Scientific Name
Morinda citrifolia L.
Synonyms
Belicea hoffimannioides Lundell, Morinda angustifolia Roth [Illegitimate], Morinda aspera Wight & Arn, Morinda asperula Standl, Morinda bracteata Roxb. [Illegitimate], Morinda chachuca Buch.-Ham, Morinda citrifolia var. bracteata Kurz, Morinda citrifolia var. elliptica Hook.f., Morinda citrifolia var. potteri O.Deg., Morinda citrifolia f. potteri (O.Deg.) H.St.John, Morinda coreia var. stenophylla (Spreng.) Chandrab., Morinda elliptica (Hook.f) Ridl., Morinda ligulata Blanco, Morinda littoralis Blanco, Morinda macrophylla Desf., Morinda mudia Buch.-Ham., Morinda multiflora Roxb., Morinda nodosa Buch.-Ham., Morinda quandrangularis G.Don, Morinda stenophylla Spreng., Morinda teysmanniana Miq., Morinda tinctoria Noronha [Invalid], Morinda tictoria var. aspera (Wight & Arn.) Hook.f., Morinda tinctoria var. multiflora (Roxb.) Hook.f., Morinda tomentosa B.Heyne ex Roth, Morinda zollingeriana Miq., Platanocephalus orientalis Crantz, Samama citrifolia (L.) Kuntze, Sarcocephalus leichhardtii F.Muell. [1]
Vernacular Name
| Malaysia | Bengkudu, kemudu, kenudu, mengkudu kechil, menkudi besar, menkudu, menkudu besar, nona, [2] mengkudu, mengkudu besar [3], mengkudu jantan, [4] bengkudu daun besar, bengkudu laki-laki [5] |
| English | Awl tree, brimstone tree, canary wood, Leichhardt’s tree, limburger tree, morinda, noni, pain killer, painkiller, togari wood, yaw-weed, [2] cheesefruit [3], Indian mulberry, [4] beach mulberry, large-leaved morinda, noni fruit, noni plant, nonu, pain killer tree, rotten cheesefruit, [5] great morinda [6] |
| India | Acchuka, achchhuka, achuhhuka, achuka, alita, aseti, ashyuka, asukha, auch, bandamaddi, bartondi, barutndi, bundamaddi, cadapilva, chaili, chayapattai, chekka, cheli, chella, darnaharidra, haladipavatemaddi, karrapitala-vam, lam-onk, lornong, lu-rong, lurong, maddi, maddichettu, manajaparvetti, manjanathi, manjanatthi, manjanatti, man-japavatta, mannanarri, mogali, molugu, nibase, nuna, nunaa, podophul, suranji, tagache, tagaroo, takote, tanakku, thogara, thogaru chettu, thogarumogali, thogoda, togara, togaramo-gali, togaree, togareemogilli, toghur, tunaon, tunavu, tunnam, uchyoota, [2] bartundi, surangi (Bengali); mhanbin, neihpahsae, yaiyae (Burmese); saraoji, (Gujarati); aal, aach, ak, ashi, barraal, surangi (Hindi); kattapitavalam, manjanathhi, manjanatthi, mannanatti, (Malayalam); aal, ach, al, aval, nagakuda, nagakunda, surangi (Marathi); hardikath (Nepalese); pindre (Oriya); achuka, achchhuka, ashyka, ashyuka (Sanskrit); ahugaha, yhugaha (Sinhalese); bankudo (Tagalog); mancanaari, munja pavattay, nunaa, periyanuna, vellainunaa (Tamil); maddi chettu, molagha, molugu, mulugu, togaru, togarumaddi, togarumogali (Telugu); achu (Urdu); al, mulgul, (Kannada), [5] bartundi (Hindi); mogali (Telugu); nagakunda (Marathi); nuna (Tamil); mannapayatta (Malayalam); tagase maddi (Kannada); surangi (Gujarati); hurdi (Benggali); bartondi (Konkani) [7] |
| China | Hai ba ji, wu ning, luo ling, [5] hai bin mu ba ji [6] |
| Indonesia | Bengkudu, bunga teratae, cengkeru, patjé, [2] pace (Javanese); cangkudu (Sundanese); mengkudu (Malay), [4] pacel, [5] kemudu, kudu (Java); kodhuk (Madura); wengkudu (Bali), [8] mengkudu laut (Javanese); kemudu, kudu, paché (Sundanese); kudu, changkudu (Sumatra); mĕkudu (Siam); yaw [9] |
| Thailand | Ka-muu-duu, mataasuea, muu duu, yae yai, yo, yo ban, yo thueen, yor ban, [2] yo baan (Central); mataa-suea (Northern); yae-yai (Karen, Mae HongSon), [4] yor [5] |
| Laos | Nho, [2] nhoo baanz [4] |
| Myanmar | Al [4] |
| Philippines | Apatot-nga-basit, bangkoro, bangkuro, nino, [2] bankoro, tumbong-aso (Tagalog); apatot (Ilokano), [4] bancudo, bangcudo (Visayan) [5] |
| Cambodia | Nhor prey, nhor thom, [2] nhoër srôk, nhoër thôm’ [4] |
| Vietnam | Chau, dau, nhau, nhau lon, nhau nui, nhau rung, rau, [2] nh[af]u l[os] chanh, ngao, nh[af]u n[us]i, [4] cây nhàu, trài nhàu [5] |
| Japan | Yaeyama-aoki [2] |
| Portugal | Pauazeitona, [3] pau-azeitona [5] |
| France | Bois douleur, [3] morinde, [4] nono [5] |
| Spain | Mora de la India, [3] huevo de reuma (Dominican Republic); noni (Puerto Rico) [5] |
| Latin America | Cobalanga, kòwòsòl zombie, pangkila, planta milagrosa, yema de huevo [2] |
| Australia | Koonjerung, tokoonja [2] |
| Papua New Guinea | Gomor, kotambul, leki, mwagum wagugu, noku, nono, oko, wal, woko [2] |
| Cameroon | Atchek, n’keng [2] |
| Netherlands | Noni, kaasvrucht, stinkend kaasvrucht [5] |
| German | Indische maulbere, indischer maulbeerbaum, indischer maulbeerstrauch, noni-baum [5] |
| Russia | Моринда Ситрифолиа , Моринда лимонолистная [5]. |
Geographical Distributions
Morinda citrifolia is possibly indigenous in tropical Asia and tropical Australia. Man and sea currents may have distributed it. It is naturalised in many tropical regions, and is now in fact almost pantropical. [4]
Botanical Description
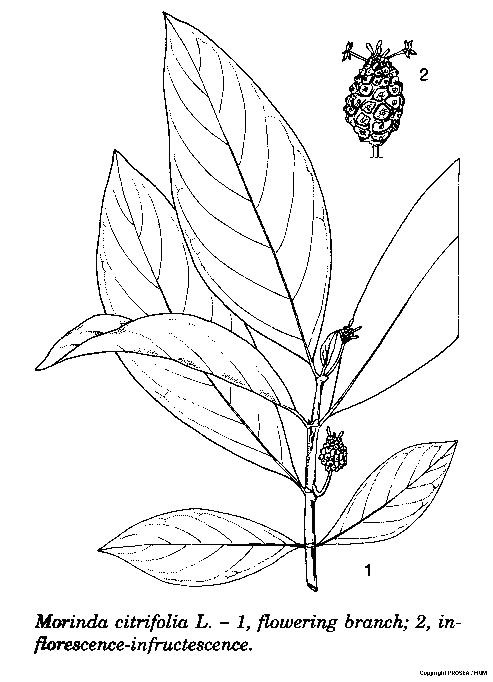
M. citrifolia is a member of Rubiaceae family. M. citrifolia grows in shady forests and on open rocky or sandy shores; it is a shrub or small-medium sized tree (3-10 m) with quadrangular or somewhat rounded branches and evergreen. [10][11]
The leaves are large, simple, alternate, broadly elliptic to oblong shape (10-30 x 5-15 cm), dark green, glossy, wavy and prominently-veined. The 5-lobed white tubular-like fragrant. [10][11]
The flower is small (1.25 cm long) and borne in a globose head (2.5 cm across). [10][11]
The heads develop into compound fruits composed of many small drupes; the fruit is ovoid, ellipsoid or roundish (3-10 x 3-6 cm) with an embossed appearance, slightly wrinkly, waxy, semi-translucent skin, and turns from green to yellow and to almost white as it ripens; the fruit surface is faintly patterned with 4- to 6-sided outlines, each with a central “eye”; the pulp is fleshy and juicy, dull-yellow or yellowish-white and gelatinous when the fruit is ripe; it has numerous hard oblong-triangular reddish-brown pits, each containing 4 seeds about 3.5 mm long. [10][11]
Cultivation
Soil Suitability and Climate Requirement
M. citrifolia is a hardy and easy growing plant. It grows well on most soils including marginal soils such as bris and peat. It can be planted in coastal areas up to 400 m above sea level. Areas with annual rainfall of 2,000-3,000 mm are ideal for good crop growth and yield. However, M. citrifolia plant is susceptible to root knot nematode. Thus, areas with high nematode population such as bris sandy soil should be avoided. [12]
Field Preparation
Land Preparation
Prior to planting, normal field operations such as land clearing, disc ploughing and rotovation are to be conducted. Ground Magnesium Limestone (GML) and Triple Super Phosphate (TSP) each at the rate of 100 g per planting hole are applied for liming and basal fertilisation purposes respectively. The size of a planting hole is 30 cm x 30 cm x 30 cm. Field drainage system should also be established in areas where waterlogged is a threat. [12]
Production of Planting Materials
M. citrifolia is propagated by seeds as the seeds can easily be collected from mature fruits. A medium size fruit can produce 200-250 seeds. Seeds are sown in sowing trays and then the seedlings should be transferred into 13 cm x 18 cm polybags. The seedlings are ready for field planting at 6 or 7-leaf stage or after 7 months in the polybags. It can also be propagated using seedlings raised from young stem cuttings. [12]
Field Planting
The recommended field planting distance is 5 m (between rows) x 3 m (within a row). This will give the population density of 667 plants/ha. The planting should be done at the beginning of the rainy season. [12]

Field maintenance
Fertilisation
Fertilisation is done using compound fertiliser NPK (12:12:17:2), processed chicken manure and GML (Ground Magnesium Limestone). Chicken manure is applied in the first year only at the rate of 1.0 kg/plant. Half of the rate is given at planting while the other half 6 months later. Compound fertiliser NPK is applied at the rate of 500, 750 and 1,000 g/plant in the 1st, 2nd and 3rd year upward, respectively. For each rate, the NPK fertiliser is divided into four equal parts and applied at 3 months intervals. GML at the rate of 300, 400 and 500 g/plant is applied in the 1st, 2nd and 3rd year upward, respectively. [12]
Weed Control
For the first five months after planting, weeds are controlled by two rounds of rotor-tillage operation. After that it can be done by spraying systemic or contact herbicides. [12]
Water management
Supplementary irrigation is required for optimum crop growth and yield. Drip irrigation system is recommended for this crop. [12]
Pest and Disease Control
The most important pest for this crop is root knot nematode (Meloidogyne incognita). The symptoms of infected plants are yellowing, stunted growth, and plants eventually die due to root damages. The nematode is controlled by using sterilised growing media at the nursery stage and using nematicide e.g. Nemacur at the early growth stage in the field. Nonetheless, planting M. citrifolia on soils with high population of nematode should be avoided. [12]
Harvesting
M. citrifolia plant starts producing yield 6 months after planting. It bears fruit throughout the year. Mature fruits are harvested at 20-25 days intervals. The average fresh fruit yields obtained by a commercial farm are 1.2, 16.0 and 18.0 t/ha for the 1st, 2nd and 3rd year respectively. After that the crop yield presumably becomes stable and can last for at least 10 years. [12]

Postharvest handling
Harvesting has to be done carefully to avoid fruit injury and contamination. The harvested fruits should immediately be transported to collection centre for cleaning, grading, storing and processing activities. [12]

Estimated cost of production
The total production cost for a 10-year production period is estimated at RM42, 500/ha. Thus, for a total yield of 161.0 t/ha, the average cost of production of fresh fruit is RM0.30/kg. The production cost was estimated based on the cost of current inputs during writing of this article. [12]
Chemical Constituent
The M. citrifolia fruits juice has been found to contain glycosides (e.g. 1-O-(3’-methylbut-3’-enyl)-β-D-glucopyranose, 1-n-butyl-4-(5’-formyl-2’-furanyl)methyl succinate, 4-epiborreriagenin, 1-n-butyl-4-methyl-2-hydroxysuccinate, 1-n-butyl-4-methyl-3-hydroxysuccinate, α-glucopyranose, β-glucopyranose, deacetylasperulosidic acid, asperulosidic acid, scopoletin). [13]
Butanol extract of the fruits has fatty acid glucosides (e.g. 1,6-di-O-octanoyl-β-D-glucopyranose, 6-O-(β-D-glucopyranosyl)-1-O-decanoyl-β-D-glucopyranose, 6-O-(β-D-glucopyranosyl)-1-O-octanoyl-β-D-glucopyranose, 6-O-(β-D-glucopyranosyl)-1-O-hexanoyl-β-D-glucopyranose, 2,6-di-O-(β-D-glucopyranosyl)-1-O-octanoyl-β-D-glucopyranose) and others (e.g. rutin, asperulosidic acid). [14][15]
Ethanol extract of the fruits contains polysaccharides (e.g. pectin polysaccharides, type II arabinogalactan, xyloglucan, heteroxylan, heteromannan) and vitamins (e.g. ascorbic acid, provitamin A). [16]
Methanol extract of the fruits has anthraquinones (e.g.1,6-dihydroxy-5-methoxy-2-methoxyanthraquinone, 1,5,7-trihydroxy-6-methoxy-2-methoxymethylanthraquinone, 1,5,15-tri-O-methylmorindol, 1,5-dimethylmorindol, 1,3-dimethoxyanthraquinone, alizarin, anthraquinone), flavonoids (e.g. quercetin, kaempferol), lignans (e.g. (+)-3,4,3’4’-tetrahydro-9,7’α-epoxylignan-7α,9’-lactone), iridoids (e.g. 6a-hydroxyadoxoside, 6b,7b-epoxy-8-epi-splendoside, morinaphthalenone, borreriagenin, citrifolinin B epimer a and b, cytidine, epi-dihydrocornin, scopoletin, isoscopoletin, 3,3’-bisdemethyltanegool, (-)-pinoresinol, (-)-3,3’-bisdemethylpinoresinol, americanin A, narcissoside), alkaloids (e.g. proxeronine, xeronine), glucosides (e.g. D-glucose, D-mannitol methyl a-D-fructofuranoside, methyl b-D-fructofuranoside, nicotifloroside, b-sitosterol 3-O-b-D-glucopyranoside, 6-O-(β-D-glucopyranosyl)-1-O-octanoyl-β-D-glucopyranose, 6-O-(β-D-glucopyranosyl)-1-O-hexanoyl-β-D-glucopyranose, 3-methyl-3-butenyl 6-O-β-D-glucopyranosyl-β-D-glucopyranoside, 2-O-(β-D-glucopyranosyl)-1-O-octanoyl-β-D-glucopyranose, 2-O-(β-D-glucopyranosyl)-1-O-hexanoyl-β-D-glucopyranose, noniosides E-H, 2,6-di-O-(β-D-glucopyranosyl)-1-O-octanoyl-β-D-glucopyranose, 6-O-(β-D-glucopyranosyl)-1-O-octanoyl-β-D-glucopyranose) and others (e.g. vanillin, asperuloside, asperulosidic acid, deacetylasperuloside, dehydromethoxygaertneroside). [17][18][19][20][21][22][23][24][25][26]
Dichloromethane extract of the fruits has been found to contain derivatives of acids (e.g. hexanoic acid, octanoic acid, decanoic acid, nonanoic acid, heptanoic acid, butanoic acid, acetic acid, butanoic acid, 2-methylbutanoic acid, 2-methylpropanoic acid, 3-methylthiopropanoic acid, benzoic acid, hexanedioic acid, undecanoic acid, lauric acid, myristic acid, palmitic acid, linoleic acid, oleic acid (Z,Z,Z)-8,11,14-eicosatrienoic acid), alcohols (e.g. 1-butanol, 3-methyl-3-buten-1-ol, 3-methyl-2-buten-1-ol,1-hexanol, benzyl alcohol, eugenol, (Z,Z)-2,5-undecadien-1-ol) and others (e.g. methyl hexanoate, ethyl hexanoate, methyl ocatanoate, methyl 5-nonenoate, methyl decanoate, methyl 3-methylthio-propanoate, ethyl octanoate, ethyl decanoate, methyl palmitate, methyl elaidate, methyl oleate, 3-hydroxy-2-butanone, 2-heptanone, (E)-6-dodeceno-γ-lactone, (Z)-6-dodeceno-γ-lactone, hexamide, limonene, scopoletin, vomifoliol). [27]
Hexane extract of the fruits contains sulfur compounds (e.g. methanethiol, S-methyl thioacetate, dimethyl disulfide, methyl 3-methylthiopropanoate), terpenes (e.g. linalool oxide, (Z)-3,7-dimethyl-1,3,6-octatriene, (+)-4-carene, D-limonene, ocimenol, terpineol), derivatives of acids (e.g. butanoic acid, decanoic acid, 2E,4Z,7Z-decatrienoic acid, hexanoic acid, octanoic acid, 2-octenoic acid, nonanoic acid, heptanoic acid, acetic acid, formic acid) and others (e.g. 1-butanol, 1-methyl-3-buten-1-ol, 1-methyl-2-buten-1-ol, 2-methyl-2-buten-1-ol, 3-methyl-3-buten-1-ol, 3-methyl-2-buten-1-ol, 3-methyl-2-butanone, benzyl alcohol, acetaldehyde, 2-methylbutanal, 3-methylbutanal, 2-pentanone, 2-hexanone, hexanal, 2-heptanone, 2-hexenal, furfural, benzaldehyde, ethanol, benzyl alcohol, ethyl acetate, butyl acetate, methyl 2-methylpropanoate, methyl butanoate, ethyl butanoate, butyl butanoate, 4-pentenyl butanoate, methyl 2-methylbutanoate, methyl 3-methylbutanoate, 3-methyl-3-buten-1-yl-3-methylbutanoate, methyl hexanoate, ethyl hexanoate, butyl hexanoate, 4-pentenyl hexanoate, 3-methyl-3-buten-1-yl hexanoate, hexyl isovalerate, methyl heptanoate, methyl ocatanoate, ethyl octanoate, butyl octanoate, 3-methyl-3-buten-1-yl octanoate, methyl 2-octenoate, methyl 3-octenoate, methyl 6-octenoate, methyl nonanate, methyl 5-nonenoate, methyl decanoate, ethyl decanoate, methyl 4-decenoate, ethyl 4-decenoate, methyl salicylate, methyl hexadecanoate). [28][29]
Chloroform and petroleum extract of M. citrifolia dried leaves was found to contain flavonoids (e.g. kaempferol 5,7-O-diarabinoside and apigenin). [30]
Aqueous extract of M. citrifolia dried leaves was found to contain alkaloids, coumarins, flavonoids (e.g. quercetin-3-O-rutinoside and kaempferol glycosides), tannins, saponins, steroids and triterpenoids. [30][31]
Methanolic extract of M. citrifolia dried leaves was found to contain flavonoids (e.g. quercetin 3,7-O-dimethyl ether, quercetin 3-O-methyl ether, kaempferol 3,4’-O-dimethyl ether, kaempferol-3-O-α-L-rhamnopyranosyl-1(1→6)-b-D-glucopyranoside and quercetin-3-O-α-L-rhamnopyranosyl-1(1→6)-b-D-glucopyranoside), iridoids (e.g. citrifoside and deacetyl asperuloside), megastigmane (e.g. roseoside), anthraquinones (e.g. 1,5,15-trimethylmorondol and 5,15-dimethylmorindol), diterpenes (e.g. phytol), triterpenes (e.g. ursolic acid, barbinervic acid, rotungenic acid, clethric acid, hederagenin, oleanolic acid and 3-O-acetylpomolic acid), fatty acids (e.g. 13-hydroxy-9,11,15-octadecatrienoic acid), coumarin (e.g. pteryxin and peucedanocoumarin III), chlorophyll derivatives (e.g. pheophorbide a, methyl pheophorbide b, methyl pheophorbide a, 151(S)-hydroxypurpurin-7 lactone dimethyl ester, 132(R)-hydroxypheophorbide a methyl ester, 151(R)-hydroxypurpurin-7 lactone dimethyl ester, 132(S)-hydroxypheophorbide a methyl ester and 13-epi-phaeophorbide a methyl ester). [32][33]
Ethanol extract of M. citrifolia dried leaves was found to contain alkaloids, carbohydrate, saponins, triterpenes (e.g. ursolic acid), phytosterols (e.g. campesterol, b-sitosterol and stigmasterol), flavonoids (e.g. quercetin-3-O–β-D-glucopyranoside, quercetin-3-O-α-L-rhamnopyranosyl-(1→6)-b-D-glucopyranoside (rutin), quercetin-3-O–β-D-glucopyranosyl-(1→2)-[ α-L-rhamnopyranosyl-(1→6)]-β-D-galacopyranoside, kaempferol-3-O-α-L-rhamnopyranosyl-(1→6)-b-D-glucopyranoside (kaempferol 3-O-rutinoside), kaempferol-3-O– β-D-glucopyranosyl-(1→2)-[ α-L-rhamnopyranosyl-(1→6)]-β-D-galacopyranoside, quercetin and kaempferol), iridoids (e.g. asperuloside, asperulosidic acid, citrifolinin B and citrifolinoside A), polyol (glycerin, D-arabinitol and sorbitol), hydrocarbon (pentadecane, hexadecane, heptadecane, octadecane and heptacosane), fatty acids (tetradecanoic acid, n-hexadecanoic acid and eicosanoic acid), diterpene (phytol), g-tocopherol, pyropheophorbide a, phenols, proteins and amino acids. [34][35][36][37][38][39][40]
Acetone-water extract of M. citrifolia dried leaves was found to contain phytosterols (e.g. campesterol, stigmasterol and β-sitosterol) and oxalic acid. [35]
Plant Part Used
Fruits and leaves. [9][10][41]
Traditional Use
The ripe fruit of M. citrifolia is traditionally used for relief of sore throat, boil, carbuncle, stomach ulcer and inflamed elbow. The fruit juice is consumed to prevent adverse effects of kava (Piper methysticum). The fruit pounded with sugar cane and kava root is used for tuberculosis. The charred unripe fruits have been applied with salt for gum disorders, while the fresh young fruit mashed with salt is applied on deep cuts and poulticed over broken bones. [9][10][41]
Traditionally, the leaves are applied to the chest or to the abdomen, for cough, enlarged spleen, in nausea, colic and fever. [9]
Preclinical Data
Pharmacology
Antinociceptive activity
Aqueous extract of M. citrifolia leaves (400 mg/kg) was administered orally to male Swiss mice (25 – 30 g) 60 minutes before the induction of abdominal constriction using acetic acid. The extract showed significant (p < 0.001) reduction in the (number of writhings = 3) compared to vehicle group (number of writhings = 13). [30]
Anti-inflammatory activity
Aqueous extract of M. citrifolia leaves (200 and 400 mg/kg) was administered subcutaneously to male Swiss mice (25 – 30 g) 60 minutes before induction of leukocyte migration into peritoneal using carrageenan. The extract (200 mg/kg) showed significant (p < 0.01) reduction in the number of leukocytes (12), meanwhile extract (400 mg/kg) showed significant (p < 0.001) reduction in the number of leukocytes (11) compared to vehicle group (25). [30]
Aqueous extract of M. citrifolia leaves administered to mouse macrophage RAW 264.7 cells with lipopolysaccharides showed significant (p < 0.05) decreased in the secretion of TNF-α (98%) compared to dexamethasone (23%) and indomethacin (25%). [31]
There is enough evidence to show that M. citrifolia as a whole possesses anti-inflammatory activity. The fruit executed the anti-inflammatory effects due to the presence of several polyphenol belonging to the coumarin, flavonoid and phenolic acid groups. [42][43][44] The anti-inflammatory activity is mediated through the antioxidant activity of some of these compounds and its direct inhibition of cyclo-oxydase COX-1 and COX-2 activities [45]. Damnacanthal, the anthraquinone isolated from the roots appears to possess these same activities too [46][47].
Antioxidant activity
Aqueous extracts of M. citrifolia leaves (1, 10 and 100 μg/mL and 1 mg/mL) showed antioxidant activity with hydroxyl radical scavenging activity inhibition concentration at 50% level (IC50) of 35 µg/mL and 30 µg/mL compared to α-tocopherol (IC50 = 28 µg/mL). [30]
Ethyl acetate extract of M. citrifolia fruit (4 mg) had strong antioxidant activity comparable to tocopherol and butylated hydroxyl toluene. [33]
Dichloromethane extract of M. citrifolia leaves (500 g/mL) showed antioxidant activity with 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity inhibition concentration at 50% of growth (IC50) of 0.20 μg/mL compared to scopoletin (IC50 = 0.25 μg/mL) and gallic acid (IC50 = 0.002 μg/mL). [48]
Methanol extract of M. citrifolia leaves (500 g/mL) showed antioxidant activity with 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity inhibition concentration at 50% of growth (IC50) of 0.26 μg/mL compared to scopoletin (IC50 = 0.25 μg/mL) and gallic acid (IC50 = 0.002 μg/mL). [48]
The methanol extract of M. citrifolia root exhibited high antioxidative activity that was not significantly (P<0.05) different from α-tocopherol or butylated hydroxyl toluene (BHT), while the methanol extracts of fruit and leaf showed negligible activities. On the other hand, the ethyl acetate extract of all parts of M. citrifolia exhibited significant antioxidative activity, which is comparable to that of both α-tocopherol and BHT. [49]
The neolignan, americanin A isolated from the methanol extract of M. citrifolia fruit showed potent antioxidant activity. [50]
Several anthraquinone isolated from the roots [51] and fruits [52] of M. citrifolia proved to be a potent antioxidant.
Screening for antioxidant activity in medicinal plants had shown that M. citrifolia do possesses antioxidant activity. The 50% ethanol extracts contain 3,3’-Bisdemethylpinoresinol, amaericanin A and quercetin, and these active constituents exhibited both tyrosine inhibitory and radical scavenging activities. [53][54][55] However, the M. citrifolia juice extract did not show any antioxidant activity when it failed to protect rats from oxygen toxicity. [56]
Antiproliferative activity
Methanol extract of M. citrifolia dried leaves (500 g/mL) showed inhibitory effect against human epidermoid carcinoma (KB) (IC50 = 186.25 ± 4.79 µg/mL) in a dose-dependent manner compared to rutin (167.00 ± 6.71 µg/mL) and scopoletin (120.00 ± 6.12 µg/mL) using MTT assay. [48]
Dichloromethane extract of M. citrifolia fresh leaves (500 g/mL) showed inhibitory effect against human epidermoid carcinoma (KB) (IC50 = 21.67 ± 1.54 µg/mL) and human cervical carcinoma (HeLa) (IC50 = 68.50 ± 2.43 µg/mL) in a dose-dependent manner compared to damnacantal (IC50 = 6.50 ± 0.5 µg/mL and IC50 = 22.00 ± 3.52 µg/mL) using MTT assay. [48]
Dichloromethane extract of M. citrifolia dried leaves (500 g/mL) showed inhibitory effect against human epidermoid carcinoma (KB) (IC50 = 39.00 ± 6.67 µg/mL) and in a dose-dependent manner compared to damnacantal (IC50 = 6.50 ± 0.5 µg/mL) using MTT assay. [48]
Antimicrobial activity
Aqueous extract of M. citrifolia leaves (256 and 512 mg/mL) showed antibacterial effect against Aeromonas hydrophila with minimum inhibitory concentration 0.625 mg/mL using microdilution assay. [30]
Methanol extract of M. citrifolia fruit (100 mg/mL) showed strong activity against gram-negative bacteria (Salmonella paratyphi A) and moderate activity against gram-positive bacteria (Bacillus subtilis, Staphylococcus aureus, Lactobacillus lactis, Streptococcus thermophilus, Pseudomonas aeruginosa) and other gram-negative bacteria (Salmonella typhi, Escherichia coli, Vibrio harveyi, Klebsiella pneumonia, Shigella flexneri, Salmonella paratyphi A, Aeromonas hydrophilam, Vibrio cholera, Chromobacterium violaceum and Enterobacter faecalis). The ethyl acetate extract showed moderate activity against all aforementioned bacteria except for P. aeruginosa and K. pneumonia. [57]
Ripe fruit juice of M. citrifolia showed antifungal activity against Candida albicans with partial growth inhibition at a concentration of 40 mg/mL and complete growth inhibition at a concentration of 50 mg/mL after 30 min contact time [27]. The methanol and ethyl acetate extracts of M. citrifolia fruit showed strong activity against Trichophyton mentagrophytes with 79.3% and 62.06% inhibition, respectively. Methanol extract of the fruit also showed moderately strong activity (close to 50% inhibition) against Penicillium sp., Fusarium sp. and Rhizopus sp. The methanol, ethyl acetate and hexane extracts weakly inhibited Candida albicans and Aspergillus sp. [58]
Aqueous extract of M. citrifolia leaves (2, 5 and 10 mg/mL) inhibited the growth of Staphylococcus aureus with inhibition zone ranging from 16 to 20 mm compared to gentamicin or nystatine (21 mm) using disc diffusion assay. [59]
Ethanol extract of M. citrifolia leaves (2, 5 and 10 mg/mL) inhibited the growth of Escherichia coli with inhibition zone ranging from 8 to 11 mm compared to gentamicin or nystatine (20 mm) using disc diffusion assay. [59]
Water extract of M. citrifolia leaves (2, 5 and 10 mg/mL) inhibited the growth of Aspergillus niger with inhibition zone ranging from 14 to 18 mm compared to gentamicin or nystatine (21 – 22 mm) using disc diffusion assay. [59]
Chloroform extract of M. citrifolia leaves (2, 5 and 10 mg/mL) inhibited the growth of Candida albicans with inhibition zone ranging from 13 to 19 mm compared to gentamicin or nystatine (17 – 21 mm) using disc diffusion assay. [59]
Alcohol extract of M. citrifolia leaves (5 and 10 mg/mL) showed antibacterial effect against Bacillus subtilis, Escherichia coliand Staphylococcus aureus with inhibition zone ranging from 1.8 to 2.4 cm compared to tetracycline (2.1 – 2.5 cm) using agar diffusion assay. [60]
Petroleum-ether extract of M. citrifolia leaves (5 and 10 mg/mL) inhibited the growth of Candida albicans and Aspergillus niger with inhibition zone ranging from 2.0 to 2.6 cm compared to tetracycline (2.9 – 3.3 cm) using agar diffusion assay. [60]
M. citrifolia does exhibit antimicrobial activities in particular antifungal. Studies had shown that the fruit extract was active against Candida albicans and Aspergilus nidulans [61]. The fruit extract did not show antibacterial activity per se but indirectly through its immunomodulatory activity was found to enhance the blood phagocytic activity against E. coli in vitro [62]. The aqueous and ethanol extract of the fruit was active against Ascaridia galli [63].
Antiangiogenic activity
Fruit juice of M. citrifolia (5% and 10%) was found to significantly inhibit (P<0.001) the initiation of new placental vessels and reduce the growth rate and proliferation of newly developing capillary sprouts compared to control explants using an in vitro angiogenesis assay. The 10% of M. citrifolia fruit juice was able to induce vessel degeneration and apoptosis of developed capillary vessels based on MTT and TUNEL assays, respectively. It was also effective in inhibiting capillary initiating of the human breast tumor explants. [64]
The fruits and the leaves of M. citrifolia exhibit antiangiogenic activity more potent that suramin but less potent to nutmeg oil. It has been suggested that Scopoletin as the compound responsible for this activity. This effect was seen when tests in a three dimensional fibrin clot matrix model using human placental vein and human breast cancer explants. [65][66]
Cytotoxic activity
Methanol (1:6 dilution of crude extract) and ethyl acetate (1:3 dilutions) extracts of M. citrifolia fruit inhibited almost 50% of the human laryngeal epithiloma (Hep2) cells [48]. The fruit extract also inhibited retinoblastoma Y79 cell lines (IC50 800 µg/mL). [67]
Antidyslipidemic activity
Aqueous-ethanolic (70%) extract of M. citrifolia fruit administered orally (1000 mg/kg) to tyloxapol-induced male Sprague Dawley rats caused significant reduction in total cholesterol level (P< 0.01) and triglyceride level (P< 0.05). The extract also significantly (P< 0.05) reduced total cholesterol, triglyceride, low density lipoprotein-cholesterol, atherogenic index and total cholesterol-high density lipoprotein-cholesterol ratio of high fat diet-induced dyslipidemic adult Sprague Dawley rat models. [68]
The aqueous ethanol extracts of the leaves, roots and fruit exhibited significant antidyslipidaemic activity and this was thought to be mediated through the inhibition of the biosynthesis, absorption and secretion of lipids [69][70]. The seed oil also showed significant anti-dyslipidaemic activity especially in the presence of hyperlipidaemia [71].
Antidiabetic activity
Fermented fruit juice of M. citrifolia given orally to male Sprague Dawley rats (2 mL/kg) twice daily for 20 days reduced fasting blood glucose by 52.6%. [72]
Two anthroquinones, damnacanthol-3-O-beta-D-primeveroside (3), lucidin 3-O-beta-D-primeveroside, isolated from the roots of M. citrifolia methanol extract were found to exhibit hypoglycaemic effects. [73]
Hepatoprotective activity
Fruit juice of M. citrifolia (20%) given to carbon tetrachloride (CCl4)-induced female Sprague Dawley rats for 7 days showed significant reduction in hepatotoxic lesions (P< 0.001), serum alanine amino transferase (P< 0.01) and aspartate aminotransferase (P< 0.05) levels. In a correlative time-dependent study, 10% of the juice displayed delayed appearance of damaged hepatocytes with a decreased number of necrotic foci (80%) and apoptosis (50%), suggesting that the juice protects the liver from acute CCl4 exposure. [73]
M. citrifolia juice with its polyphenol contents (gentisic acid, p-hydroxybenoic acid and chlorogenic acid) were found to prevent fatty liver in hamsters fed with a high-fat diet. This is via regulations of antioxidative and anti-inflammatory responses. [74]
Pre-treatment with 20% M. citrifolia juice in drinking water was able to protect Sprague-Dawley rats from toxic effects of CCl4 suggesting that M. citrifolia juice can protect the liver from extrinsic toxin exposure. [75]
Immunomodulatory activity
M. citrifolia fruit juice and juice concentrate (1 and 5 mg/mL) stimulated cannabinoid 2 (CB2) (54-224%) but inhibited cannabinoid 1 (CB1) (14-172%) receptors in vitro. The fruit juice administered orally (100 mL/day) to male mice for 16 days decreased the production of IL-4, but increased the production of IFN-γ cytokines. [76]
M. citrifolia fruit had been found to have the ability to stimulate the immune response. This activity is centred upon the humoral immune system. It was noted that the polysaccharide-rich-substance in the fruit juice could be responsible for the activity via its ability to stimulate the release of several mediators from effector cells including TNF-alfa, IL-1beta, IL-10, IL-12 p70, IFN-gamma and NO. There is also evidence that the fruit juice modulates immune system via activation of the CB2 receptors but inhibits the CB1 receptors thus increasing the IRN-gamma and decreasing the IL-4 production. Furthermore, by increasing the percentage of granulocytes and NK cells in peripheral blood, peritoneum and spleen it is able to exercise antitumour activity. By treating dendritic cells with fermented M. citrifolia juice it was found that there is increase in the production of splenocytes especially B cells and lg class switching cluster. [77][78][79]
Anticancer activity
The fruit juice of M. citrifolia was found to exhibit antitumor activity in the Lewis lung (LLC) peritoneal carcinomatosis model. Therapeutic administration of M. citrifolia significantly enhanced the duration of survival of inbred syngeneic LLC tumor bearing mice. It did not exert significant cytotoxic effects in an adapted culture of LLC cells, LLC1, but could activate peritoneal exudates cells (PEC) to impart profound toxicity when co-cultured with the tumor cells. [80]
The roots M. citrifolia contain damnacanthal, an anthraquinone compound exhibiting selective tumor antiproliferative activity. The apoptotic activity involved sustained activation of the p38 MAPK pathway leading to the transcription of the death receptor family genes encoding DR5/TRAIL and TNF-R1/TNF-alpha genes as well as the p53-regulated Bax gene. Such activity was seen in colorectal cancer cells. [81][82][83]
The fruit has been reported to have cancer preventive properties. This was mediated through carcinogen-DNA adduct formation and the antioxidant activity of the fruit juice. The polysaccharide-rich substance (Noni-ppt) may be one of the components responsible for this activity. Noni-ppt had produced a cure rate of between 25-45 % in allogeneic mice and this activity was completely abolished by the concomitant administration of specific inhibitors of macrophages (2-chloroadenosine), T-cells (cyclosporine) or natural killer (NK) cells (anti-asialo GM1 antibody). [84][85] The methanol extract exhibited tumor cell-selective antiproliferative effects against human laryngeal carcinoma, breast cancer (MCF7) and neuroblastoma (LAN5) [86]. This anti-growth effect resulted from the induction of apoptosis confirmed by the positive results from the Terminal deoxynucleotidyl transferased dUTO Nick End Labeling (TUNEL) analysis, active caspase-3 cells in tissues, and caspase-cleaved cytokeratin 18 elevation in serum in Ehrlich ascites tumor in female Balb-c mice treated with M. citrifolia [87].
Analgesic activity
The lyophilised aqueous extract of roots of M. citrifolia was evaluated for analgesic and behavioural effects in mice. The extract did not exhibit any toxic effects but did show a significant, dose-related, central analgesic activity in the writhing and hotplate tests; this effect was confirmed by the antagonistic action of naloxone. Furthermore, administration of M. citrifolia extract at high dosages decreased all behavioural parameters in the two compartment test, the light/dark choice situation test, and the staircase test; together with the induced sleeping time, these results are suggestive of sedative properties. [88]
M. citrifolia fruit was found to reduce stress-induced impairment of cognitive function accompanied by vasculature improvement in mice. Results obtained show that the administration of M. citrifolia fruit juice protects brains from stress-induced impairment of cognitive function and that this protective effect may be related to improvement in stress-induced decreases in blood vessel density in the hippocampal dentate gyrus. [89]
Antitubercular activity
A crude ethanol extract and hexane fraction from M. citrifolia show antitubercular activity. The major constituents of the hexane fraction are E-phytol, cycloartenol, stigmasterol, beta-sitosterol, campesta-5,7,22-trien-3beta-ol and the ketosteroids stigmasta-4-en-3-one and stigmasta-4-22-dien-3-one. E-Phytol, a mixture of the two ketosteroids, and the epidioxysterol derived from campesta-5,7,22-trien-3beta-ol all show pronounced antitubercular activity. [90]
Neurological activity
The methanol extract of M. citrifolia fruit and its butanol and aqueous partitions exhibited significant affinity to the gamma-aminobutyric acid A (GABAa) inhibitory neurotransmitter receptors and showed 75% binding inhibition of the agonist radioligand [3H] muscimol at a concentration of 100 µg/ml. In the fruit is present competitive ligand(s) which may bind to the GABAa receptor as an agonist inducing its anxiolytic and sedative effects. [91]
The fruit of M. citrifolia proved to have capabilities of improving cognitive functions in beta-amyloid induced cognitive dysfunction in mice. The ethyl acetate extract showed the following positive activities such as improved both short-term and long-term memory, decreased in escape latency, increased in behavioural alteration, reduced acetylcholinesterase activity, decreased Monoamine oxidase-A levels and increased in serotonin and dopamine level. [92]
In scopolamine-induced cognitive impairment in mice, the ethanol extract of M. citrifolia and its chloroform and ethyl acetate fractions significantly improved memory and cerebral blood flow. There were also attenuation of the increased oxidative stress and AChE activity induced by scopolamine. [93]
The free intake of 10% M. citrifolia fruit juice was found to protect effects on neuronal damage after ischaemia. This effect was believed to be mediated by the suppression of ischaemic stress-induced glucose intolerance. It was also found that neurological deficit scores were decreased after reperfusion and that there was reduced infarct volume in mice pretreated with the juice. [94][95]
Methanol extract of M. citrifolia fruit was able to significantly reduce cage climbing behavior in apomorphine and methamphetamine induced stereotypy behavior in mice in a dose dependent manner. This indicates that M. citrifolia has antidopaminergic effects thus possessing anti-psychotic-like activity. [96]
Aged mice pre-treated with increasing dose of M. citrifolia fruit juice out performed young and aged control mice in forced swim test and rotarod test. This shows that the juice has ability to combat fatigue, improve endurance and increase overall physical performance. [97]
Antiosteoporotic activity
In a study on the effects of anthraquinones derived from extracts of roots of M. citrifolia, the investigators found that 1, 3, 8-trihydroxy-2-methoxy-anthraquinone (1), 2-hydroxy-1-methoxy-anthraquinone (2) and rubiadin (3) are potential inhibitors of bone resorption. The aqueous leaf extract could increase alkaline phosphates activity, and excite the formation of matrix containing mineralized nodules in human periodontal ligament cells. In other words the aqueous leaf extract promote osteogenic differentiation and matrix mineralization useful in bone and periodontal tissue regeneration. [98]
Antimelanogenesis activity
The fruit, the leaves and the seeds of M. citrifolia exhibited antimelanogenesis activity with the seed extracts being the most potent. From the seeds two lignans were isolated and proved to be the compounds responsible for this activity. 3,3’-bisdemethylpinoresinol and americanin A were found to inhibit melanogenesis by down regulation of the levels of phosphorylation of p38 mitogen-activated protein kinase, resulting in suppression of tyrosinase expression. In the leaves, 13 compounds were found to be responsible for this activity. From the fruits most of the saccharide fatty acid esters, hemiteroene glycosides and iridoid glycosides were responsible for the inhibitory effects. All these compounds do not exhibit toxicity to the cells. [99][100]
Gastrokinetic activity
The aqueous extract of the fruit of M. citrifolia and its component active principle, Scopoletin, has the ability to induce contraction of rat gastric fundus mediated through 5-HT(4) receptor. They also significantly inhibited gastric acid secretion and pepsin activity in pylorus ligated rats. At the same time they strongly increased gastrointestinal transit of charcoal meal better than cisapride. Thus, scopoletin could be utilized in treating gastro-oesophageal inflammatory diseases because of its anti-secretory and prokinetic activity including an inhibitory activity on serotonin, free radicals and cytokine-mediated inflammation. [101]
Wound healing activity
The fruit and leaves of M. citrifolia exhibited accelerated wound healing process in mice. This activity is probably due to its ligand binding to the PDGF and A(2A) receptors. [102][103][104]
Antigout activity
M. citrifolia fruit juice was found to inhibit xanthine oxidase concentration and this is the probable mechanism by which it ameliorates gout and gout-like diseases. [105]
Antihypertensive activity
The aqueous-ethanolic extract of the roots of M. citrifolia exhibited spasmolytic and vasodilator effect which were mediated through blockade of voltage dependent calcium channels and release of intracellular calcium. [106]
Toxicity
Acute toxicity
Oral single dose acute toxicty study of hot water and ethanol extracts of M. citrifolia leaves (2000 mg/kg body weight) on male and female Jcl:ICR mice (6-weeks old; 25 mice per gender) respectively showed no toxic effects on the parameters observed, including behaviours and body weights. No treatment-related differences in weight gain between extract-treated and water-treated groups. All mice were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. [35]
Oral single dose acute toxicity study of M. citrifolia dried fruit powder on female Sprague Dawley rats (aged between 8 and 12 weeks old) showed no toxic effects on the parameters observed, including behaviors, body weight, food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. LD50 value was determined as > 2000 mg/kg. [107]
In acute oral toxicity studies with rats, the juice and puree of Tahitian M. citrifolia fruit had LD50 values higher than 15,000 mg/kg body weight, [108] while the fruit concentrate had LD50 value higher than 5000 mg/kg body weight [109].
Sub-acute toxicity
Freeze-dried powder of hot water and ethanol extracts of M. citrifolia leaves (200 mg per mouse per day) respectively was mixed into the feed pellet and given to male and female Jcl:ICR mice (6-weeks old; 25 mice per gender). All mice were observed for 28 days prior to necropsy. The study showed no toxic effects on the parameters observed, including behaviours and body weights. No treatment-related differences in weight gain between extract pre-mixed feed and no extract pre-mixed feed groups. No death was found throughout the study period. Necropsy revealed no significant abnormality. [35]
Aqueous extract of M. citrifolia fruit (5.1% total solids) administered via oral gavage at a dose of 1000 mg/kg body weight to both male and female Sprague Dawley rats for 28 days showed no adverse effects on body weight, food consumption, haematological, clinical chemistry and histopathological parameters. [109]
Freeze-dried powder of aqueous extract of M. citrifolia leaves (1000 mg/kg body weight/day) was mixed into drinking water (mg of freeze-dried powder of extract/500 mL water) and given to male and female Wistar rats (7 to 8-weeks old, 120-160 body weight; five rats per gender). The study showed no significant different on the parameters observed and measured, including behaviours, body weights, organ weights and biochemical & haematological parameters, between extract pre-mixed drinking water and no extract pre-mixed drinking water groups. All mice were observed for 28 days prior to necropsy. Necropsy revealed no significant abnormality. [110]
Subchronic toxicity
Freeze-dried powder of hot water and ethanol extracts of M. citrifolia leaves (20 mg per mouse) respectively was mixed into the feed pellet and given to male and female Jcl:ICR mice (6-weeks old; 25 mice per gender) daily. All mice were observed for 90 days prior to necropsy. The study showed no toxic effects on the parameters observed, including behaviours and body weights. No treatment-related differences in weight gain between extract pre-mixed feed and no extract pre-mixed feed groups. No death was found throughout the study period. Necropsy revealed no significant abnormality. [35]
Freeze-dried powder of aqueous extract of M. citrifolia leaves (100, 300 and 1000 mg/kg body weight/day) was mixed into drinking water (mg of freeze-dried powder of extract/500 mL water) and given to male and female Wistar rats (7 to 8-weeks old, 120-160 body weight; 10 rats per gender). The study showed no significant different on the parameters observed and measured, including behaviours, body weights, organ weights and biochemical parameters, between extract pre-mixed drinking water and no extract pre-mixed drinking water groups. All mice were observed for 90 days prior to necropsy. Necropsy revealed no significant abnormality. However, the haemoglobin levels for male rats treated with 300 and 1000 mg/kg dose were significantly (p < 0.01) lower (8.5 ± 0.4 mmol/L and 8.8 ± 0.4 mmol/L) than control group (9 – 12 mmol/L). The haemoglobin levels for female rats treated with 1000 mg/kg dose were significantly (p < 0.05) lower than control group (9 – 11 mmol/L). In addition, the differential leukocyte count in lymphocytes for male rats treated with all three doses were significantly (p < 0.01) higher (80 – 90%) than control group (60 – 70%). Whilst differential leukocyte count in lymphocytes for female rats treated with 1000 mg/kg dose was significantly (p < 0.05) higher (80 – 90%) than control group (70 – 80%). For the differential leukocyte count in neutrophils, male rats treated with all three doses and showed significant (p < 0.05) lower values (10 – 20%) than control group (30 – 40%) whilst female rats treated with 1000 mg/kg dose also showed significant (p < 0.05) lower value (10 – 20%) than control group (20 – 30%). After 28 days of recovery period, the haemoglobin and differential leukocytes counts in both genders were not observed anymore.[111]
Fruit juice of Tahitian M. citrifolia fruit was administered by oral gavage at a daily dose of up to 80 mL/kg for 13-weeks to Sprague Dawley rats showed no adverse clinical signs, abnormal food consumption and weight gain, as well as no negative impact on the clinical chemistry, haematological and histopathological parameters. The No Observed Adverse Effect Level (NOAEL) was 80 mL/kg body weight/day. [112][112]
Cytotoxicity
Aqueous extract of M. citrifolia leaves (500, 1000 and 2000 mg/kg body weight/day) respectively was given orally to male and female NMRI albino mice (22 ± 2 g body weight; 5 rats per gender) for two days and sacrificed on Day 3. The mice femurs were dissected to obtain bone marrow smears. The study showed no significant difference (p > 0.05) in the ratio of polychromatic erythrocytes to normochromatic erythrocytes frequencies (1.29 – 1.40) for both gender compared to water-treated group (1.13 – 1.27). [113]
Mutagenicity
Aqueous extract of M. citrifolia leaves (500, 1000 and 2000 mg/kg body weight/day) respectively was given orally to male and female NMRI albino mice (22 ± 2 g body weight; 5 rats per gender) for two days and sacrificed on Day 3. The mice femurs were dissected to obtain bone marrow smears. The study showed no significant difference (p > 0.05) in the frequencies of micronucleated polychromatic erythrocyte (0.12 – 0.30) vs water-treated group (0.18 – 0.20). [111]
Clinical Data
Clinical findings
Safety study
A double-blind randomized study on the safety of M. citrifolia juice was conducted with 96 healthy individuals being given graduated concentration of M. citrifolia juice 4 times a day for 28 days. Hematology, biochemistry, urinalysis, vital signs and adverse events measurements were taken at 0, 2 and 4 weeks as well as during a two-week follow up (week 6). Electrocardiogram was done at 0 and 6 weeks. A reduction in adverse event report was observed in the M. citrifolia group as compared to the placebo group. [113]
Anti-inflammatory activity
In a randomized double-blind placebo controlled trial to study the anti-inflammatory activity of noni capsules in women with primary dysmenorrhea, there was no significant difference in mean bleeding score or pain score between the noni group and the control group. [114]
Antiemetic activity
A randomized double blind, placebo-controlled trial to evaluate the efficacy of M. citrifolia in prevention of postoperative nausea and vomiting in patients considered as high risk of developing PONV after various types of surgery was done. The results indicated that M. citrifolia has antiemetic activity and prophylactic used of the extract at 600 mg did effectively reduce the incidence of early postoperative nausea. [115]
Antioxidant activity
In a human study on the antioxidant activity of M. citrifolia juice in smokers demonstrated after 30 days, the target group showed a reduction in the mean superoxide anion radical and lipid hydroperoxide. [116]
Precautions
No documentation.
Pregnancy/Breast Feeding
M. citrifolia should not be used during pregnancy and lactation. [117]
Adverse reaction
Reported adverse effects of the M. citrifolia fruit juice include sedation, nausea, vomiting, anorexia, hypersensitivity, and hyperkalaemia. [117]
Interaction & Depletion
Interaction with drug
M. citrifolia juice can reduce the efficacy of warfarin [118][119]. Additive toxicity has been reported when M. citrifolia juice was combined with conventional chemotherapy such as vincristine, 5-fluorouracil and doxorubicin[120]. The ability of M. citrifolia juice to enhance the effects of insulin in animal models could be used advantageously or otherwise. Patients on antidiabetic drugs should closely monitor their blood glucose level if they choose to consume M. citrifolia juice. [121][122][123][122][123]
Contraindications
Contraindicate in people with renal disease especially those with hyperkalaemia. People with hypersensitivity to M. citrifolia should avoid using it. [117]
Case Report
A man with chronic renal insufficiency on dietary restriction of potassium developed hyperkalamaemia (5.8 mmol/l). Investigation showed that he had been taking M. citrifolia juice. Analysis of the juice showed a potassium level of 56 mmol/l. [118][121][124][125]
A 45 year old man developed very high liver transaminase activities and raised lactate dehydrogenase activity without any evidence of viral hepatitis, Epstein-Barr virus or cytomegalovirus infection, autoimmune hepatitis, Budd-Chiari syndrome, haemochromatosis or Wilson’s disease. Liver biopsy confirmed acute hepatitis and there was an inflammatory infiltrate with numerous eosinophils in the portal tract. The patient admitted to taking M. citrifolia fruit juice for three weeks. Upon cessation of the juice his transaminase activity normalized quickly and was within the reference ranges 1 month later. [125][126]
38 woman developed acute liver injury associated with M. citrifolia juice consumption on a long-term (9 months) anticonvulsant therapy. Liver biopsy was consistent with severe, predominantly hepatocellular type of injury. Upon cessation of both medication and provide with corticosteroid therapy, the patient recovered fully in 5 months. [127]
A 14 year old previously health boy developed acute hepatotoxicity after consuming M. citrifolia juice. [128]A 29 year old man with previous toxic hepatitis associated with small doses of paracetamol developed sub-acute hepatic failure following consumption of 1.5 L M. citrifolia juice over 3 weeks. He had to undergo liver transplantation. [129]
A 62 year old woman without evidence of previous liver disease developed an episode of self-limiting acute hepatitis following consumption of 2 L M. citrifolia juice for over 3 months. [129]
A 24 year old female patient developed fulminant hepatitis and impending liver failure with no evidence of apparent usual causes of hepatotoxicity. A fine needle liver biopsy ruled out an autoimmune hepatitis but showed signs indicating drug-induced toxicity. She admitted to taking M. citrifolia juice for 4 weeks prior to the development of the condition. After cessation of the M. citrifolia juice her liver enzymes returned to normal within a month. [130]
Poisonous Management
No documentation.
Line Drawing
References
- The Plant List. Ver1.1. Morinda citrifolia L. [homepage on the Internet]. c2013 [updated 2012 Mar 23; cited 2017 July 17]. Available from: http://www.theplantlist.org/tpl1.1/record/kew-129789.
- Quattrocchi U. CRC world dictionary of medicinal and poisonous plants: Common names, scientific names, eponyms, synonyms, and etymology. Volume IV M-Q. Boca Raton, Florida: CRC Press, 2012; p. 195-196.
- Species in GBIF (Global Biodiversity Information Facility) Backbone Taxonomy. Morinda citrifolia L. [homepage on the Internet]. c2015 [updated 2015 Mar 2; cited 2017 July 17] Available from: http://www.gbif.org/species/5339879/vernaculars.
- JJ Groenendijk. Morinda citrifolia L. In: Lemmens RHMJ, Wulijarni-Soetjipto N, editors. Plant Resources of South-East Asia No. 3: Dye and tannin-producing plants. Pudoc, Netherlands: Wageningen, 1991; p. 94-96.
- Porcher Michel H. Sorting Morinda names: Multilingual Multiscript Plant Name Database (M.M.P.N.D) – a work in progress (1995-2020). School of Agriculture and Food Systems. Faculty of Land & Food Resources. The University of Melbourne, Australia. 2000.
- Flora of China. Morinda citrifolia. [homepage on the Internet]. No date [cited 2017 July 17]. Available from: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200022155.
- Flowers of India. Morinda citrifolia Linn. [homeoage on the Internet]. No date [cited 20 May 2016]. Available from: http://www.flowersofindia.net/catalog/slides/Great%20Morinda.html.
- Thomas ANS. Tanaman obat tradisional, volume 1. Kanisius; 1989, p. 62-64.
- Burkill IH. A dictionary of the economic products of the Malay Peninsula. Volume 2. London: Published on behalf of the governments of the Straits settlements and Federated Malay states by the Crown agents for the colonies, 1935; p. 1493-1494.
- Morton JF. The ocean-going noni, or Indian mulberry (Morinda citrifolia, Rubiaceae) and some of its ‘‘colourful’’ relatives. Ecological Botany. 1992;46:241–256.
- Dittmar A. Morinda citrifolia L. Use in indigenous Samoan medicine. Journal of Herbs, Spices and Medicine Plants. 1993;1:77-92.
- Ghawas MM. Mengkudu (Morinda citrifolia Linn.). In: Penanaman tumbuhan ubatan & beraroma. Musa Y, Ghawas MM, Mansor P, editors. Serdang: MARDI, 2005; p. 124-129.
- Samoylenko V, Zhao J, Dunbar DC, Khan IA, Rushing JW, Muhammad I. New constituents from noni (Morinda citrifolia) fruit juice. J Agric Food Chem. 2006;54(17):6398-6402.
- Kim HK, Kwon MK, Kim JN, et al. Identification of novel fatty acid glucosides from the tropical fruit Morinda citrifolia L. Phytochemistry Letters. 2010;3(4):238-241.
- Wang M, Kikuzaki H, Csiszar K, et al. Novel trisaccharide fatty acid ester identified from the fruits of Morinda citrifolia (noni). J Agric Food Chem. 1999;47(12):4880-4882.
- Bui AKT, Bacic A, Pettolino F. Polysaccharide composition of the fruit juice of Morinda citrifolia (noni). Phytochemistry. 2006;67(12):1271-1275.
- Lin CF, Ni CL, Huang YL, Sheu SJ, Chen CC. Lignans and anthraquinones from the fruits of Morinda citrifolia. Nat Prod Res. 2007;21(13):1199-1204.
- Akihisa T, Matsumoto K, Tokuda H, et al. Anti-inflammatory and potential cancer chemopreventive constituents of the fruits of Morinda citrifolia (noni). J Nat Prod. 2007;70(5):754-757.
- Siddiqui BS, Sattar FA, Ahmad F, Begum S. Isolation and structural elucidation of chemical constituents from the fruits of Morinda citrifolia Linn. Archives of Pharmacal Research (Seoul). 2007;30(8):919-923.
- Deng S, West BJ, Jensen CJ, Basar S, Westendorf J. Development and validation of an RP-HPLC method for the analysis of anthraquinones in noni fruits and leaves. Food Chem. 2009;116(2):505-508.
- Deng S, West BJ. Antidepressant effects of noni fruit and its active principals. Asian J Med Sci. 2011;3(2):79-83.
- Su BN, Pawlus AD, Jung HA, Keller WJ, McLaughlin JL, Kinghorn AD. Chemical constituents of the fruits of Morinda citrifolia (Noni) and their antioxidant activity. J Nat Prod. 2005;68(4):592-595.
- Chan-Blanco Y, Vaillant F, Mercedes Perez A, Reynes M, Brillouet JM, Brat P. The noni fruit (Morinda citrifolia L.): A review of agricultural research, nutritional and therapeutic properties. J Food Compos Anal. 2006;19(6–7):645-654.
- Heinicke R. The pharmacologically active ingredients of noni. Bulletin of the National Tropical Botanical Garden. 1985;15:10-14.
- Wang M, Kikuzaki H, Jin Y, et al. Novel glycosides from noni (Morinda citrifolia). J Nat Prod. 2000;63(8):1182-1183.
- Dalsgaard PW, Potterat O, Dieterle F, Paululat T, Kuhn T, Hamburger M. Noniosides E – H, new trisaccharide fatty acid esters from the fruit of Morinda citrifolia (noni). Planta Medica. 2006;72(14):1322-1327.
- Farine JP, Legal L, Moreteau B, Le Quere JL. Volatile components of ripe fruits of Morinda citrifolia and their effects on Drosophila. Phytochemistry. 1996;41(2):433-438.
- Wei GJ, Ho CT, Huang AS. Analysis of volatile compounds in noni fruit (Morinda citrifolia L.) juice by steam distillation-extraction and solid phase microextraction coupled with GC/AED and GC/MS. J Food Drug Anal. 2011;19(1):33-39.
- Basar S, Westendorf J. Identification of (2E, 4Z, 7Z)- decatrienoic acid in noni fruit and its use in quality screening of commercial noni products. Food Analytical Methods. 2011;4:57-65.
- Serafini MR, Santos RC, Guimaraes AG, et al. Morinda citrifolia Linn extract possesses antioxidant activities and reduces nociceptive behavior and leukocyte migration. J Med Food. 2011;14(10):1159-1166.
- Saraphanchotiwitthaya A, Sripalakit P. Anti-inflammatory effect of Morinda citrifolia leaf extract on macrophage RAW 264.7 cells. Science Asia. 2015;41:5-11.
- Saidin SH, Yusuf UK, Rahmat A, Sukari AM. Determination of flavonoid components from Morinda citrifolia (Mengkudu) and their antioxidant activity. Pertanika J Trop Agric Sci. 2005;28(2):111-119.
- Takashima J, Ikeda Y, Komiyama K, Hayashi M, Kishida A, Ohsaki A. New constituents from the leaves of Morinda citrifolia. Chem Pharm Bull. 2007;55(2):343-345.
- Kochuthressia KP, Jaseentha MO. Phytochemical investigation of active compound in Morinda citrifolia leaves. Asian J Biochem Pharm Res. 2015;4(5):98-103.
- West BR, Tani H, Palu AK, Tolson CB, Jensen CK. Safety tests and antinutrient analyses of noni (Morinda citrifolia L.) leaf. J Sci Food Agric. 2007;87:2583-2588.
- Deng S, West BJ, Jensen J. Simultaneous characterization and quantitation of flavonol glycosides and aglycones in noni leaves using a validated HPLC-UV/MS method. Food Chem. 2008;111(2):526-529.
- Sang S, Cheng X, Zhu N, et al. Iridoid glycosides from the leaves of Morinda citrifolia. J Nat Prod. 2001; 64(6):799-800.
- Sang S, Cheng X, Zhu N, et al. Flavonol glycosides and novel iridoid glycoside from the leaves of Morinda citrifolia. J Agric Food Chem. 2001; 49(9):4479-4481.
- Rivera A, Cedillo L, Hernandez F, Castillo V, Sanchez A, Castaneda D. Bioactive constituents in ethanolic extract leaves and fruit juice of Morinda citrifolia. Ann Biol Res. 2012;3(2):1044-1049.
- Ahmad VU, Bano S. Isolation of b-sitosterol and ursolic acid from Morinda citrifolia Linn. J Chem Soc Pak. 1980;2(2):71.
- Dixon AR, Heather M, Etkin LN. Ferment this: The transformation of noni, a traditional Polynesian medicine (Morinda citrifolia, Rubiaceae). Economic Botany. 1999;53(1):51-68.
- Basar S, Uhlenhut K, Högger P, Schöne F, Westendorf J. Analgesic and antiinflammatory activity of Morinda citrifolia L. (Noni) fruit. Phytother Res. 2010;24(1):38-42.
- Song HS, Park SH, Ko MS, Jeong JM, Sohn UD, Sim SS. Morinda citrifolia inhibits both cytosolic Ca-dependent Phospholipase A(2) and Secretory Ca-dependent Phospholipase A(2). Korean J Physiol Pharmacol. 2010;14(3):163-167.
- Serafini MR, Santos RC, Guimarães AG, et al. Morinda citrifolia Linn leaf extract possesses antioxidant activities and educes nociceptive behaviour and leukocyte migration. J Med Food. 2011;14(10):1159-1166.
- Dussosoy E, Brat P, Bony E, et al. Characterization, anti-oxidative and anti-inflammatory effects of Costa Rican noni Juice (Morinda citrifolia L.) J Ethnopharmacol. 2011;133(1):108-115.
- Okusada K, Nakamoto K, Nishida M, et al. The antinociceptive and anti-inflammatory action of the CHCl3-soluble phase and its main active component, damnacanthal, isolated from the root of Morinda citrifolia. Biol Pharm Bull. 2011;34(1):103-107.
- Nualsanit T, Rojanapanthu P, Gritsanapan W, Kwankitpraniti T, Min KW, Baek SJ. Damnacanthal-induced anti-inflammation is associated with inhibition of NF-kB activity. Inflamm Allergy Drug Targets. 2011;10(6):455-463.
- Thani W, Vallisuta O, Siripong P, Ruangwises N. Anti-proliferative and antioxidative activities of Thai noni/Yor (Morinda citrifolia L.) leaf extract. Southeast Asian J Trop Med Public Health. 2010;41(2):482-489.
- Zin ZM, Abdul-Hamid A, Osman A. Antioxidative activity of extracts from Mengkudu (Morinda citrifolia L.) root, fruit and leaf. Food Chem. 2002;78(2):227-231.
- Kamiya K, Tanaka Y, Endang H, Umar M, Satake T. Chemical constituents of Morinda citrifolia fruits inhibit copper-induced low-density lipoprotein oxidation. J Agric Food Chem. 2004;52(19):5843-5848.
- Pawlus AD, Su BN, Keller WJ, Kinghorn AD. An anthraquinone with potent quinine reductase-inducing activity and other constituents of the fruits of Morinda citrifolia (noni). J Nat Prod. 2005;68(12):1720-1722.
- Kamiya K, Tanaka Y, Endang H, Umar M, Satake T. New anthraquinone and iridoid from the fruits of Morinda citrifolia. Chem Pharm Bull (Tokyo). 2005;53(12):1597-1599.
- Jagetia GC, baliga MS. The evaluation of nitric oxide scavenging activity of certain Indian medicinal plants in vitro: A preliminary study. J Med Food 2004;7(3):343-348.
- Basu S, Hazra B. Evaluation of nitric oxide scavenging activity, in vitro and ex vivo, of selected medicinal plants traditionally used in inflammatory diseases. Phytother Res. 2006;20(10):896-900.
- Masuda M, Murata K, Fukuhama A, et al. Inhibitory effects of constituents of Morinda citrifolia seeds on elastase and tyrosinase. J Nat Med. 2009;63(3):267-273.
- Berg JT, Furusawa E. Failure of juice or juice extract from the noni plant (Morinda citrifolia) to protect rats against oxygen toxicity. Hawaii Med J. 2007;66(2):41-44.
- Jayaraman SK, Manoharan MS, Illanchezian S. Antibacterial, antifungal and tumor cell suppression potential of Morinda citrifolia fruit extracts. Int J Integr Biol. 2008;3(1):44-49.
- Jainkittivong A, Butsarakamruha T, Langlais RP. Antifungal activity of Morinda citrifolia fruit extract against Candida albicans. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2009;108(3):394-398.
- Usha R, Sangeetha S, Palaniswamy M. Antimicrobial activity of a rarely known species, Morinda citrifolia L. Ethnobotanical Leaflets. 2010;14:306-311.
- Kumar KT, Panda DS, Nanda UN, Khuntia S. Evaluation of antibacterial, antifungal and anthelmintic activity of Morinda citrifolia L. (noni). International Journal of PharmaTech Research. 2010;2(2):1030-1032.
- Baneries S, Johnson AD, Csiszar K, Wansley DL, McGeady P. An extract of Morinda citrifolia interferes with the serum-induced formation of filamentous structures in Candida albicans and inhibits germination of Aspergillus nidulans. Am J Chin Med. 2006;34(3):503-509.
- Schäfer M, Sharp P, Brooks VJ, et al. Enhanced bactericidal activity against Escherichia coli in calves fed Morinda citrifolia (Noni) puree. J Vet Intern Med. 2008;22(2):499-502.
- Brito DR, Fernandes RM, Fernandes MZ, Ferreira MD, Rolim FR, da Silva Filho ML. [Anthelmintic activity of aqueous and ethanolic extracts of Morinda citrifolia fruit on Ascaridia galli]. Rev Bras Parasitol Vet. 2009;18(4):32-36.
- Hornick CA, Myers A, Sadowska-Krowicka H, Anthony CT, Woltering EA. Inhibition of angiogenic initiation and disruption of newly established human vascular networks by juice from Morinda citrifolia (noni). Angiogenesis. 2003;6(2):143-149.
- Piaru SP, Mahmud R, Abdul Majid AM, Mahmoud Nassar ZD. Antioxidant and antiangiogenic activities of the essential oils of Myristica fragrans and Moridna citrifolia. Asian Pac J Trop Med. 2012;5(4):294-298.
- Beh HK, Seow LJ, Asmawi MZ, et al. Anti-angiogenic activity of Morinda citrifolia extracts and its chemical constituents. Nat Prod Res. 2012;26(16):1492-1497.
- Gangadharan S, Thiyagarajan SL, Subramanian K, Narayanasamy M. Induction of caspase-3 dependdent apoptosis in Y79 cells by fruit extracts of Morinda citrifolia. J Biotechnol. 2008;136:S75-S98.
- Mandukhail SU, Aziz N, Gilani AH. Studies on antidyslipidemic effects of Morinda citrifolia (noni) fruit, leaves and root extracts. Lipids in Health and Disease. 2010;9(1):88-93.
- Lin YL, Chou CH, Yang DJ, Chen JW, Tzang BS, Chen YC. Hypolipidemic and antioxidative effects of noni (Morinda citrifolia L.) juice on high-fat/cholesterol-dietary hamsters. Plant Foods Hum Nutr. 2012;67(3):294-302.
- Wang MY, Peng L, Weidenbacher-Hoper V, Deng S, Anderson G, West BJ. Noni juice improves serum lidid profiles and other risk markers in cigarette smokers. ScientificWorldJournal. 2012;2012:594657.
- Pazoc DC, Jiménez FE, Garduño L, López VE, Cruz MC. Hypolipidemic effect of seed oil of noni (Morinda citrifolia). Nat prod Commun. 2011;6(7):1005-1008.
- Wang MY, Nowicki D, Anderson G, Jensen J, West B. Liver protective effects of Morinda citrifolia (noni). Plant Foods for Human Nutrition. 2008;63(2):59-63.
- Kamiya K, Hamabe W, Harada S, Murakami R, Tokuyama S, Satake T. Chemical constituents of Morinda citrifolia roots exhibit hypoglycemic effects in streptozotocin-induced diabetic mice. Bio Pharm Bull. 2008;31(5):935-938.
- Lin YL, Chang YY, Yang DJ, Tzang BS, Chen YC. Beneficial effects of noni (Morinda citrifolia L.) juice on livers of high-fat dietary hamsters. Food Chem. 2013;140(1-2):31-38.
- Wang MY, Anderson G, Nowicki D, Jensen J. Hepatic protection by noni fruit juice against CCl(4)-induced chronic liver damage in female SD rats. Plant Foods Hum Nutr. 2008;63(3):141-145.
- Palu AK, Kim AH, West BJ, Deng S, Jensen J, White L. The effects of Morinda citrifolia L. (noni) on the immune system: Its molecular mechanisms of action. J Ethnopharmacol. 2008;115:502-506.
- Nayak S, Mengi S. Immunostimulant activity of noni (Morinda citrifolia) on T and B lymphocytes. Pharma Biol. 2010;48(7):724-731.
- Li J, Stickel SL, Bouton-Verville H, et al. Fermented Noni exudates (fNE): A mediator between immune system and anti-tumor activity. Oncol Rep. 2008;20(6):1505-1509.
- Zhang X, Li J, Wong DK, Wagner TE, Wei Y. Fermented Noni Exudate-treated dendritic cells directly stimulate B lymphocyte proliferation and differentiation. Oncol Rep. 2009;21(5):1147-1152.
- Hirazumi A, Furusawa E. An immunomodulatory polysaccharide-rich substance from the fruit juice of Morinda citrifolia (noni) with antitumour activity. Phytother Res. 1999;13(5):380-387.
- Hiwasaa T, Araseb Y, Chena Z, et al. Stimulation of ultraviolet-induced apoptosis of human fibroblast UVr -1 cells by tyrosine kinase inhibitors. FEBS Lett. 1999;444(2-3):173-176.
- Lin FL, Hs JL, Chou CH, Wu WJ, Chang CI, Liu HJ. Activation of p38 MAPK by damancanthal mediates apoptosis in SKHep 1 cells through the DR5/TRAIL and TNFR1/TNF-alpha and p53 pathways. Eur J Pharmacol. 2011;650(1):120-129.
- Nualsanit T, Rojanapanthu P, Gritsanapan W, Lee SH, Lawson D, Baek SJ. Damancanthal, a noni component, exhibit antitumorigenic activity in human colorectal cancer cells. J Nutr Biochem. 2012;23(8):915-923.
- Wang MY, Su C. Cancer preventive effect of Morinda citrifolia (Noni). Ann N Y Aced Sci. 2001;952:161-168.
- Furusawa E, Hirazumi A, Story S, Jensen J. Antitumour potential of a polysaccharide-rich substance from the fruit juice of Morinda citrifolia (Noni) on sarcoma 180 ascites tumour in mice. Phytother Res. 2003;17(10):1158-1164.
- Arpornsuwan T, Punjanon T. Tumor cell-selective antiproliferative effect of the extract from Morinda citrifolia fruits. Pythother Res. 2006;20(6):515-517.
- Taskin EI, Akgün-Dar K, Kapucu A, et al. Apoptosis-inducing effects of Morinda citrifolia L. and doxorubicin on the Ehrlich ascites tumor in Balb-c mice. Cell Biochem Funct. 2009;27(8):542-546.
- Younos C, Rolland A, Fleurentin J, Lanthers MC, Misslin R, Mortier F. Analgesic and behavioural effects of Morinda citrifolia. Planta Med. 1990;56(5):430-434.
- Muto J, Hosung L, Uwaya A, Isami F, Ohno M, Mikami T. Morinda citrifolia fruit reduces stress-induced impairment of cognitive function accompanied by vasculature improvement in mice. Physiol Behav. 2010;101(2):211-217.
- Saludes JP, garson MJ, Franzblau SG, Aguinaldo AM. Antitubercular constituents from the hexane fraction of Morinda citrifolia Linn. (Rubiaceae). Phytother Res. 2002;16(7):683-685.
- Deng S, West BJ, Palu AK, Zhou BN, Jensen CJ. Noni as an anxiolytic and sedative: A mechanism involving its gamma-aminobutyric aciderfic effects. Phytomedicine. 2007;14(7-8):517-522.
- Muralidharan P, Kumar VR, Balamurugan G. Protective effect of Morinda citrifolia fruits on beta-amyloid (25-35) inducedcognitive dysfunction in mice: An experimental and biochemical study. Phytother Res. 2010;24(2):252-258.
- Pachauri SD, Tota S, Khandelwal K, et al. Protective effect of fruits of Morinda citrifolia L. on scopolamine induced memory impairment in mice: A behavioural, biochemical and cerebral blood flow study. J Ethnopharmacol. 2012;139(1):34-41.
- Harada S, Hamabe W, Kamiya K, Satake T, Yamamoto J, Tokuyama S. Preventive effect of Morinda citrifolia fruit juice on neuronal damage induced by focal ischemia. Biol Pharm Bull. 2009;32(3):405-409.
- Harada S, Fujita-Hamabe W, Kamiya K, Muzishina Y, Satake T, Tokuyama S. Morinda citrifolia fruit juice prevents ischemia neuronal damage through suppression of the development of post-ischemic glucose intolerance. J Nat Med. 2010;64(4):468-473.
- Pandy V, Narasingam M, Mohamed Z. Antipsychotic-like activity of Noni (Morinda citrifolia Linn.) in mice. BMC Complement Altern Med. 2012;12:186.
- Ma DL, West BJ, Su CX, Gao JH, Liu TZ, Liu YW. Evaluation of the ergogenic potential of noni juice. Phytother Res. 2007;21(11):1100-1101.
- Boonanantanasarn K, Janebodin K, Suppakpatana P, et al. Morinda citrifolia leaves enhance osteogenic differentiation and mineralization of human periodontal ligament cells. Dent Mater J. 2012;3(5):863-871.
- Akihisa T, Tochizawa S, Takahashi N, et al. Melanogenesis-inhibitory saccharide fatty acid esters and other constituents of the fruits of Morinda citrifolia (noni). Chem Biodivers. 2012;9(6):1172-1187.
- Masuda M, Itoh K, Murata K, et al. Inhibitory effects of Moridna citrifolia extract and its constituents on melanogenesis in murine B16 melanoma cells. Biol Pharm Bull. 2012;35(1):78-83.
- Mahattanadul S, Ridtitid W, Nima S, Phdoongsombut N, Ratanasuwon P, Kasiwong S. Effects of Morinda citrifolia aqueous fruit extract and its biomarker scopoletin on reflux esophagitis and gastric ulcer rats. J Ethnopharmacol. 2011;134(2):243-250.
- Nayak BS, Isitor GN, Maxwell A, Bhogadi V, Ramdath DD. Wound-healing activity of Morinda citrifolia fruit juice on diabetes-induced rats. J Wound Care. 2007;16(2):83-86.
- Nayak BS, Sandiford S, Maxwell A. Evaluation of the wound-healing activity of ethanolic extract of Morinda citrifolia L. leaf. Evid Based Complement Alternat Med. 2009;6(3):351-356.
- Palu A, Su C, Zhou BN, West B, Jensen J. Wound healing effects of noni (Morinda citrifolia L.) levaes: A mechanism involving its PDGF/A2A receptor ligand binding and promotion of wound closure. Pythother Res. 2010;24(10):1437-1441.
- Palu A, Deng S, West B, Jensen J. Xanthine oxidase inhibiting effects of noni (Morinda citrifolia) fruit juice. Phytother Res. 2009;23(12):1790-1791.
- Gilani AH, Mandukhail SU, Iqbal J, et al. Antispasmodic and vasodilator activities of Morinda citrifolia root extract are mediated through blockade of voltage dependent calcium channels. BMC Complement Altern Med. 2010;10:2.
- Teh BP, Hamzah NF, Rosli SNS, Yahaya MAF, Zakiah I, Murizal Z. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2012. Report No.: HMRC 11-045/01/MC/F/K.
- Scientific Committee on Food. Opinion of the Scientific Committee on Food on Tahitian Noni® Juice. European Commission. Health & Consumer Protection Directorate-General. 11 December 2002.
- Nima S, Kasiwong S, Ridtitid W, Thaenmanee N, Mahattanadul S. Gastrokinetic activity of Morinda citrifolia aqueous fruit extract and its possible mechanism of action in human and rat models. J Ethnopharmacol. 2012;142:354-361.
- West BJ, White LD, Jensen CJ, Palu AK. A double-blind clinical safety study of noni fruit juice. Pac Health Dialog. 2009;15(2):21-32.
- Lagarto A, Bueno V, Merino N, Vega Y. Safety evaluation of Morinda citrifolia (noni) leaves extract: Assessment of genotoxicity, oral short term and subchronic toxicity. J Intercult Ethnopharmacol. 2013;2(1):15-20.
- Fletcher HM, Dawkins J, Rattray C, Wharfe G, Reid M, Gordon-Strachan G. Morinda citrifolia (Noni) as an anti-inflammatory treatment in women with primary dysmenorrhoea: A randomised double-blind placebo-controlled trial. Obstet Gynecol Int. 2013;2013:195454.
- West BJ, White LD, Jensen CJ, Palu AK. A double-blind clinical safety study of noni fruit juice. Pac Health Dialog. 2009;15(2):21-32.
- Flether HM, Dawkins J, Rattray C, Wharfe G, Reid M, Gordon-Strachan G. Morinda citrifolia (Noni) as an anti-inflammatory treatment in women with primary dysmenorrhoea: A randomized double-blind placebo-controlled trial. Obstet Gynecol Int. 2013;(2013):195454.
- Prapaitrakool S, Itharat A. Morinda citrifolia Linn. for prevention of postoperative nausea and vomiting. J Med Assoc Thai. 2010;93 Suppl 7:S204-S209.
- Wang MY, Lutfiyya MN, Weidenbacher-Hoper V, Anderson G, Su CX, West BJ. Antioxidant activity of noni juice in heavy smokers. Chem Cent J. 2009;3:13.
- Koh HL. A guide to medicinal plants: An illustrated, scientific and medicinal approach. Singapore: World Scientific Publications; 2009, p. 103-104.
- Carr ME, Klotz J, Bergeron M. Coumarin resistance and the vitamin supplement “Noni”. Am J Hematol. 2004;77(1):103.
- Sarubin-Fragakis A, Thomson C. The health professional’s guide to popular dietary supplements. American Dietetic Associati; 2007, p. 388-390.
- Cassileth BR, Yeung KS, Gubili J. Herb-drug interactions in oncology. Shelton, USA: PMPH-USA; 2010, p. 488.
- Lee SY, Park SL, Hwang JT, Yi SH, Nam YD, Lim SI. Antidiabetic effect of Morinda citrifolia (Noni) fermented by Cheonggukjang in KK-A(y) diabetic mice. Evid Based Complement Alternat Med. 2012;2012:163280.
- Nayak BS, Marshall JR, Isitor G, Adogwa A. Hypoglycemic and hepatoprotective activity of fermented fuir juice of Morinda citrifolia (Noni) in diabetic rats. Evid Based Complement Alternat Med. 2011;(2011):875293.
- Nerurkar PV, Nishioka A, Eck PO, Johns LM, Volper E, Nerurkar VR. Regulation of glucose metabolism via hepatic forkhead transcription factor 1 (FoxO1) by Morinda citrifolia (noni) in high-fat diet-induced obese mice. Br J Nutr. 2012;108(2):218-228.
- Meller BA, Scott MK, Sowinski KM, Prag KA. Noni juice (Morinda citrifolia): A hidden potential for hyperkalemia? Am J Kidney Dis. 2000;35(2):310-312.
- Aronson JK. Meyler’s side effects of herbal medicines. Amsterdam: Elsevier BV; 2009, p. 203-204.
- Milloniq G, Stadlmann S, Vogel W. Herbal hepatotoxicity: Acute hepatitis caused by a Noni preparation (Morinda citrifolia). Eur J Gastroenterol Hepatol. 2005;17(4):445-447.
- Mrzljak A, Kosuta I, Skrtic A, Kanizai TF, Vrhovac R. Drug-indiced liver injury associated with Noni (Morinda citrifolia) juice and Phenobarbital. Case Rep Gastroenterol. 2013;7(1):19-24.
- Yu EL, Sivagnanam M, Ellis L, Huang JS. Acite hepatotoxicity ad=fter ingestion of Moridna citrifolia (Noni Berry) juice in a 14-year-old boy. J Pediatr Gastroenterol Nutr. 2011;52(2):222-224.
- Stadbauer V, Fickert P, Lackner C, et al. Hepatotoxicity of NONI juice: Report of two cases. World J Gastroenterol. 2005;11(30):4758-4760.
- Yuce B, Gulberg V, Diebold J, Gerbes AL. Hepatitis induced by Noni juice from Morinda citrifolia: A rare cause of hepatotoxicity or the tip of the iceberg? Digestion. 2006;73(2-3):167-170.


