Mengkudu Leaves
Morinda citrifolia L.
Rubiaceae
DEFINITION
Mengkudu leaves consist of the powder of dried leaves of Morinda citrifolia L. (Rubiaceae).
SYNONYM
Belicea hoffimannioides Lundell, Morinda bracteata Roxb., Morinda elliptica (Hook.f.) Ridl., Morinda litoralis Blanco, Morinda macrophylla Desf., Morinda multiflora Roxb., Morinda stenophylla Spreng., Samama citrifolia (L.) Kuntze, Sarcocephalus leichhardtii F.Muell [ 1 , 2 ].
VERNACULAR NAMES
Noni, indian mulberry (English); Mengkudu, mengkudu besar, mengkudu jantan (Malay); Hai ba ji, wu ning luo ling kuo (Chinese); Nunaakai, nunavu (Tamil) [ 2 , 3 , 4 ].
CHARACTER
| Colour | Green (fresh leaf), dark green (powder) |
| Odour | Characteristic |
| Taste | Characteristic |
IDENTIFICATION
Plant Morphology
M. citrifolia is a shrub or small-medium sized tree, 3–10 m tall with quadrangular or somewhat rounded branches and evergreen, grows in shady forest and in open rocky or sandy shores. Leaves large, simple, alternate, broadly elliptic to oblong shape, 10–30 cm x 5–15 cm, dark green, glossy, wavy and prominently-veined. Flowers small, 1.25 cm long, 5-lobed, white, tubular-like, fragrant, borne in a globose head with 2.5 cm across. Fruits, head develop into compound fruits composed of many small drupes, ovoid, ellipsoid or roundish, 3–10 cm x 3–6 cm with an embossed appearance, slightly wrinkly, waxy, semi-translucent skin, and turn from green to yellow and to almost white as it ripens; the fruits surface is faintly patterned with 4–6 sided outlines, each with a central ‘’eye’’; the pulp is fleshy and juicy, dull-yellow or yellowish-white and gelatinous when the fruit is ripe; it has numerous hard oblong-triangular reddish-brown pits, each containing 4 seeds about 3.5 mm long [ 5 , 6 ].
Microscopy
Powdered material consists of fragment of adaxial epidermis cell with straight to wavy anticlinal wall; abaxial epidermis cell with stomata; fragment of parenchyma cell; fragment of vessels (i.e. spiral, spiral with thickening and scalariform vessel); fragment of fibre and a group of acicular calcium oxalate crystal [ 7 ].
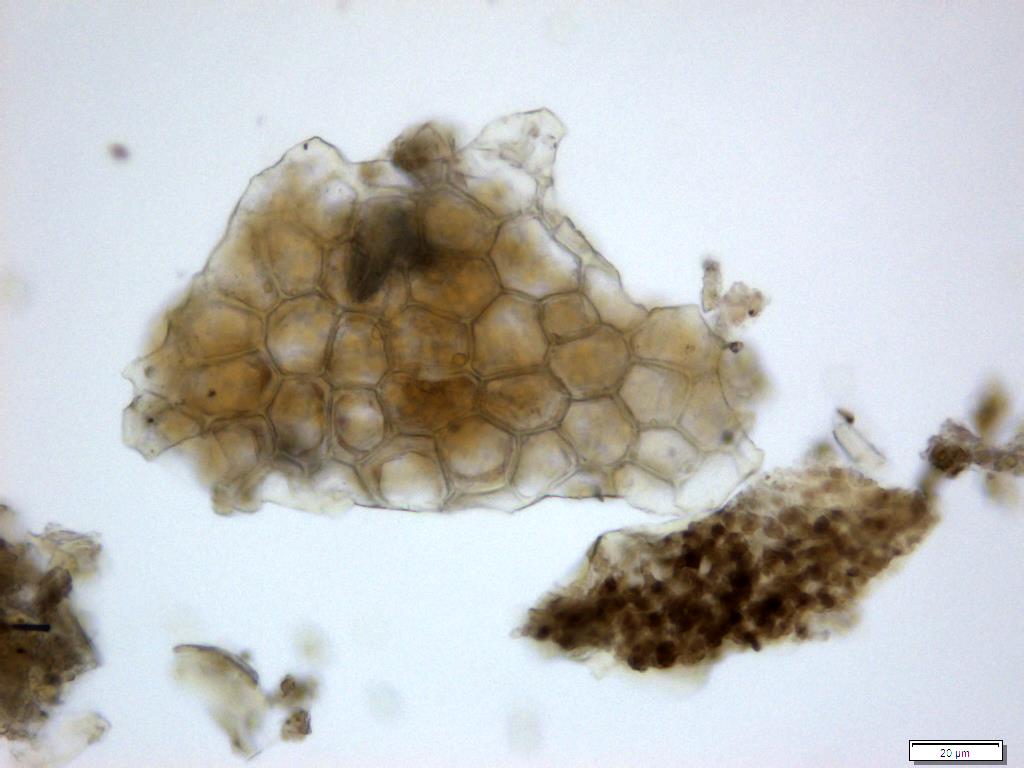
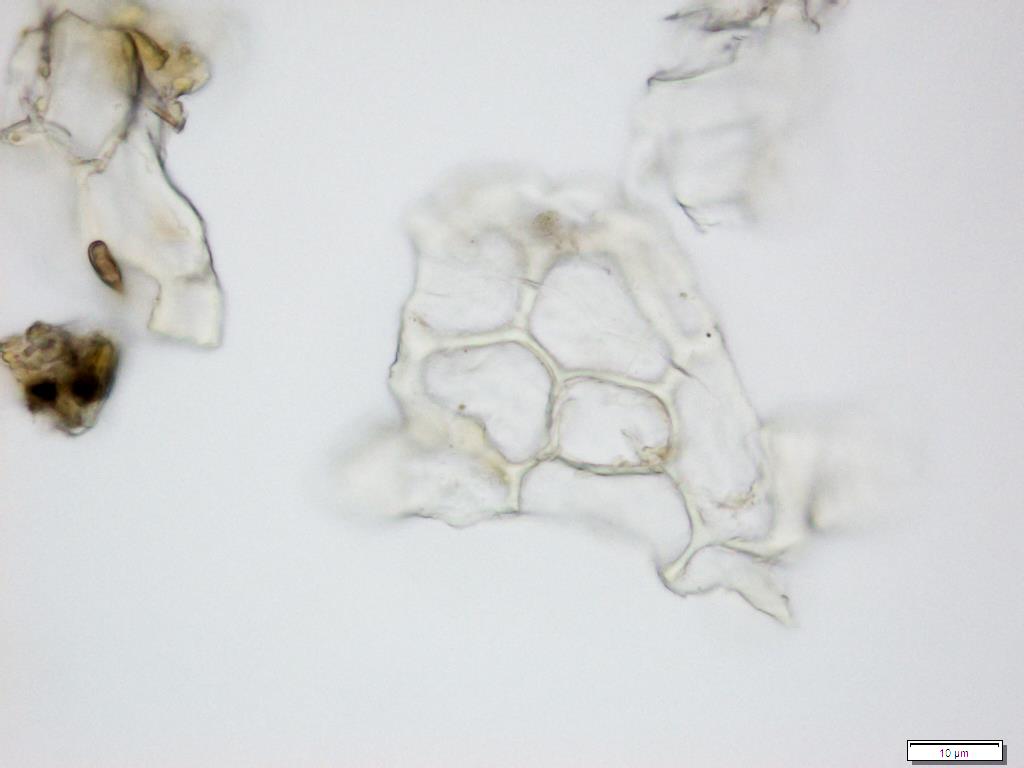
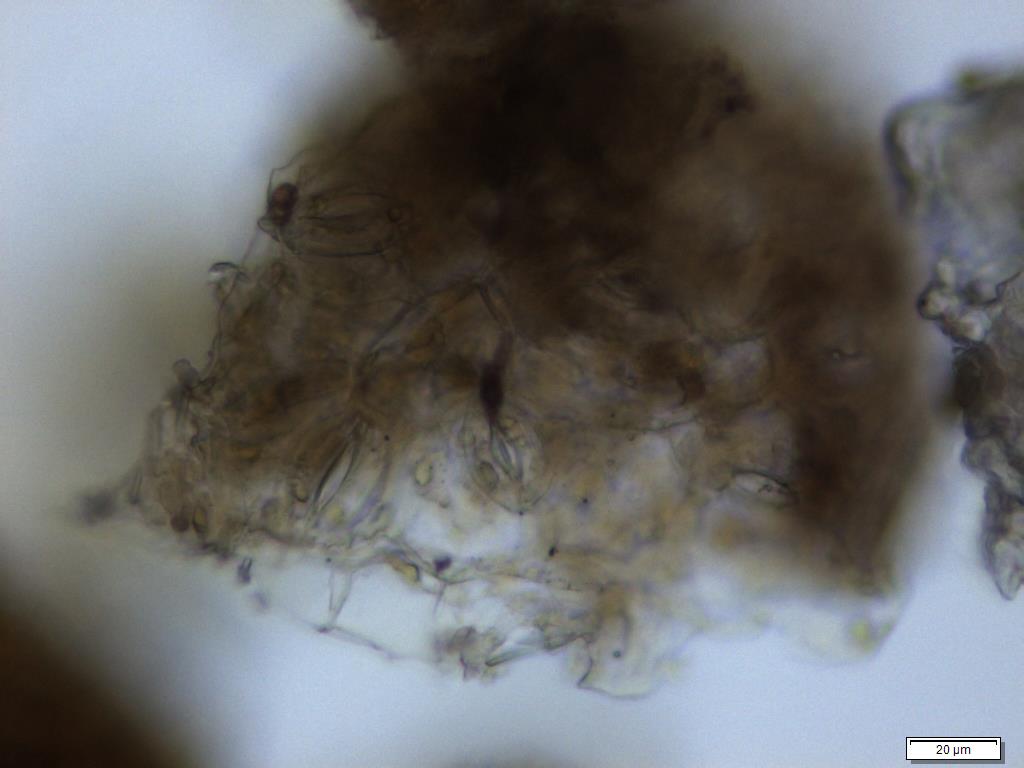
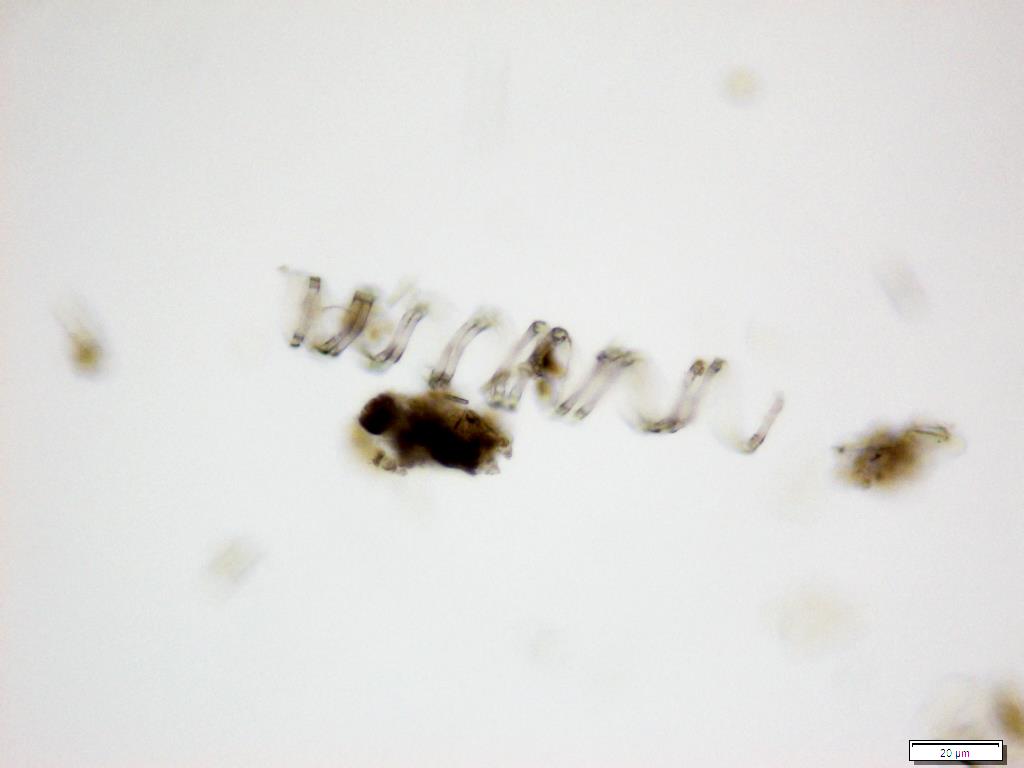

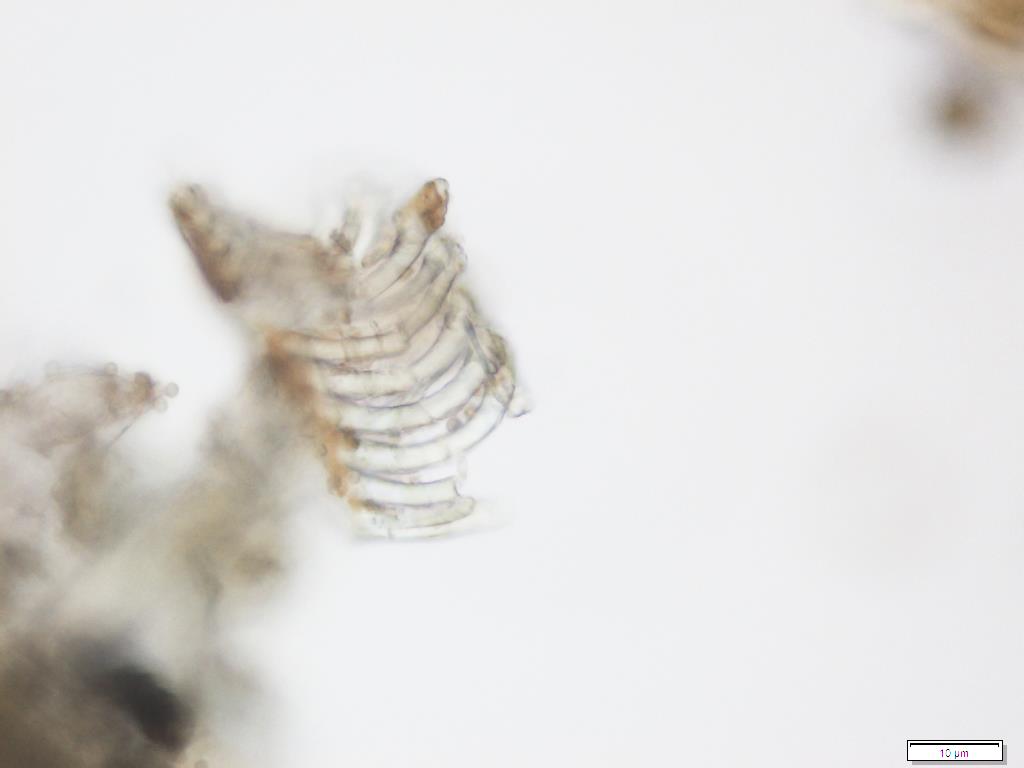



Figure 2 : Microscopic characters of Morinda citrifolia leaves powder of 0.355 mm size. (a) Adaxial epidermis cells (magnification 20x); (b) parenchyma cells (magnification 40x); (c) stomata cells (magnification 20x); (d) spiral vessel (magnification 20x); (e) scalariform vessel (magnification 40x); (f) spiral thickening vessel (magnification 40x); (g) fragment of scalariform and spiral thickening vessels (magnification 20x); (h) fibres (magnification 40x); (i) acicular crystal (magnification 40x). [Scale bars: a = 20 µm; b = 10 µm; c = 20 µm; d = 20 µm; e = 10 µm; f = 10 µm; g = 20 µm; h = 10 µm; i = 10 µm]
Colour Tests
Observed colour of solution after treatment with various reagents:
| NaOH (5%) | Yellow |
| KOH (5%) | Yellow |
Thin Layer Chromatography (TLC)
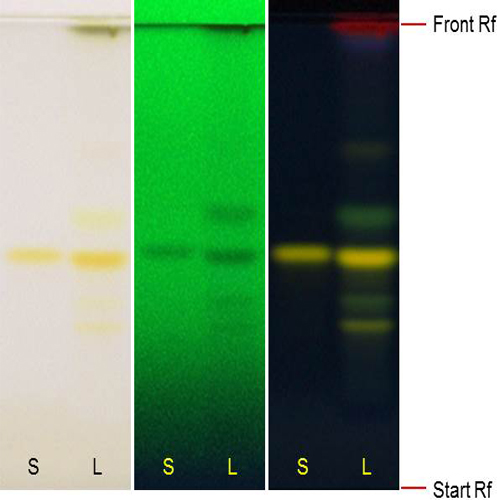
Figure 3 : TLC chromatogram of rutin (S) and ethanol extract of Morinda citrifolia dried leaves powder (L) observed under (a) visible light after derivatisation, (b) UV at 254 nm before derivatisation and (c) UV at 366 nm after derivatization.
| Test Solutions | Weigh about 0.5 g of M. citrifolia dried leaf powder of 0.355 mm size in a 14 mL screw-capped vial. Add 5 mL of ethanol and sonicate for 15 minutes at ambient temperature. Filter the solution through filter paper. Evaporate to dryness and reconstitute with 2 mL methanol. Use the solution as test solution. |
| Standard solution | Dissolve rutin standard [CAS no.: 153-18-4] in methanol to produce 1.0 mg/mL solution. |
| Stationary Phase | HPTLC silica gel pre-coated plate 60 F254, 10 x 10 cm |
| Mobile phase | Ethyl acetate : acetic acid : formic acid : water; (100 : 11 : 11 : 26) (v/v/v/v) |
| Application |
|
| Development distance | 7 cm |
| Drying | Air drying |
| Detection |
|
High Performance Liquid Chromatography (HPLC)
| Test solution | Weigh about 0.5 g of M. citrifolia dried leaf powder of 0.355 mm size in a 14 mL screw-capped vial. Add 5 mL of ethanol and sonicate for 15 minutes at ambient temperature. Filter the solution and evaporate to dryness. Reconstitute with 2 mL methanol and use the solution as test solution. | ||||||||||||||||||
| Standard solution | Dissolve rutin standard [CAS no.: 153-18-4] in methanol to produce 1.0 mg/mL solution. | ||||||||||||||||||
| Chromatographic system |
Detector: PDA 356 nm Column: C18 (5 µm, 4.6 mm i.d. x 250 mm) (Luna, Phenomenex if necessary) Column oven temperature: Ambient Flow rate: 1.0 mL/min Injection volume: 5 µL for rutin; 10 µL for test solution |
||||||||||||||||||
| Mobile Phase (gradient mode) |
|
||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the standard solutions (1.0 mg/mL). The requirements of the system suitability parameters are as follow:
|
||||||||||||||||||
| Acceptance criteria |
|
(a)
(b)
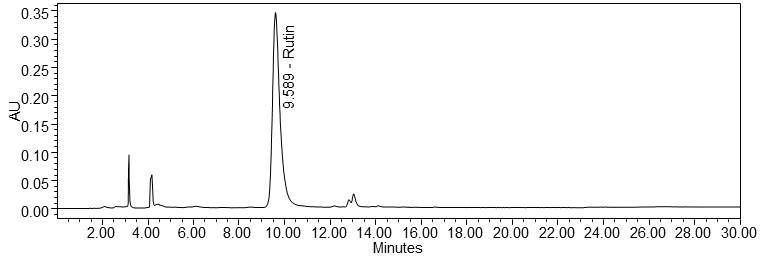
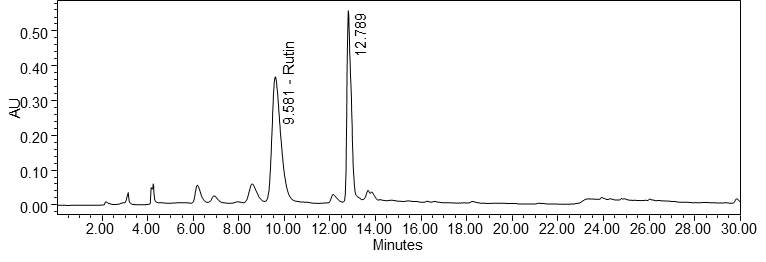
Figure 4 : Whole HPLC chromatogram of (a) rutin standard solution (1.0 mg/mL) at tr= 9.589 min and (b) ethanol extract of Morinda citrifolia dried leaves powder showing peak corresponding to rutin standard solution at tr= 9.581 min.
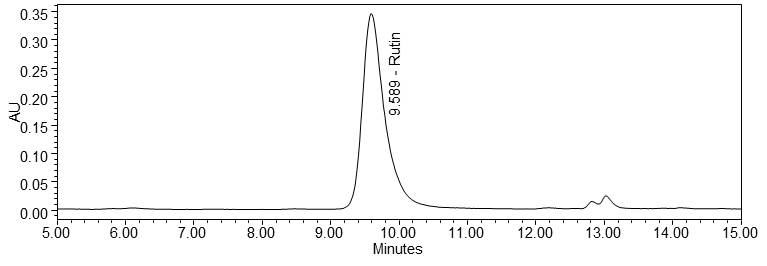
(a)
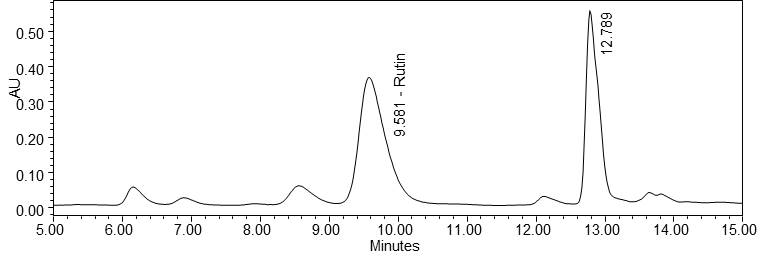
(b)
Figure 5 : HPLC chromatogram highlighting the elution region of rutin in (a) rutin standard solution (1.0 mg/mL) at tr= 9.589 min and (b) ethanol extract of Morinda citrifolia dried leaves powder showing peak corresponding to rutin standard solution at tr= 9.581 min.
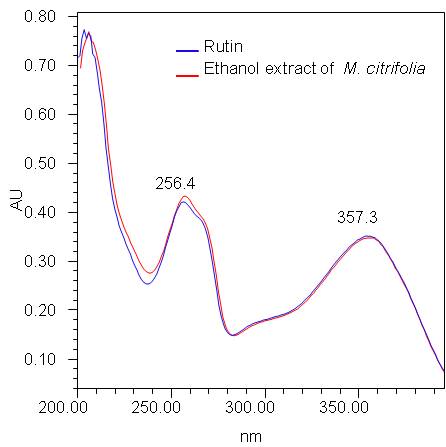
Figure 6 : UV spectrum of rutin standard solution (1.0 mg/mL) and ethanol extract of Morinda citrifolia dried leaves powder.
PURITY TESTS
The purity tests are based on M. citrifolia dried leaves powder of 0.355 mm particle size.
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 9% |
| Acid-insoluble ash | Not more than 1% |
| Loss on Drying |
| Not more than 15% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 27% |
| Cold method | Not less than 11% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 13% |
| Cold method | Not less than 7% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Aqueous extract of M. citrifolia dried leaves was found to contain alkaloids, coumarins, flavonoids (e.g. quercetin-3-O-rutinoside and kaempferol glycosides), tannins, saponins, steroids and triterpenoids [ 8 , 9 ].
Methanolic extract of M. citrifolia dried leaves was found to contain flavonoids (e.g. quercetin 3,7-O-dimethyl ether, quercetin 3-O-methyl ether, kaempferol 3,4’-O-dimethyl ether, kaempferol-3-O-α-L-rhamnopyranosyl-1(1→6)-b-D-glucopyranoside and quercetin-3-O-α-L-rhamnopyranosyl-1(1→6)-b-D-glucopyranoside), iridoids (e.g. citrifoside and deacetyl asperuloside), megastigmane (e.g. roseoside), anthraquinones (e.g. 1,5,15-trimethylmorondol and 5,15-dimethylmorindol), diterpenes (e.g. phytol), triterpenes (e.g. ursolic acid, barbinervic acid, rotungenic acid, clethric acid, hederagenin, oleanolic acid and 3-O-acetylpomolic acid), fatty acids (e.g. 13-hydroxy-9,11,15-octadecatrienoic acid), coumarin (e.g. pteryxin and peucedanocoumarin III), chlorophyll derivatives (e.g. pheophorbide a, methyl pheophorbide b, methyl pheophorbide a, 151(S)-hydroxypurpurin-7 lactone dimethyl ester, 132(R)-hydroxypheophorbide a methyl ester, 151(R)-hydroxypurpurin-7 lactone dimethyl ester, 132(S)-hydroxypheophorbide a methyl ester and 13-epi-phaeophorbide a methyl ester) [ 10 , 11 ].
Ethanol extract of M. citrifolia dried leaves was found to contain alkaloids, carbohydrate, saponins, triterpenes (e.g. ursolic acid), phytosterols (e.g. campesterol, b-sitosterol and stigmasterol), flavonoids (e.g. quercetin-3-O–β-D-glucopyranoside, quercetin-3-O-α-L-rhamnopyranosyl-(1→6)-b-D-glucopyranoside (rutin), quercetin-3-O–β-D-glucopyranosyl-(1→2)-[ α-L-rhamnopyranosyl-(1→6)]-β-D-galacopyranoside, kaempferol-3-O-α-L-rhamnopyranosyl-(1→6)-b-D-glucopyranoside (kaempferol 3-O-rutinoside), kaempferol-3-O– β-D-glucopyranosyl-(1→2)-[ α-L-rhamnopyranosyl-(1→6)]-β-D-galacopyranoside, quercetin and kaempferol), iridoids (e.g. asperuloside, asperulosidic acid, citrifolinin B and citrifolinoside A), polyol (glycerin, D-arabinitol and sorbitol), hydrocarbon (pentadecane, hexadecane, heptadecane, octadecane and heptacosane), fatty acids (tetradecanoic acid, n-hexadecanoic acid and eicosanoic acid), diterpene (phytol), g-tocopherol, pyropheophorbide a, phenols, proteins and amino acids [ 12 , 13 , 14 , 15 , 16 , 17 , 18 ].
Acetone-water extract of M. citrifolia dried leaves was found to contain phytosterols (e.g. campesterol, stigmasterol and β-sitosterol) and oxalic acid [ 13 ].
Chloroform and petroleum extract of M. citrifolia dried leaves was found to contain flavonoids (e.g. kaempferol 5,7-O-diarabinoside and apigenin) [ 8 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally, the leaves are applied to the chest or to the abdomen, for cough, enlarged spleen, in nausea, colic and fever [ 19 ].
Biological and pharmacological activities supported by experimental data
Antioxidant activity
Aqueous extracts of M. citrifolia leaves (1, 10 and 100 μg/mL and 1 mg/mL) showed antioxidant activity with hydroxyl radical scavenging activity inhibition concentration at 50% level (IC50) of 35 µg/mL and 30 µg/mL compared to α-tocopherol (IC50 = 28 µg/mL) [ 8 ].
Dichloromethane extract of M. citrifolia leaves (500 g/mL) showed antioxidant activity with 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity inhibition concentration at 50% of growth (IC50) of 0.20 μg/mL compared to scopoletin (IC50 = 0.25 μg/mL) and gallic acid (IC50 = 0.002 μg/mL) [ 20 ].
Methanol extract of M. citrifolia leaves (500 g/mL) showed antioxidant activity with 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity inhibition concentration at 50% of growth (IC50) of 0.26 μg/mL compared to scopoletin (IC50 = 0.25 μg/mL) and gallic acid (IC50 = 0.002 μg/mL) [ 20 ].
Antimicrobial activity
Aqueous extract of M. citrifolia leaves (2, 5 and 10 mg/mL) inhibited the growth of Staphylococcus aureus with inhibition zone ranging from 16 to 20 mm compared to gentamicin or nystatine (21 mm) using disc diffusion assay [ 21 ].
Ethanol extract of M. citrifolia leaves (2, 5 and 10 mg/mL) inhibited the growth of Escherichia coli with inhibition zone ranging from 8 to 11 mm compared to gentamicin or nystatine (20 mm) using disc diffusion assay [ 21 ].
Aqueous extract of M. citrifolia leaves (256 and 512 mg/mL) showed antibacterial effect against Aeromonas hydrophila with minimum inhibitory concentration 0.625 mg/mL using microdilution assay [ 8 ].
Alcohol extract of M. citrifolia leaves (5 and 10 mg/mL) showed antibacterial effect against Bacillus subtilis, Escherichia coli and Staphylococcus aureus with inhibition zone ranging from 1.8 to 2.4 cm compared to tetracycline (2.1 – 2.5 cm) using agar diffusion assay [ 22 ].
Water extract of M. citrifolia leaves (2, 5 and 10 mg/mL) inhibited the growth of Aspergillus niger with inhibition zone ranging from 14 to 18 mm compared to gentamicin or nystatine (21 – 22 mm) using disc diffusion assay [ 21 ].
Chloroform extract of M. citrifolia leaves (2, 5 and 10 mg/mL) inhibited the growth of Candida albicans with inhibition zone ranging from 13 to 19 mm compared to gentamicin or nystatine (17 – 21 mm) using disc diffusion assay [ 21 ].
Petroleum-ether extract of M. citrifolia leaves (5 and 10 mg/mL) inhibited the growth of Candida albicans and Aspergillus niger with inhibition zone ranging from 2.0 to 2.6 cm compared to tetracycline (2.9 – 3.3 cm) using agar diffusion assay [ 22 ].
Antinociceptive activity
Aqueous extract of M. citrifolia leaves (400 mg/kg) was administered orally to male Swiss mice (25 – 30 g) 60 minutes before the induction of abdominal constriction using acetic acid. The extract showed significant (p < 0.001) reduction in the (number of writhings = 3) compared to vehicle group (number of writhings = 13) [ 8 ].
Anti-inflammatory activity
Aqueous extract of M. citrifolia leaves (200 and 400 mg/kg) was administered subcutaneously to male Swiss mice (25 – 30 g) 60 minutes before induction of leukocyte migration into peritoneal using carrageenan. The extract (200 mg/kg) showed significant (p < 0.01) reduction in the number of leukocytes (12), meanwhile extract (400 mg/kg) showed significant (p < 0.001) reduction in the number of leukocytes (11) compared to vehicle group (25) [ 8 ].
Aqueous extract of M. citrifolia leaves administered to mouse macrophage RAW 264.7 cells with lipopolysaccharides showed significant (p < 0.05) decreased in the secretion of TNF-α (98%) compared to dexamethasone (23%) and indomethacin (25%) [ 9 ].
Antiproliferative activity
Methanol extract of M. citrifolia dried leaves (500 g/mL) showed inhibitory effect against human epidermoid carcinoma (KB) (IC50 = 186.25 ± 4.79 µg/mL) in a dose-dependent manner compared to rutin (167.00 ± 6.71 µg/mL) and scopoletin (120.00 ± 6.12 µg/mL) using MTT assay [ 20 ].
Dichloromethane extract of M. citrifolia fresh leaves (500 g/mL) showed inhibitory effect against human epidermoid carcinoma (KB) (IC50 = 21.67 ± 1.54 µg/mL) and human cervical carcinoma (HeLa) (IC50 = 68.50 ± 2.43 µg/mL) in a dose-dependent manner compared to damnacantal (IC50 = 6.50 ± 0.5 µg/mL and IC50 = 22.00 ± 3.52 µg/mL) using MTT assay [ 20 ].
Dichloromethane extract of M. citrifolia dried leaves (500 g/mL) showed inhibitory effect against human epidermoid carcinoma (KB) (IC50 = 39.00 ± 6.67 µg/mL) and in a dose-dependent manner compared to damnacantal (IC50 = 6.50 ± 0.5 µg/mL) using MTT assay [ 20 ].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute toxicity
Oral single dose acute toxicty study of hot water and ethanol extracts of M. citrifolia leaves (2000 mg/kg body weight) on male and female Jcl:ICR mice (6-weeks old; 25 mice per gender) respectively showed no toxic effects on the parameters observed, including behaviours and body weights. No treatment-related differences in weight gain between extract-treated and water-treated groups. All mice were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality [ 13 ].
Oral single dose acute toxicity study on female Sprague Dawley rats ( aged between 8 and 12 weeks old) using aqueous extract of M. citrifolia leaves showed no toxic effect on the parameters observed, including behaviours, body weight, food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. Half lethal dose (LD50) is more than 2,000 mg/kg body weight [ 23].
Sub-acute toxicity
Freeze-dried powder of hot water and ethanol extracts of M. citrifolia leaves (200 mg per mouse per day) respectively was mixed into the feed pellet and given to male and female Jcl:ICR mice (6-weeks old; 25 mice per gender). All mice were observed for 28 days prior to necropsy. The study showed no toxic effects on the parameters observed, including behaviours and body weights. No treatment-related differences in weight gain between extract pre-mixed feed and no extract pre-mixed feed groups. No death was found throughout the study period. Necropsy revealed no significant abnormality [ 13 ].
Freeze-dried powder of aqueous extract of M. citrifolia leaves (1000 mg/kg body weight/day) was mixed into drinking water (mg of freeze-dried powder of extract/500 mL water) and given to male and female Wistar rats (7 to 8-weeks old, 120-160 body weight; five rats per gender). The study showed no significant different on the parameters observed and measured, including behaviours, body weights, organ weights and biochemical & haematological parameters, between extract pre-mixed drinking water and no extract pre-mixed drinking water groups. All mice were observed for 28 days prior to necropsy. Necropsy revealed no significant abnormality [ 24 ].
Sub-chronic toxicity
Freeze-dried powder of hot water and ethanol extracts of M. citrifolia leaves (20 mg per mouse) respectively was mixed into the feed pellet and given to male and female Jcl:ICR mice (6-weeks old; 25 mice per gender) daily. All mice were observed for 90 days prior to necropsy. The study showed no toxic effects on the parameters observed, including behaviours and body weights. No treatment-related differences in weight gain between extract pre-mixed feed and no extract pre-mixed feed groups. No death was found throughout the study period. Necropsy revealed no significant abnormality [ 13 ].
Freeze-dried powder of aqueous extract of M. citrifolia leaves (100, 300 and 1000 mg/kg body weight/day) was mixed into drinking water (mg of freeze-dried powder of extract/500 mL water) and given to male and female Wistar rats (7 to 8-weeks old, 120-160 body weight; 10 rats per gender). The study showed no significant different on the parameters observed and measured, including behaviours, body weights, organ weights and biochemical parameters, between extract pre-mixed drinking water and no extract pre-mixed drinking water groups. All mice were observed for 90 days prior to necropsy. Necropsy revealed no significant abnormality. However, the haemoglobin levels for male rats treated with 300 and 1000 mg/kg dose were significantly (p < 0.01) lower (8.5 ± 0.4 mmol/L and 8.8 ± 0.4 mmol/L) than control group (9 – 12 mmol/L). The haemoglobin levels for female rats treated with 1000 mg/kg dose was significantly (p < 0.05) lower than control group (9 – 11 mmol/L). In addition, the differential leukocyte count in lymphocytes for male rats treated with all three doses were significantly (p < 0.01) higher (80 – 90%) than control group (60 – 70%). Whilst differential leukocyte count in lymphocytes for female rats treated with 1000 mg/kg dose was significantly (p < 0.05) higher (80 – 90%) than control group (70 – 80%). For the differential leukocyte count in neutrophils, male rats treated with all three doses and showed significant (p < 0.05) lower values (10 – 20%) than control group (30 – 40%) whilst female rats treated with 1000 mg/kg dose also showed significant (p < 0.05) lower value (10 – 20%) than control group (20 – 30%). After 28 days of recvory period, the haemoglobin and differential leukocytes counts in both gender were not observed anymore [ 24 ].
Cytotoxicity
Aqueous extract of M. citrifolia leaves (500, 1000 and 2000 mg/kg body weight/day) respectively was given orally to male and female NMRI albino mice (22 ± 2 g body weight; 5 rats per gender) for two days and sacrificed on Day 3. The mice femurs were dissected to obtain bone marrow smears. The study showed no significant difference (p > 0.05) in the ratio of polychromatic erythrocytes to normochromatic erythrocytes frequencies (1.29 – 1.40) for both gender compared to water-treated group (1.13 – 1.27) [ 24 ].
Mutagenicity
Aqueous extract of M. citrifolia leaves (500, 1000 and 2000 mg/kg body weight/day) respectively was given orally to male and female NMRI albino mice (22 ± 2 g body weight; 5 rats per gender) for two days and sacrificed on Day 3. The mice femurs were dissected to obtain bone marrow smears. The study showed no significant difference (p > 0.05) in the frequencies of micronucleated polychromatic erythrocyte (0.12 – 0.30) vs water-treated group (0.18 – 0.20) [ 24 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The Plant List [Internet] Morinda citrifolia L. [cited on 8th August 2016]. Available from: http://www.theplantlist.org/tpl1.1/record/kew-129789.
- Groenendijk JJ. Plant Resources of South-East Asia No. 3: Dye and tannin-producing plants. In: Lemmens, R.H.M.J. and Wulijarni-Soetjipto, N. (Editors). Pudoc, Wageningen, The Netherlands. 1991; p.94-96.
- Database on flora of China. [Internet] Morinda citrifolia L. [cited on 20 May 2016]. Available from: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200022155.
- Database on flower of India. [Internet] Morinda citrifolia Linn. [cited on 20 May 2016]. Available from: http://www.flowersofindia.net/catalog/slides/Great%20Morinda.html.
- Rastogi P, Mehrotra BN.Compendium of Indian medicinal plants. PID: New Delhi. 1990.
- Porcher Michel H. Sorting Morinda names; Multilingual Multiscript Plant Name Database (M.M.P.N.D) – a work in progress (1995-2020). School of Agriculture and Food Systems. Faculty of Land & Food Resources. The University of Melbourne Australia. 2000.
- Malaysian Herbal Monograph Committee. Malaysian Herbal Monograph. Vol. 2. Malaysia: Forest Research Institute Malaysia. 2009; p.79-84.
- Serafini MR, Santos RC, Guimaraes AG, Almeida dos Santos JP, Conceicao Santos AD, Almeida Alves I, Gelain DP, Lima Nogueira PC, Quintans-Junior LJ, Rigoldi Bonjardim L, Souza Araujo AA. Morinda citrifolia Linn extract possesses antioxidant activities and reduces nociceptive behavior and leukocyte migration. Journal of Medical Food. 2011;14(10):1159-1166.
- Saraphanchotiwitthaya A, Sripalakit P. Anti-inflammatory effect of Morinda citrifolia leaf extract on macrophage RAW 264.7 cells. Science Asia. 2015;41:5-11.
- Saidatul Husni S, Umi Kalsom Y, Asmah R, Aspollah MS. Determination of flavonoid components from Morinda citrifolia (Mengkudu) and their antioxidant activity. Pertanika Journal of Tropical Agricutural Science. 2005;28(2):111-119.
- Takashima J, Ikeda Y, Komiyama K, Hayashi M, Kishida A & Ohsaki A. New constituents from the leaves of Morinda citrifolia. Chem. Pharm. Bull. 2007;55(2):343-345.
- Kochuthressia KP and Jaseentha MO. Phytochemical investigation of active compound in Morinda citrifolia leaves. Asian Journal of Biochemical and Pharmaceuticals Research. 2015;4(5):98-103.
- West BR, Tani H, Palu AK, Tolson CB, Jensen CK. Safety tests and antinutrient analyses of noni (Morinda citrifolia L.) leaf. Journal of the Science of Food and Agriculture. 2007;87:2583-2588.
- Deng S, West BJ, Jensen J. Simultaneous characterization and quantitation of flavonol glycosides and aglycones in noni leaves using a validated HPLC-UV/MS method. Food Chemistry. 2008;111(2):526-529.
- Sang S, Cheng X, Zhu N, Wang M, Jhoo J-W, Stark RE, Badmaev V, Ghai G, Rosen RT & Ho C-T. Iridoid glycosides from the leaves of Morinda citrifolia. J. Nat. Prod. 2001; 64(6):799-800.
- Sang S, Cheng X, Zhu N, Stark RE, Badmaev V, Ghai G, Rosen RT & Ho C-T. Flavonol glycosides and novel iridoid glycoside from the leaves of Morinda citrifolia. J. Agric. Food Chem. 2001; 49(9):4479-4481.
- Rivera A, Cedillo L, Hernandez F, Castillo V, Sanchez A, Castaneda D. Bioactive constituents in ethanolic extract leaves and fruit juice of Morinda citrifolia. Annals of Biological Research. 2012;3(2):1044-1049.
- Ahmad VU & Bano S. Isolation of b-sitosterol and ursolic acid from Morinda citrifolia Linn. J. Chem. Soc. Pak. 1980;2(2):71.
- Burkill IH. A dictionary of the economic products of the Malay peninsula. Vol. 2. London; Published on behalf of the governments of the Straits settlements and Federated Malay states by the Crown agents for the colonies. 1966; p.1518-1519.
- Thani W, Vallisuta O, Siripong P, Ruangwises N. Anti-proliferative and antioxidative activities of thai nonni/yor (Morinda citrifolia L.) leaf extract. Southeast Asian Journal Tropical Medicine Public Health. 2010;41(2):482-489.
- Usha R, Sangeetha S, Palaniswamy M. Antimicrobial activity of a rarely known species, Morinda citrifolia L. Ethnobotanical Leaflets. 2010;14:306-3011.
- Kumar KT, Panda DS, Nanda UN, Khuntia S. Evaluation of antibacterial, antifungal and anthelmintic activity of Morinda citrifolia L. (noni). International Journal of PharmaTech Research. 2010;2(2):1030-1032.
- Teh BP, Lee MG, Nor Liyana MY, Wan Abdul Hakim WL, Emylyn M. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of health; 2016. Report no.: HMRC 11-045/01/MC/L/J.
- Lagarto A, Bueno V, Merino N, Piloto J, Valdes O, Apricio G, Bellma A, Couret M, Vega Y. Safety evaluation of Morinda citrifolia (noni) leaves extract: assessment of genotoxicity, oral short term and subchronic toxicity. Journal of Intercultural Ethnopharmacology. 2013;2(1):15-2






