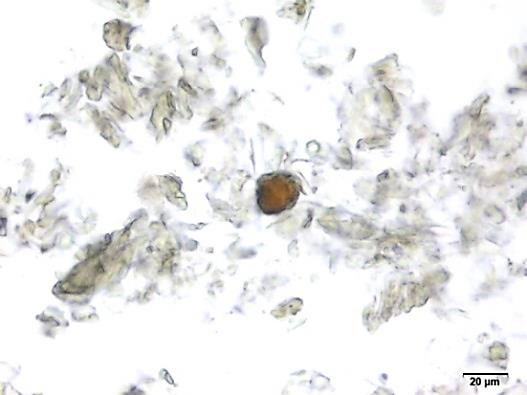
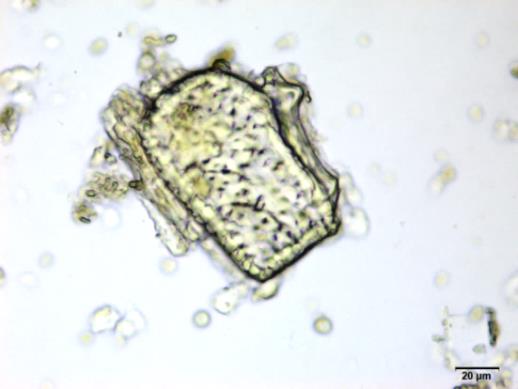
Figure 2 : Microscopic characters of M. charantia fruit powder.(a) Spiral thickening vessels; (b) spiral thickening vessels attached to parenchyma; (c) prismatic calcium oxalate crystals; (d) parenchyma cells; (e) starch granules; (f) agglomerated starch granules; (g) single fibers; (h) fibers in group; (i) oil globule; (j) sclereid cell. [Scale bars: a, e, g = 5 mm; b-d, f, h-j =20 mm]
Colour Tests
Observed colour of solution after treatment with various reagents:
| H2SO4 (conc.) | Light brown |
| NH4OH (25%) | Light yellow |
Thin Layer Chromatography (TLC)
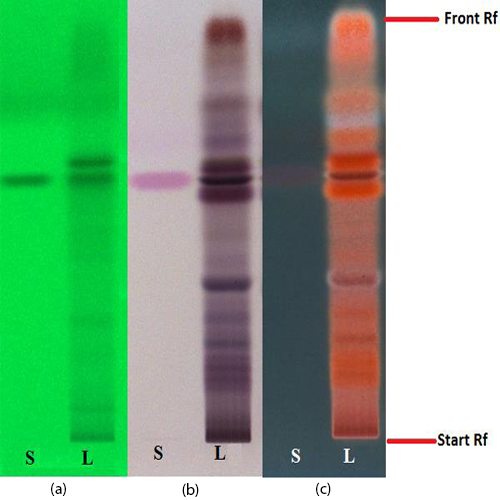
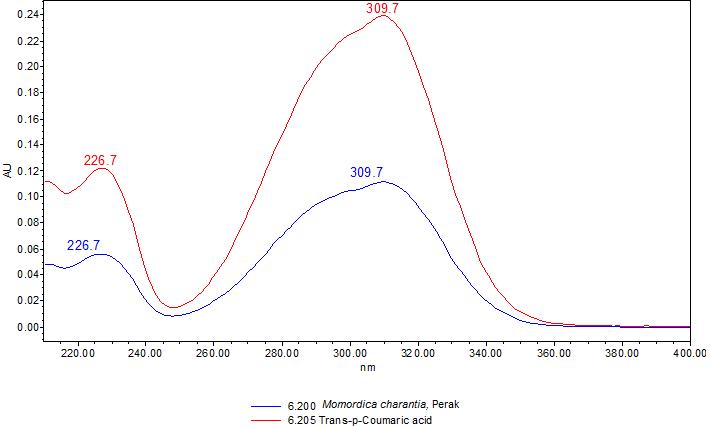
Figure 3 : TLC chromatogram of trans-p-coumaricacid(S) and ethyl acetate fraction of ethanol extract of M. charantia dried fruit powder (L) observed under (a) UV at 254 nm before spraying; (b) visible light and (c) UV at 366 nm after derivatized with anisaldehyde reagent.
| Test Solutions | Weigh about 3.0 g of M. charantia dried fruit powder in a 250 mL conical flask. Add 30 mL of ethanol and sonicate the mixture for 15 min at room temperature. Filter the mixture with filter paper (Whatman no. 1) into round bottom flask. Evaporate the filtrate to dryness. Dissolve the dried filtrate with 20 mL of aqueous and transfer into separating funnel. Add 30 mL x 3 times of ethyl acetate and shake well. Collect the ethyl acetate layer (upper layer). Evaporate to dryness. Reconstitute with 1.5 mL ethanol. Use the filtrate as test solution. |
| Standard solution | Dissolve trans-p-coumaric acid standard [CAS no.: 501-98-4] in ethanol to produce a standard concentration 0.20 mg/mL. |
| Stationary Phase | HPTLC Silica gel 60 F254, 10 x 10 cm |
| Mobile phase | Toluene : acetone : formic acid (38 : 35 : 5) (v /v /v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
High Performance Liquid Chromatography (HPLC)
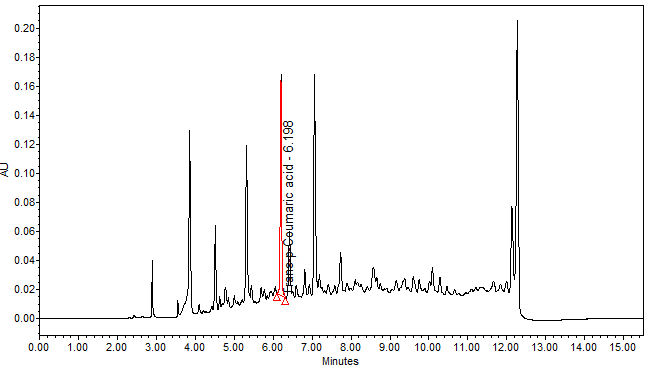
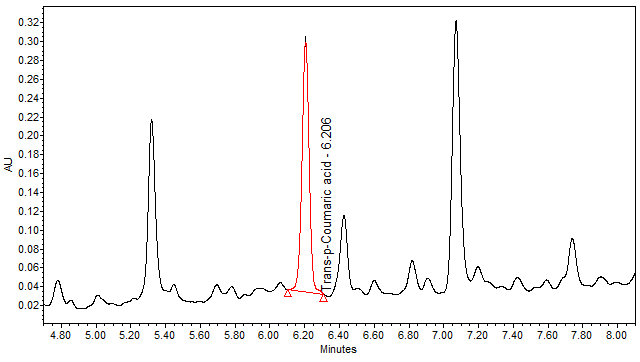
| Test solution | Weigh about 3.0 g of M. charantia dried fruit powder in a 250 mL conical flask. Add 30 mL of ethanol and sonicate the mixture for 15 min at room temperature. Filter the mixture with filter paper (Whatman no. 1) into round bottom flask. Evaporate to dryness. Dissolve the dried filtrate with 20 mL of aqueous and transfer into separating funnel. Add 30 mL x 3 times of ethyl acetate and shake well. Collect the ethyl acetate layer (upper layer). Evaporate to dryness. Reconstitute with 1 mL ethanol, filter the mixture with 0.45 µm syringe filter into HPLC vial. Use the filtrate as test solution. | |||||||||||||||
| Standard solution | Dissolve trans-p-coumaric acid standard [CAS no.: 501-98-4] in ethanol to produce a standard concentration 0.05 mg/mL. | |||||||||||||||
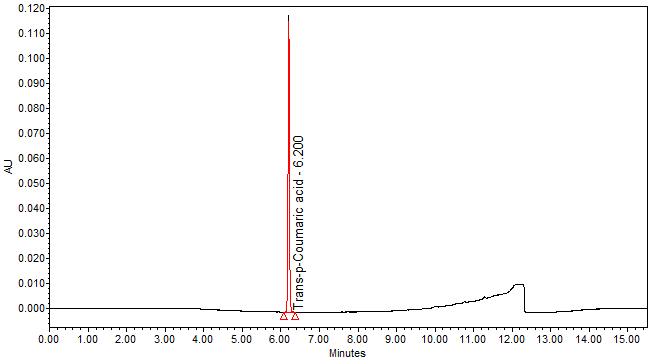
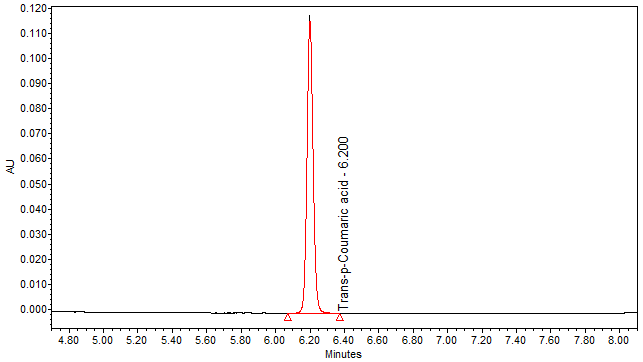
| Chromatographic system |
Detector: UV 310 nm Column: Polar encapped C18 (4µ, 250 x 4.6mm) (Synergi Hydro RP Phenomenex unless necessary) Column oven temperature: 30oC Flow rate: 1.2 mL/min Injection volume: 1 µL for standard; 10 µL for sample extract |
|||||||||||||||
| Mobile Phase (gradient mode) |
|
|||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of trans-p-coumaric acid solutions (0.05 mg/mL). The requirements of the system suitability parameters are as follow:
|
|||||||||||||||
| Acceptance criteria |
|
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 12% |
| Acid-insoluble ash | Not more than 1% |
| Loss on Drying |
| Not more than 9% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 40% |
| Cold method | Not less than 30% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 16% |
| Cold method | Not less than 7% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Aqueous methanol (20%) extract of the M. charantia fruits has been reported to contain flavonoids (e.g. kaempferol, quercetin, luteolin) [ 12 ].
Methanol extract of the M. charantia fruits has been reported to contain cucurbitane-type triterpene (e.g. momordicatin) and phenolics (e.g. gallic acid, protocatechuic acid, gentisic acid, vanillic acid, chlorogenic acid, syringic acid, epicatechin, tannic acid, catechin, caffeic acid, p-coumaric acid, benzoic acid) [ 10 , 11 ].
Ethanol extract of the M. charantia fruits has been reported to contain cucurbitane-type triterpene (e.g. charantin) [ 9 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
The juice of M. charantia fruits is used to purify the blood, gargle for abdominal disease [ 13 ], tonic [ 13 , 14 ] high blood glucose level, chronic colitis and dysentery [ 14 ]. The cooked fruits are eaten to stop runny nose, flux and cough [ 14 ].
The leaves are ingested to improve appetite [ 15 , 16 ], relieve headache and stimulate lactation [ 15 ]. A decoction of the leaves (also the fruits juice) is used for constipation [ 13 , 14 , 16 ] fever and vermifuge [ 13 , 14 ]. Crushed leaves in poultice or ointment are applied externally to treat skin diseases, burns, scalds and stomachache on children [ 13 , 16 ] while the leaves as decoction are employed as skin lotion [ 15 ] and wound healing (also the fruits macerated in oil) [ 13 , 16 ].
The flower in infusion is also taken to treat asthma [ 13 ]. The roots of the plants are used as astringent, abortifacient and external treatment of haemorrhoids [ 13 , 15 ].The plants also can be used to treat diarrhea in children [ 13 ], flatulence and delirium [ 14 ].
Biological and pharmacological activities supported by experimental data
Antihyperglycaemic activity
Ethanol (70%) extract of M. charantia fruits (24.8 mg/100 g body weight/day) administered subcutaneously to alloxan-induced diabetic male and female Sprague Dawley rats (120-150 g) for a duration of 30 days significantly (p < 0.01) reduce the serum glucose level (75.2 ± 2.9 mg/dL) compared to the initial level (before administration: 252.0 ± 10.6 mg/dL)
[ 17 ].
The juice of M. charantia fruits (5.7 mg/100 g body weight/day) administered subcutaneously to alloxan-induced diabetic male and female Sprague Dawley rats (12-150 g) for a duration of 30 days significantly (p < 0.01) reduce the serum glucose level (189.2 ± 10.0 mg/dL) compared to the initial level (before administration: 249.2 ± 9.8 mg/dL) [ 17 ].
Antioxidant activity
Methanol extract of M. charantia green flesh cultivar fruits inhibited the growth of HT 1080 human fibrosarcoma cells with inhibition concentration at 50% growth (IC50) of 0.37 mg/mL compared to doxorubicin after 48 hour incubation using resaruzin assay [ 18 ].
Aqueous extract of M. charantia green flesh cultivar fruits inhibited the growth of HT 1080 human fibrosarcoma cells with IC50 of 0.55 mg/mL compared to doxorubicin after 48 hour incubation using resaruzin assay [ 18 ].
Antileishmania activity
Aqueous extract of M. charantia fruits significantly (p < 0.05) inhibit (100% inhibition) the growth of Leishmania donovani prosmatigotes (1.27 x 106 cells/mL) with inhibition concentration at 50% of growth (IC50) of 0.6 mg/mL compared to sodium antimony gluconate (IC50 = 12 mg/mL) and amphotericin B (IC50 = 0.1 mg/mL). Meanwhile, the phytochemical compound momordicatin of M. charantia fruits significantly (p < 0.05) inhibited (100% inhibition) the growth of L. donovani prosmatigotes (1.27 x 106 cells/mL) with IC50 of 0.02 mg/mL compared to sodium antimony gluconate (IC50 = 12 mg/mL) and amphotericin B (IC50 = 0.1 mg/mL) using the same assay [ 19 ].
Aqueous extract of M. charantia fruits administered intramuscularly on alternate days to Syrian Golden hamsters (aged four weeks old) with visceral leishmaniasis for a duration of 15 days significantly (p < 0.05) reduced (100%) splenic parasite burden (0%) in dose dependent manner (0-400 mg/kg). The phytochemical compound (momordicatin) also showed significant (p < 0.05) dose dependent (0-20 mg/kg) reduction (100%) in the splenic parasite burden (0%) [ 19 ].
Trypanocidal activity
Aqueous extracts of M. charantia fruits inhibited the growth of Trypanosoma brucei bloodstream form in dose-dependent manner (10, 3, 1, 0.3 and 0.1% v/v) with minimum inhibitory concentration (MIC) of 3% v/v (Chinese and Indian variety) while 50% growth inhibition (GI50) of 1.28 ± 0.12% v/v (Chinese variety) and GI50 of 0.53 ± 0.01% v/v (Indian variety) after 48 hour incubation of the trypanosomes using trypan blue staining assay [ 20 ].
The filtrate isolated from aqueous extracts of M. charantia fruits inhibited the growth of T. brucei bloodstream form with MIC of 10% v/v (Chinese variety) and 3% v/v (Indian variety), GI50 of 1.14 ± 0.1% v/v (Chinese variety) and 1.15 ± 0.02% v/v (Indian variety) while 90% growth inhibition (GI90) of 5.76 ± 0.69% v/v (Indian variety) after 48 hour incubation of the trypanosomes using trypan blue staining assay [ 20 ].
Cytotoxic activity
Aqueous extracts of M. charantia fruits obtained at local Asian food markets in the United Kingdom (percentage GI) (10% v/v) showed cytotoxicity effect with 50% growth inhibition (GI50)of 3.17 ± 1.33% to 3.49 ± 0.69% on human myeloid leukaemia HL-60 cells (HL-60) compared to water (control) after 48 hour incubation using trypan blue staining assay [ 20 ].
Aqueous extracts of M. charantia fruits (3% v/v) showed cytotoxicity effect (70-85% lysis) on trypanosomes compared to water (control) after one hour incubation using fluorescence-activated cell sorting analysis [ 20 ].
Anti-inflammatory activity
Ethanol (95%) extract of M. charantia fruits (50 µg/mL) significantly (p < 0.05) decreased the level of nitric oxide production (NO) by 36% in lipopolysaccharide (LPS)-stimulated murine monocytic macrophages (RAW 264.7) compared to gentisic acid treated group (154 µg/mL; NO: 28%). The extract also reduced nitric oxide synthase expression level (iNOS) by 34% relative intensity reduction compared to LPS treated group (iNOS: 0%). A decrease of 29% relative intensity in the pro-interleukin-1β expression level (pro-IL-1β) was also observed in ethanol treated group compared to LPS treated group (pro-IL-1β: 0%) [ 20 ].
Clinical studies
A randomized double-blind, placebo-controlled, two-arm, parallel trial to study the hypoglycemic and antiglycation activities of M. charantia was conducted involving 38 patients with type 2 diabetes mellitus who have reversible glycation product (A1C) ≥ 6.5% and aged at least 20 years old. These patients were divided into two groups i.e a group given 6g/day M. charantia dried pulp powder capsule (consist of 6.26 ± 0.28 mg charantin) and another given placebo, at 30 minutes before meals thrice daily (2 g/meal) for 16 weeks. The patients were followed up every four weeks. No drop-out was reported. The A1C level significantly (p < 0.05) decreased (-0.50 ± 0.45%) from eight weeks (-0.27 ± 0.30%) compared to placebo (0.31 ± 0.15%) and baseline (-0.23 ± 0.26%). No significant changes in the level of fasting blood glucose (FPG: 117.63-11.16 ± 17.05 mg/dL, p = 0.16) compared to baseline (FPG: 117.63 mg/dL) were observed [ 23 ].
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute toxicity
Ethanol (70%) extract of M. charantia fruits was administered subcutaneously to mice. Two hours after injection, there were toxicity signs observed (depression, shallow deep respiration and weak heart beats) and animals died (LD10 = 268.6 mg/100 g body weight, LD50 = 362.34 mg/100 g body weight, LD100 = 502.8 mg/100 g body weight). Histology examination revealed congestion of internal organs (lung, heart and mucous membrane of the eyes), changes in blood colour to dark brown and flabby heart with blood engorged
[ 17 ].
Ethanol (80%) extract of M. charantia fruits (300 and 2000 mg/kg) administered orally as a single dose to female Sprague Dawley rats (aged eight to 12 weeks old) showed no significant toxic effect on the parameters observed which includes body weight, food and water intakes when compared to control. However, the extract (2000 mg/kg) showed significant (p < 0.05) reduction in red blood cells (RBC: 5.28 ± 0.47 x 106/mm3), (WBC: 3.03 ± 0.39 x 103/mm3), haemoglobins (Hb: 14.30 ± 0.55 g/dL) and packed cell volume (PCV: 46.46 ± 2.00%) compared to control (RBC: 7.43 ± 0.29 x 106/mm3, Hb: 15.95 ± 0.35 g/dL, PCV: 55.93 ± 2.46%). All rats were observed for 14 days and no death was found throughout the study period (LD50 < 2000 mg/kg) [ 22 ].
The juice of M. charantia fruits was administered subcutaneously to mice. There were toxicity signs observed (abdominal convulsions, nervous disturbance, jumping and fast respiration) and animals died. LD10 was 42 mg/100 g body weight, LD50 was 91.9 mg/100 g body weight and LD100 was 188.2 mg/100 g body weight. Histology examination revealed congestion of internal organs (lung, heart, abdomen and intestine), heart with blood engorged and cyanosed mucous membrane of eye [ 17 ].
Oral single dose acute toxicity study using aqueous extract of M. charantia fruits on female Sprague Dawley rats (aged between 8 and 12 weeks old) showed no toxic effect on the parameters observed which includes behaviors, body weight, food and water intakes. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. Approximate lethal dose (LD50) is more than 2,000 mg/kg body weight [ 24 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Side effects
M. charantia dried pulp powder capsule (consist of 6.26 ± 0.28 mg charantin) which was given 30 min before meals thrice daily (2 g/meal) to type 2 diabetes mellitus patients showed significant (p < 0.05) side effects (diarrhea: 8 patients, flatulence: 4 patients) observed compared to placebo (diarrhea: 0 patients, flatulence: 2 patients) [ 23 ].
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The Plant List 2012 [1st December 2014]. Available from: http://www.theplantlist.org/tpl1.1/record/kew-2372864.
- Flora Zambesiaca 1978 [1st December 2014]. Available from: http://apps.kew.org/efloras/namedetail.do?flora=fz&qry=key&taxon=3824&keyid=852#SYNONYMS.
- Complementary and Alternative Healing University. 2013 [cited 2014 March 2014]. Available from: http://alternativehealing.org/ku_gua.htm.
- The Ayurvedic Pharmacopoeia of India [cited 2014 March 2014]. 2]. Available from: http://www.ayurveda.hu/api/API-Vol-2.pdf.
- Khare CP. Indian Medicinal Plants: An Illustrated Dictionary: Springer Science & Business Media. 2004;p.900.
- China Fo. MOMORDICA Linnaeus, Sp. Pl. 2: 1009. 1753 2011. Available from: flora.huh.harvard.edu/china/PDF/PDF19/Momordica.pdf.
- Saifi A, Namdeo K, Chauhan R, Dwivedi J. Evaluation of pharmacognostical, phytochemical and antidiabetic activity fruits of Momordica charantia Linn. Asian Journal of Pharmaceutical and Clinical Research. 2014;7(4).
- Aswar P, Kuchekar B. Phytochemical, microscopic, antidiabetic, biochemical and histopathological evaluation of Momordica charantia fruits. International Journal of Pharmacy and Pharmaceutical Sciences. 2012;4(1):325-331.
- Thomas C, Reddy P, Devanna N. Impact of cooking on charantin estimated from bitter melon fruits using high performance thin layer chromatography. Int Res J Pharm. 2012;3(6):149-154.
- Gupta S, Raychaudhuri B, Banerjee S, Das B, Mukhopadhaya S, Datta SC. Momordicatin purified from fruits of Momordica charantia is effective to act as a potent antileishmania agent. Parasitology International. 2010;59(2):192-197.
- Horax R, Hettiarachchy N, Islam S. Total phenolic contents and phenolic acid constituents in 4 varieties of bitter melons (Momordica charantia) and antioxidant activities of their extracts. Journal of food science. 2005;70(4):C275-C280.
- Agarwal M, Kamal R. Studies on flavonoid production using in vitro cultures of Momordica charantia L. 2007.
- Burkill IH. A dictionary of the economic products of the Malay Peninsula. Vol. II. Malaysia, Ministry of Agriculture. 1935;p.1485-1486.
- Wiart C. Medicinal plants of Southeast Asia, second edition. Malaysia, Pearson Malaysia Sdn Bhd.. 2002;p.99.
- Zakaria M, Mohd MA. Traditional Malay medicinal plants. Kuala Lumpur, Malaysia; Institut Terjemahan Negara Malaysia Berhad. 2010;p.100.
- Perry LM. Medicinal plants of East and Southeast Asia: Attributed Properties and Uses. Massachusetts: MIT Press. 1980;p.117.
- Batran SAESE, El-Gengaihi SE, Shabrawy OAE. Some toxicological studies of Momordica charantia L. on albino rats in normal and alloxan diabetic rats. Journal of Ethnopharmacology. 2006;108:236-242.
- Lu YL, Liu YH, Liang WL, Chyuan JH, Cheng KT, Liang HJ, Hou WC. Antibacterial and cytotoxic activities of different wild bitter gourd cultivars (Momordica charantia L. var. abbreviate Seringe). Botanical Studies. 2011;52:427-434.
- Gupta S, Raychaudhuri B, Banerjee S, Das B, Mukhopadhaya S, Datta SC, Momordicatin purified from fruits of Momordica charantia is effective to act as a potent antileishmania agent. Parasitology International. 201;59:192–197.
- A Phillips E, W Sexton D, Steverding D. Bitter melon extract inhibits proliferation of Trypanosoma brucei bloodstream forms in vitro. Experimental Parasitology. 2013;133(3):353-356.
- Lii CK, Chen HW, Yun WT, Liu KL. Suppressive effects of wild bitter gourd (Momordica charantia Linn. var. abbreviate ser.) fruit extracts on inflammatory response in RAW 264.7 macrophages. Journal of Ethnopharmacology. 2009;122:227-233.
- Nurul HR, Noriham A, Noorain H, Azizah AH, Farah Amna O. Acute oral toxicity effects of Momordica charantia on Sprague Dawley rats. International Journal of Bioscience, Biochemistry and Bioinformatics. 2013;3(4):408-410.
- Trakoon-oson W, Sotanaphun U, Phanachet P, Porasuphatana S, Udomsubpayakul U, Komindr S. Pilot study: hypoglycemic and antiglycation activities of bitter melon (Momordica charantia L.) in type 2 diabetic patients. Journal of Pharmacy Research. 2013;6:859-864.
- Teh BP, Hamzah NF, Norazila Z, Nor Liyana MY, Wan Abdul Hakim WL. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2014. Report no.: HMRC 11-045/01/MC/F2/A.