Mengkudu Fruit
Morinda citrifolia L.
Rubiaceae
DEFINITION
Mengkudu fruit consists of dried partially ripe fruits of M. citrifolia L. (Rubiaceae) [ 1 ].
SYNONYM
Morinda bracteata Roxb [ 2 ].
VERNACULAR NAMES
Mengkudu, mengkudu besar (Malay); hai ba ji, wu ning luo ling kuo (Chinese); nunaakai, nunavu(Tamil); Indian mulberry (English) [ 2 , 3 , 4 , 5 ].
CHARACTER
The fruit is yellow to green in colour with bitter taste. The ripe fruit has a strong butyric acid-like rancid smell [ 6 ].
IDENTIFICATION
Plant Morphology
M. citrifolia grows in shady forests and on open rocky or sandy shores; it is a shrub or small-medium sized tree (3-10 m) with quadrangular or somewhat rounded branches and evergreen. The leaves are large, simple, alternate, broadly elliptic to oblong shape (10-30 x 5-15 cm), dark green, glossy, wavy and prominently-veined. The 5-lobed white tubular-like fragrant flower is small (1.25 cm long) and borne in a globose head (2.5 cm across). The heads develop into compound fruits composed of many small drupes;the fruit is ovoid, ellipsoid or roundish (3-10 x 3-6 cm) with an embossed appearance, slightly wrinkly, waxy, semi-translucent skin, and turns from green to yellow and to almost white as it ripens; the fruit surface is faintly patterned with 4- to 6-sided outlines, each with a central “eye”; the pulp is fleshy and juicy, dull-yellow or yellowish-white and gelatinous when the fruit is ripe; it has numerous hard oblong-triangular reddish-brown pits, each containing 4 seeds about 3.5 mm long [ 6 , 7 ].
Microscopy
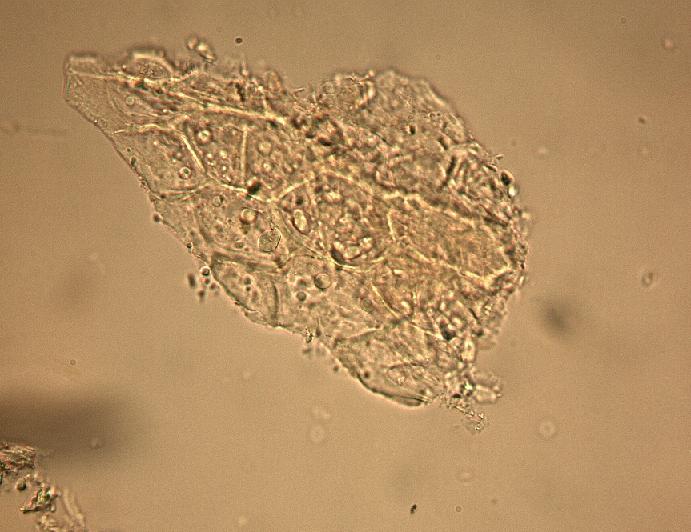
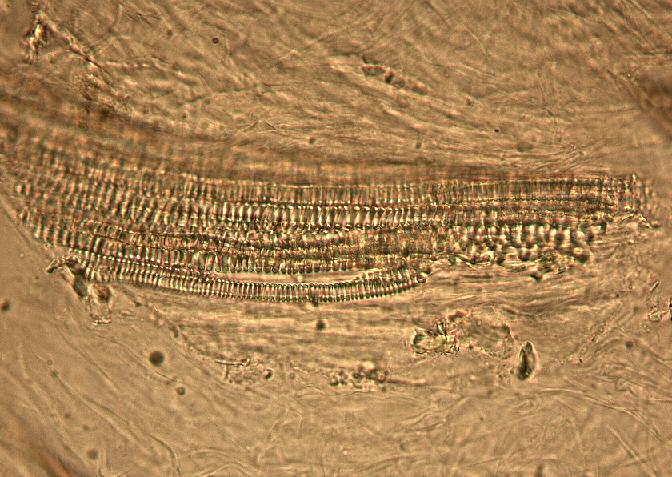
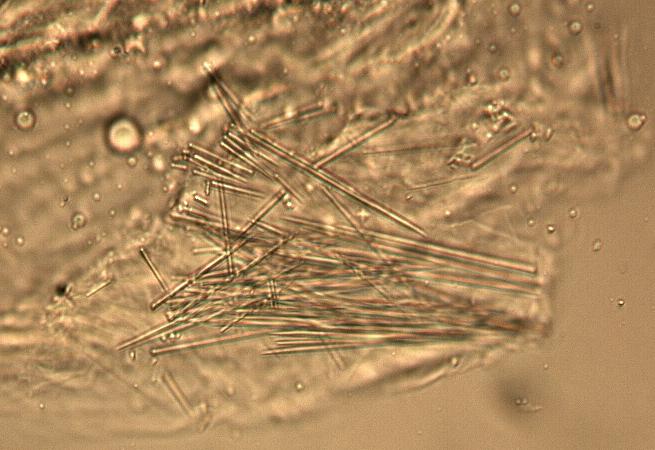
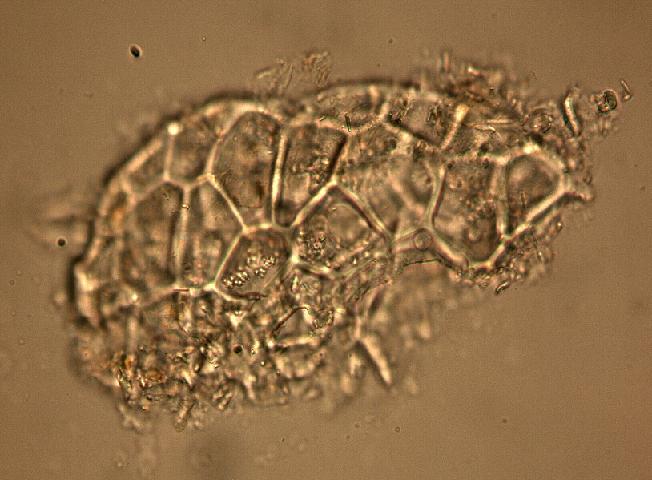
The fruit powder contains thin-walled parenchyma cells, exocarp and sparsely distributed thin strands of vascular tissue containing tracheary elements with annular, spiral and rarely, pitted secondary walls; acicular calcium oxalate crystals, starch granules and oil globules [ 1 , 8 ].
Observed colour of solution after treatment with various reagents : Colour Tests
| H2SO4 (conc.) | Brown to dark brown |
| 5% NaOH | Light brown to brown |
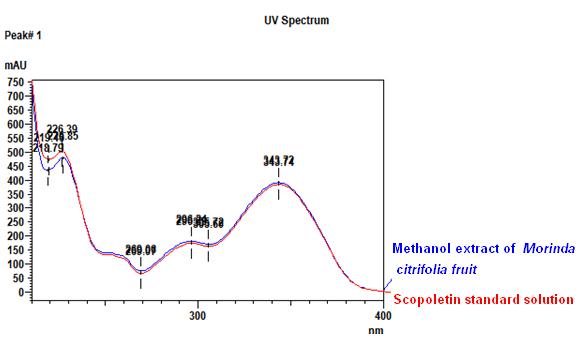
Thin Layer Chromatography (TLC)
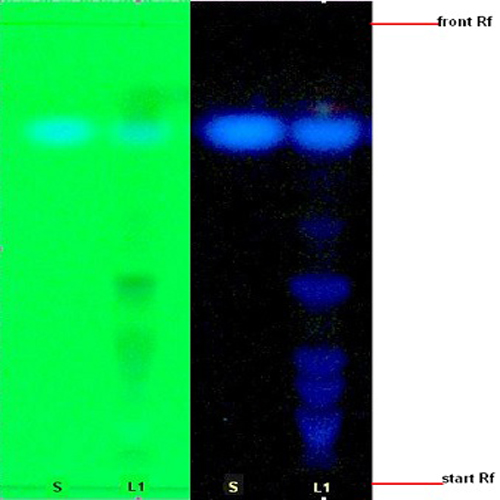
Figure 3 : TLC profiles of scopoletin (S) and ethanol extracts of M. citrifolia fruits (L) observed under (a) UV at 254 nm and (b) UV at 366 nm.
| Test Solutions | Weigh about 1.0 g of M. citrifolia dried fruit powder in a 50 mL screw-capped conical flask and add 10 mL of ethanol into the flask. Shake the mixture for 15 min. Filter the mixture and evaporate the filtrate on a water bath to dryness. Reconstitute the residue with 0.5 mL of methanol. |
| Standard solution | Dissolve 2.0 mg of scopoletin standard in 20 mL of methanol to give 100 µg/mL solution. |
| Stationary Phase | HPTLC Silica gel 60 F254, 5 x 10 cm. |
| Mobile phase |
n-Butanol-acetic acid-water, 5:1:4 (v/v) Preparation of butanol-acetic acid-water, 5:1:4 (v/v) Saturate butanol with water at the ratio of 1:1 (v/v), in a separation funnel for 12 hr. Collect the upper organic layer and saturate it with acetic acid and water at a ratio of 5:1:4 (v/v) in a separation funnel for 12 hr. Collect the upper organic layer and use as the mobile phase. |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
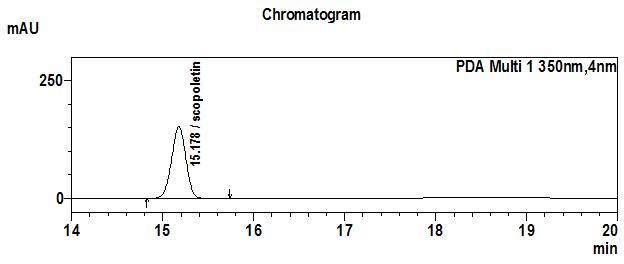
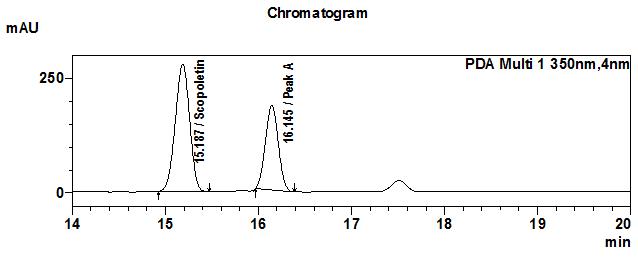
High Performance Liquid Chromatography (HPLC)
| Test solution | Extract about 1.0 g of M. citrifolia dried fruit powder in a 50 mL screw-capped conical flask and add 4 mL of methanol. Sonicate in a water bath of 50°C for 60 min. Cool the mixture and allow the insoluble matter to settle. Filter 3 mL of the upper solution through a 0.45 µm syringe filter and inject the filtrate into the HPLC column. | ||||||||||||||||||
| Standard solution | Dissolve 2.0 mg of scopoletin standard with 50 mL of methanol to produce 40 µg/mL solution. | ||||||||||||||||||
| Chromatographic system |
Detector: UV 350 nm |
||||||||||||||||||
| Mobile Phase (gradient mode) |
|
||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the scopoletin standard solution (40 µg/mL). The requirements of the system suitability parameters are as follow:
|
||||||||||||||||||
| Acceptance criteria |
|
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 6% |
| Acid-insoluble ash | Not more than 1% |
| Loss on Drying |
| Not more than 9% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 39% |
| Cold method | Not less than 30% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 17% |
| Cold method | Not less than 10% |
SAFETY TEST
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
The fruits juice has been found to contain glycosides (e.g. 1-O-(3’-methylbut-3’-enyl)-β-D-glucopyranose, 1-n-butyl-4-(5’-formyl-2’-furanyl)methyl succinate, 4-epiborreriagenin, 1-n-butyl-4-methyl-2-hydroxysuccinate, 1-n-butyl-4-methyl-3-hydroxysuccinate, α-glucopyranose, β-glucopyranose, deacetylasperulosidic acid, asperulosidic acid, scopoletin) [ 9 ].
Butanol extract of the fruits has fatty acid glucosides (e.g. 1,6-di-O-octanoyl-β-D-glucopyranose, 6-O-(β-D-glucopyranosyl)-1-O-decanoyl-β-D-glucopyranose, 6-O-(β-D-glucopyranosyl)-1-O-octanoyl-β-D-glucopyranose, 6-O-(β-D-glucopyranosyl)-1-O-hexanoyl-β-D-glucopyranose, 2,6-di-O-(β-D-glucopyranosyl)-1-O-octanoyl-β-D-glucopyranose) and others (e.g. rutin, asperulosidic acid) [ 10 , 11 ].
Ethanol extract of the fruits contains polysaccharides (e.g. pectin polysaccharides, type II arabinogalactan, xyloglucan, heteroxylan, heteromannan) and vitamins (e.g. ascorbic acid, provitamin A) [ 12 ].
Methanol extract of the fruits has anthraquinones (e.g.1,6-dihydroxy-5-methoxy-2-methoxyanthraquinone, 1,5,7-trihydroxy-6-methoxy-2-methoxymethylanthraquinone, 1,5,15-tri-O-methylmorindol, 1,5-dimethylmorindol, 1,3-dimethoxyanthraquinone, alizarin, anthraquinone), flavonoids (e.g. quercetin, kaempferol), lignans (e.g. (+)-3,4,3’4’-tetrahydro-9,7’α-epoxylignan-7α,9’-lactone), iridoids (e.g. 6a-hydroxyadoxoside, 6b,7b-epoxy-8-epi-splendoside, morinaphthalenone, borreriagenin, citrifolinin B epimer a and b, cytidine, epi-dihydrocornin, scopoletin, isoscopoletin, 3,3’-bisdemethyltanegool, (-)-pinoresinol, (-)-3,3’-bisdemethylpinoresinol, americanin A, narcissoside), alkaloids (e.g. proxeronine, xeronine), glucosides (e.g. D-glucose, D-mannitol methyl a-D-fructofuranoside, methyl b-D-fructofuranoside, nicotifloroside, b-sitosterol 3-O-b-D-glucopyranoside, 6-O-(β-D-glucopyranosyl)-1-O-octanoyl-β-D-glucopyranose, 6-O-(β-D-glucopyranosyl)-1-O-hexanoyl-β-D-glucopyranose, 3-methyl-3-butenyl 6-O-β-D-glucopyranosyl-β-D-glucopyranoside, 2-O-(β-D-glucopyranosyl)-1-O-octanoyl-β-D-glucopyranose, 2-O-(β-D-glucopyranosyl)-1-O-hexanoyl-β-D-glucopyranose, noniosides E-H, 2,6-di-O-(β-D-glucopyranosyl)-1-O-octanoyl-β-D-glucopyranose, 6-O-(β-D-glucopyranosyl)-1-O-octanoyl-β-D-glucopyranose) and others (e.g. vanillin, asperuloside, asperulosidic acid, deacetylasperuloside, dehydromethoxygaertneroside) [ 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 ].
Dichloromethane extract of the fruits has been found to contain derivatives of acids (e.g. hexanoic acid, octanoic acid, decanoic acid, nonanoic acid, heptanoic acid, butanoic acid, acetic acid, butanoic acid, 2-methylbutanoic acid, 2-methylpropanoic acid, 3-methylthiopropanoic acid, benzoic acid, hexanedioic acid, undecanoic acid, lauric acid, myristic acid, palmitic acid, linoleic acid, oleic acid (Z,Z,Z)-8,11,14-eicosatrienoic acid), alcohols (e.g. 1-butanol, 3-methyl-3-buten-1-ol, 3-methyl-2-buten-1-ol,1-hexanol, benzyl alcohol, eugenol, (Z,Z)-2,5-undecadien-1-ol) and others (e.g. methyl hexanoate, ethyl hexanoate, methyl ocatanoate, methyl 5-nonenoate, methyl decanoate, methyl 3-methylthio-propanoate, ethyl octanoate, ethyl decanoate, methyl palmitate, methyl elaidate, methyl oleate, 3-hydroxy-2-butanone, 2-heptanone, (E)-6-dodeceno-γ-lactone, (Z)-6-dodeceno-γ-lactone, hexamide, limonene, scopoletin, vomifoliol) [ 23 ].
Hexane extract of the fruits contains sulfur compounds (e.g. methanethiol, S-methyl thioacetate, dimethyl disulfide, methyl 3-methylthiopropanoate), terpenes (e.g. linalool oxide, (Z)-3,7-dimethyl-1,3,6-octatriene, (+)-4-carene, D-limonene, ocimenol, terpineol), derivatives of acids (e.g. butanoic acid, decanoic acid, 2E,4Z,7Z-decatrienoic acid, hexanoic acid, octanoic acid, 2-octenoic acid, nonanoic acid, heptanoic acid, acetic acid, formic acid) and others (e.g. 1-butanol, 1-methyl-3-buten-1-ol, 1-methyl-2-buten-1-ol, 2-methyl-2-buten-1-ol, 3-methyl-3-buten-1-ol, 3-methyl-2-buten-1-ol, 3-methyl-2-butanone, benzyl alcohol, acetaldehyde, 2-methylbutanal, 3-methylbutanal, 2-pentanone, 2-hexanone, hexanal, 2-heptanone, 2-hexenal, furfural, benzaldehyde, ethanol, benzyl alcohol, ethyl acetate, butyl acetate, methyl 2-methylpropanoate, methyl butanoate, ethyl butanoate, butyl butanoate, 4-pentenyl butanoate, methyl 2-methylbutanoate, methyl 3-methylbutanoate, 3-methyl-3-buten-1-yl-3-methylbutanoate, methyl hexanoate, ethyl hexanoate, butyl hexanoate, 4-pentenyl hexanoate, 3-methyl-3-buten-1-yl hexanoate, hexyl isovalerate, methyl heptanoate, methyl ocatanoate, ethyl octanoate, butyl octanoate, 3-methyl-3-buten-1-yl octanoate, methyl 2-octenoate, methyl 3-octenoate, methyl 6-octenoate, methyl nonanate, methyl 5-nonenoate, methyl decanoate, ethyl decanoate, methyl 4-decenoate, ethyl 4-decenoate, methyl salicylate, methyl hexadecanoate) [ 24 , 25 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
The ripe fruit of M. citrifolia is traditionally used for relief of sore throat, boil, carbuncle, stomach ulcer and inflamed elbow. The fruit juice is consumed to prevent adverse effects of kava (Piper methysticum). The fruit pounded with sugar cane and kava root is used for tuberculosis. The charred unripe fruits have been applied with salt for gum disorders, while the fresh young fruit mashed with salt is applied on deep cuts and poulticed over broken bones [ 3 , 6 , 26 ].
Biological and pharmacological activities supported by experimental data
Antioxidant activity
Ethyl acetate extract of M. citrifolia fruit (4 mg) had strong antioxidant activity comparable to tocopherol and butylated hydroxyl toluene [ 26 ].
Antimicrobial activity
Methanol extract of M. citrifolia fruit (100 mg/mL) showed strong activity against gram-negative bacteria (Salmonella paratyphi A) and moderate activity against gram-positive bacteria (Bacillus subtilis, Staphylococcus aureus, Lactobacillus lactis, Streptococcus thermophilus, Pseudomonas aeruginosa) and other gram-negative bacteria (Salmonella typhi, Escherichia coli, Vibrio harveyi, Klebsiella pneumonia, Shigella flexneri, Salmonella paratyphi A, Aeromonas hydrophilam, Vibrio cholera, Chromobacterium violaceum and Enterobacter faecalis). The ethyl acetate extract showed moderate activity against all aforementioned bacteria except for P. aeruginosa and K. pneumonia [ 28 ].
Antifungal activity
Ripe fruit juice of M. citrifolia showed antifungal activity against Candida albicans with partial growth inhibition at a concentration of 40 mg/mL and complete growth inhibition at a concentration of 50 mg/mL after 30 min contact time [ 26 ]. The methanol and ethyl acetate extracts of M. citrifolia fruit showed strong activity against Trichophyton mentagrophytes with 79.3% and 62.06% inhibition, respectively. Methanol extract of the fruit also showed moderately strong activity (close to 50% inhibition) against Penicillium sp., Fusarium sp. and Rhizopus sp. The methanol, ethyl acetate and hexane extracts weakly inhibited Candida albicans and Aspergillus sp [ 29 ].
Anti-angiogenic activity
Fruit juice of M. citrifolia (5% and 10%) was found to significantly inhibit (P<0.001) the initiation of new placental vessels and reduce the growth rate and proliferation of newly developing capillary sprouts compared to control explants using an in vitro angiogenesis assay. The 10% of M. citrifolia fruit juice was able to induce vessel degeneration and apoptosis of developed capillary vessels based on MTT and TUNEL assays, respectively. It was also effective in inhibiting capillary initiating of the human breast tumor explants
[ 30 ].
Cytotoxic activity
Methanol (1:6 dilution of crude extract) and ethyl acetate (1:3 dilution) extracts of M. citrifolia fruit inhibited almost 50% of the human laryngeal epithiloma (Hep2) cells [ 27 ]. The fruit extract also inhibited retinoblastoma Y79 cell lines (IC50 800 µg/mL) [ 31 ].
Antidyslipidemic activity
Aqueous-ethanolic (70%) extract of M. citrifolia fruit administered orally (1000 mg/kg) to tyloxapol-induced male Sprague Dawley rats caused significant reduction in total cholesterol level (P< 0.01) and triglyceride level (P< 0.05). The extract also significantly (P< 0.05) reduced total cholesterol, triglyceride, low density lipoprotein-cholesterol, atherogenic index and total cholesterol-high density lipoprotein-cholesterol ratio of high fat diet-induced dyslipidemic adult Sprague Dawley rat models [ 32 ].
Hypoglycemic activity
Fermented fruit juice of M. citrifolia given orally to male Sprague Dawley rats (2 mL/kg) twice daily for 20 days reduced fasting blood glucose by 52.6% [ 34 ].
Hepatoprotective activity
Fruit juice of M. citrifolia (20%) given to carbon tetrachloride (CCl4)-induced female Sprague Dawley rats for 7 days showed significant reduction in hepatotoxic lesions (P< 0.001), serum alanine amino transferase (P< 0.01) and aspartate aminotransferase (P< 0.05) levels. In a correlative time-dependent study, 10% of the juice displayed delayed appearance of damaged hepatocytes with a decreased number of necrotic foci (80%) and apoptosis (50%), suggesting that the juice protects the liver from acute CCl4 exposure [ 34 ].
Immunomodulatory effect
M. citrifolia fruit juice and juice concentrate (1 and 5 mg/mL) stimulated cannabinoid 2 (CB2) (54-224%) but inhibited cannabinoid 1 (CB1) (14-172%) receptors in vitro. The fruit juice administered orally (100 mL/day) to male mice for 16 days decreased the production of IL-4, but increased the production of IFN-γ cytokines [ 35 ].
SAFETY INFORMATION
Preclinical study(toxicology studies)
Acute toxicity
Oral single dose acute toxicity study of M. citrifolia dried fruit powder on female Sprague Dawley rats (aged between 8 and 12 weeks old) showed no toxic effects on the parameters observed, including behaviours, body weight, food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. Half lethal dose (LD50) is more than 2,000 mg/kg body weight [ 36 ].
In acute oral toxicity studies with rats, the juice and puree of Tahitian M. citrifolia fruit had LD50 values higher than 15,000 mg/kg body weight [ 37 , 38 ], while the fruit concentrate had LD50 value higher than 5000 mg/kg body weight [ 39 ].
The ethanol and aqueous extracts of M. citrifolia fruit were administered intraperitoneally at a single dose to 21-day old female mice. The LD50 of the ethanol and aqueous extracts were found to be 3500 mg/kg and 7500 mg/kg respectively [ 40 ].
Sub-acute toxicity
Aqueous extract of M. citrifolia fruit (5.1% total solids) administered via oral gavage at a dose of 1000 mg/kg body weight to both male and female Sprague Dawley rats for 28 days showed no adverse effects on body weight, food consumption, haematological, clinical chemistry and histopathological parameters [ 41 ].
Sub-chronic toxicity
Fruit juice of Tahitian M. citrifolia fruit was administered by oral gavage at a daily dose of up to 80 mL/kg for 13-weeks to Sprague Dawley rats showed no adverse clinical signs, abnormal food consumption and weight gain, as well as no negative impact on the clinical chemistry, haematological and histopathological parameters. The No Observed Adverse Effect Level (NOAEL) was 80 mL/kg body weight/day [ 42 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Adverse reaction
Reported adverse effects of the M. citrifolia fruit juice include allergic reactions, diarrhoea, nausea and skin rash [ 43 ]. Contraindication Owing to possible teratogenicity, the aqueous extract and juice of M. citrifolia fruit should not be taken during pregnancy [ 44 ].
Warnings
M. citrifolia fruit juice include allergic reactions, diarrhoea, nausea and skin rash [ 43 ]. Consumers with liver disorders should seek advice from their health care professionals before taking any fruit juice preparations containing M. citrifolia. There were cases reported on hepatotoxicity after consuming noni juice but no convincing evidence for a causal relationship could be established which suggests that some individuals have a particular sensitivity for hepatotoxic effects to noni fruit products [ 45 ].
DOSAGE
The recommended daily intake of M. citrifolia fruit juice is 30 mL/day (equivalent to around 0.5 mL/kg body weight for a 60 kg adult) [ 46 ].
STORAGE
Store below 30˚C. Protect from light and moisture.
REFERENCES
- Malaysian herbal monograph. Volume 2. Kuala Lumpur: Forest Research Institute Malaysia. 2009.
- Plant resources of South-East Asia. No. 12. (3): medicinal and poisonous plants 3. Leiden: Backhuys. 2003.
- Burkill, IH. A dictionary of the economic products of the Malay Peninsula. Malaysia: Ministry of Agriculture. 1966;Vol 2:p.1493-1494.
- Rastogi P, Mehrotra BN. Compendium of Indian medicinal plants. PID: New Delhi. 1990.
- Porcher Michel H. Sorting Morinda names: Multilingual Multiscript Plant Name Database (M.M.P.N.D) – a work in progress (1995-2020). School of Agriculture and Food Systems. Faculty of Land & Food Resources. The University of Melbourne, Australia. 2000.
- Morton JF. The ocean-going noni, or Indian mulberry (Morinda citrifolia, Rubiaceae) and some of its ‘‘colourful’’ relatives. Ecological Botany. 1992;46:241–256.
- Dittmar A. Morinda citrifolia L.-use in indigenous Samoan medicine. Journal of Herbs, Spices and Medicine Plants. 1993;1:77-92.
- Nayak S, Mengi S. Preliminary physicochemical and phytochemical evaluation of Morinda citrifolia fruit extractives. International Journal of Pharmacy and Pharmaceutical Sciences. 2010;2(4):150-154.
- Samoylenko V, Zhao J, Dunbar DC, Khan IA, Rushing JW, Muhammad I. New constituents from noni (Morinda citrifolia) fruit juice. Journal of Agricultural and Food Chemistry. 2006;54(17):6398-6402.
- Kim HK, Kwon MK, Kim JN, Kim CK, Lee YJ, Shin HJ, Lee J, Lee HS. Identification of novel fatty acid glucosides from the tropical fruit Morinda citrifolia L. Phytochemistry Letters. 2010;3(4):238-241.
- Wang M, Kikuzaki H, Csiszar K, Boyd CD, Maunakea A, Fong SF, Ghai G, Rosen RT, Nakatani N, Ho CT. Novel trisaccharide fatty acid ester identified from the fruits of Morinda citrifolia (noni). Journal of Agricultural and Food Chemistry. 1999;47(12):4880-4882.
- Bui AKT, Bacic A, Pettolino F. Polysaccharide composition of the fruit juice of Morinda citrifolia (noni). Phytochemistry. 2006;67(12):1271-1275.
- Lin CF, Ni CL, Huang YL, Sheu SJ, Chen CC. Lignans and anthraquinones from the fruits of Morinda citrifolia. Natural Product Research. 2007;21(13):1199-1204.
- Akihisa T, Matsumoto K, Tokuda H, Yasukawa K, Seino K, Nakamoto K, Kuninaga H, Suzuki T, Kimura Y. Anti-inflammatory and potential cancer chemopreventive constituents of the fruits of Morinda citrifolia (noni). Journal of Natural Products. 2007;70(5):754-757.
- Siddiqui BS, Sattar FA, Ahmad F, Begum S. Isolation and structural elucidation of chemical constituents from the fruits of Morinda citrifolia Linn. Archives of Pharmacal Research (Seoul). 2007;30(8):919-923.
- Deng S, West BJ, Jensen CJ, Basar S, Westendorf J. Development and validation of an RP-HPLC method for the analysis of anthraquinones in noni fruits and leaves. Food Chemistry. 2009;116(2):505-508.
- Deng S, West BJ. Antidepressant effects of noni fruit and its active principals. Asian Journal of Medical Sciences. 2011;3(2):79-83.
- Su BN, Pawlus AD, Jung HA, Keller WJ, McLaughlin JL, Kinghorn AD. Chemical constituents of the fruits of Morinda citrifolia (noni) and their antioxidant activity. Journal of Natural Products. 2005;68(4):592-595.
- Chan-Blanco Y, Vaillant F, Mercedes Perez A, Reynes M, Brillouet JM, Brat P. The noni fruit (Morinda citrifolia L.): a review of agricultural research, nutritional and therapeutic properties. Journal of Food Composition and Analysis. 2006;19(6–7):645-654.
- Heinicke R. The pharmacologically active ingredients of noni. Bulletin of the National Tropical Botanical Garden. 1985;15:10-14.
- Wang M, Kikuzaki H, Jin Y, Nakatani N, Zhu N, Csiszar K, Boyd C, Rosen RT, Ghaig G, Ho CT. Novel glycosides from noni (Morinda citrifolia). Journal of Natural Products. 2000;63(8):1182-1183.
- Dalsgaard PW, Potterat O, Dieterle F, Paululat T, Kuhn T, Hamburger M. Noniosides E – H, new trisaccharide fatty acid esters from the fruit of Morinda citrifolia (noni). Planta Medica. 2006;72(14):1322-1327.
- Farine JP, Legal L, Moreteau B, Le Quere JL. Volatile components of ripe fruits of Morinda citrifolia and their effects on Drosophila. Phytochemistry. 1996;41(2):433-438.
- Wei GJ, Ho CT, Huang AS. Analysis of volatile compounds in noni fruit (Morinda citrifolia L.) juice by steam distillation-extraction and solid phase microextraction coupled with GC/AED and GC/MS. Journal of Food and Drug Analysis. 2011;19(1):33-39.
- Basar S, Westendorf J. Identification of (2E, 4Z, 7Z)- decatrienoic acid in noni fruit and its use in quality screening of commercial noni products. Food Analytical Methods. 2011;4:57-65.
- Dixon AR, Heather M, Etkin LN. Ferment this: the transformation of noni, a traditional Polynesian medicine (Morinda citrifolia, Rubiaceae). Economic Botany. 1999;53(1):51-68.
- Zin ZM, Hamid AA, Osman A. Antioxidative activity of extracts from mengkudu (Morinda citrifolia L.) root, fruit and leaf. Food Chemistry. 2002;78:227-231.
- Jayaraman SK, Manoharan MS, Illanchezian S. Antibacterial, antifungal and tumor cell suppression potential of Morinda citrifolia fruit extracts. International Journal of Integrative Biology. 2008;3(1):44-49.
- Jainkittivong A, Butsarakamruha T, Langlais RP. Antifungal activity of Morinda citrifolia fruit extract against Candida albicans. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2009;108(3):394-398.
- Hornick CA, Myers A, Sadowska-Krowicka H, Anthony CT, Woltering EA. Inhibition of angiogenic initiation and disruption of newly established human vascular networks by juice from Morinda citrifolia (noni). Angiogenesis. 2003;6(2):143-149.
- Gangadharan S, Thiyagarajan SL, Subramanian K, Narayanasamy M. Induction of caspase-3 dependdent apoptosis in Y79 cells by fruit extracts of Morinda citrifolia. Journal of Biotechnology. 2008;136:S75-S98.
- Mandukhail SU, Aziz N, Gilani AH. Studies on antidyslipidemic effects of Morinda citrifolia (noni) fruit, leaves and root extracts. Lipids in Health and Disease. 2010;9(1):88-93.
- Nayak BS, Marshall JR, Isitor G, Adogwa A. Hypoglycemic and hepatoprotective activity of fermented fruit juice of Morinda citrifolia (noni) in diabetic rats. Evidence-Based Complementary and Alternative Medicine. 2011;2011.
- Wang MY, Nowicki D, Anderson G, Jensen J, West B. Liver protective effects of Morinda citrifolia (noni). Plant Foods for Human Nutrition. 2008;63(2):59-63.
- Palu AK, Kim AH, West BJ, Deng S, Jensen J, White L. The effects of Morinda citrifolia L. (noni) on the immune system: its molecular mechanisms of action. Journal of Ethnopharmacology. 2008;115:502-506.
- Teh BP, Hamzah NF, Rosli SNS, Yahaya MAF, Zakiah I, Murizal Z. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2012. Report No.: HMRC 11-045/01/MC/F/K.
- Product Safety Labs (PSL). Acute oral toxicity study in rats – limit test: Tahitian noni juice. Product Safety Labs (Eurofins Scientific, Inc.), East Brunswick, New Jersey, US (6 October 1999).
- Product Safety Labs (PSL). Acute oral toxicity study in rats – limit test: Tahitian noni puree. Product Safety Labs (Eurofins Scientific, Inc.), East Brunswick, New Jersey, US (6 October 1999).
- Product Safety Labs (PSL). Acute oral toxicity study in rats – limit test: Tahitian noni concentrate. Product Safety Labs (Eurofins Scientific, Inc.), East Brunswick, New Jersey, US (3 September 1999).
- Sawpheeyah N, Srirat K, Wibool R, Niwan T, Sirima M. Gastrokinetic activity of Morinda citrifolia aqueous fruit extract and its possible mechanism of action in human and rat models. Journal of Ethnopharmacology. 2012;142:354-361.
- Mancebo A, Scull I, Gonzalez Y, Arteaga ME, Gonzales BO, Fuentes D, Hernandez O, Correa M. Ensayo de toxicidad a dosis repetidas (28 días) por vía oral del extracto acuoso de Morinda citrifolia en ratas Sprague Dawley. Critical Reviews in Toxicology. 2002;19:73-78.
- Glerup P. Tahitian noni Juice – a 13-week oral (gavage) toxicity study in rats. Scantox Biologisk Laboratorium, Lille Skensved, Denmark (Lab no. 39978). 2001.
- Opinion on a request from the commission related to the safety of noni juice (juice of the fruits of Morinda citrifolia). [Internet] The European Food Safety Authority Journal; 2006 [cited 11 Nov 2012] Available from: http://www.efsa.europa.eu/en/efsajournal/doc/998.pdf.
- Marques NFQ, Marques APBM, Iwano AL, Golin M, De-Carvalho RR, Paumgartten FJR, Dalsenter PR. Delayed ossification in Wistar rats induced by Morinda citrifolia L. exposure during pregnancy. Journal of Ethnopharmacology. 2010;128(1):85-91.
- Scientific opinion of the panel on dietetic products nutrition and allergies on a request from the European commission on the safety of ‘Morinda citrifolia’ (noni) fruit puree and concentrate’ as a novel food ingredient.[Internet] The European Food Safety Authority Journal. 2009;998:1-16: [cited 11 Nov 2012] Available from: http://www.efsa.europa.eu/de/scdocs/doc/s998.pdf.
- Opinion of the Scientific Committee on food on Tahitian noni juice.[Internet] SCF/CS/NF/DOS/18 ADD 2 Final 2002: [cited 11 Nov 2012] Available from: http://ec.europa.eu/food/fs/sc/scf/out151_en.pdf.