Scientific Name
Mucuna pruriens (L.) DC. [1]
Synonyms
Carpogon capitatus Roxb., Carpogon niveus Roxb.Dolichos pruriens L., Marcanthus cochinchinense Lour., Mucuna axillaris Baker, Mucuna bernieriana Baill., Mucuna cochinchinense (Lour.)A.Chev., Mucuna cochinchinensis (Lour.)A.Chev., Mucuna esquirolii H.Lev., Mucuna luzoniensis Merr., Mucuna lyonii Merr., Mucuna minima Haines, Mucuna nivea (Roxb.) DC., Mucuna prurita (L.) Hook., Mucuna sericophylla Perkins, Mucuna velutina Hassk., Negretia mitis Blanco, Stizolobium capitatum (Roxb.)Kuntze, Stizolobium cochinchinense (Lour.)Burk, Stizolobium niveum (Roxb.)Kuntze, Stizolobium pruritum (Wight) Piper, Stizolobium velutinum (Hassk.) Piper & Tracy [1]
Vernacular Name
| Malaysia | Kacang babi, kekaras gatal.[2] |
| English | Velvet bean [2] |
| Indonesia | Kara benguk (Javanese); kowas (Sundanese); kekara juleh (Moluccas).[2] |
| Thailand | Mamui (central).[2] |
| Laos | Tam nhe. [2] |
| Philippines | Sabawel.[2] |
| Cambodia | Khnhae.[2] |
| Vietnam | D[aaj]u m[ef]o r[uf]ng [2] |
| France | Pois mascate, pois velus.[2] |
| United States of America | Cowitch. [2] |
Geographical Distributions
Mucuna pruriens is probably a native of tropical South or Southeast Asia, and has been widely distributed throughout the tropics. It was introduced into Florida in 1876, from where its range was extended into temperate and subtropical areas by breeding. In the south-eastern United States, it used to be the most important cover crop grown in combination with maize in an area of about 1 000 000 ha around 1920. Later, soya bean and commercial fertilisers rapidly replaced it and it disappeared from agricultural statistics in 1965. As a cover crop, it is now most important in Australia, Hawaii, the Fiji Islands, Indonesia, Malaysia and the Philippines. [2]
Botanical Description
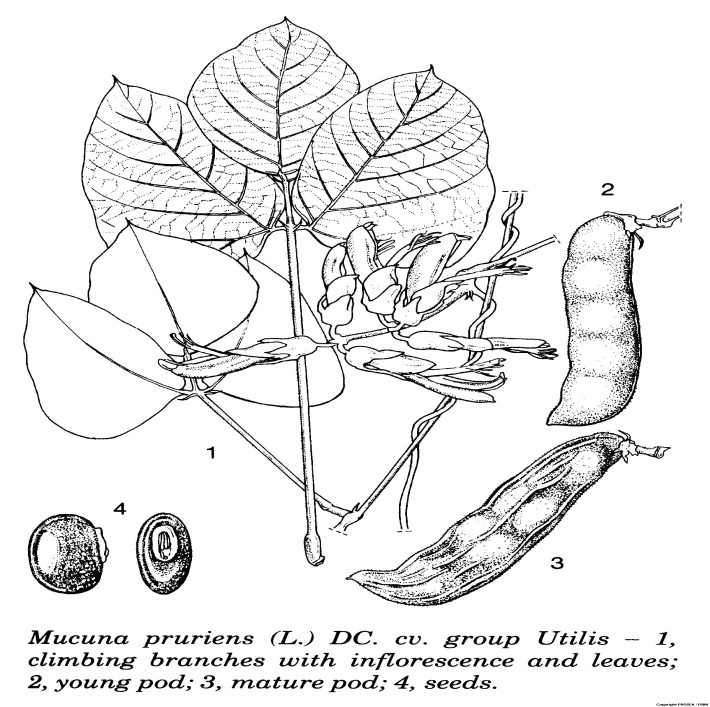
M. pruriens is an annual climbing shrub in the family Fabaceae. Mucuna pruriens is a vigorous, climbing, pubescent annual herb which can grow up to 2-18 m long. The stem is slender, cylindrical, slightly pubescent with white, straight, short and long hairs and nearly hairless.
The 3-foliolate leaves are arranged alternate. The stipules are 0.5 cm long, caducous, subulate, white-hairy outside and hairless inside. The petiole is (3-)4-9(-13.5) cm long, slightly grooved above and generally slightly pubescent. The rachis is (0.5-)1-2 cm long, grooved above and slightly pubescent. The stipels are slender. The lateral leaflets are conspicuously asymmetrical, obovate, rhombic, ovate or elliptical, measuring (5-)7-15(-19) cm x (3- )5-12( -17) cm and with symmetrical terminal leaflets. The apex is acute to acuminate-mucronate, rounded at base, and covered with appressed, grey or silvery hairs that turn black when dry.
The inflorescence is an axillary raceme, up to 32 cm long, 1-many-flowered and silvery pubescent. The tubercled rachis is without lateral branchlets. The bracts are 5-10 mm long, early caducous and narrowly triangular-elliptical. The pedicel is 1.5-10 mm long, and with two bracteoles measuring 10 mm x 2 mm near the base of the sepal. The sepal is bell-shaped, with tube 4-7 mm long, 5-lobed, appressed silvery pubescent outside and hairless inside. The upper pair of lobes is connate while the other 3 lobes are subequal, triangular, measure 3-9 mm long and acute. The petal is blackish-purple, pale lilac or white. It is clawed and auricled. The upper part of it is hood-shaped, much shorter than other petals, measuring 17-22 mm x 11-15 mm, fleshy especially towards the base and rounded at the top. The wings are narrowly obovate, measuring 32-35 mm x 8-10 mm, fleshy especially towards the base, rounded at the top and finely and patently pubescent at the base. The keel measures about 35 mm x 5 mm, narrow in the middle, entirely split dorsally, ciliolate at the edges, nearly hairless towards the top, ventrally split near the base and apex, with apical part hard and ending in a horny tip. The 10 stamens are in two bundles.
The fruit is an oblong, (1-)3(-7)seeded pod with oblique top, somewhat compressed laterally, slightly bulging over the seeds, measuring 4-13 cm x 1-2 cm and finely pubescent with white to light brown hairs. The valves are thick and leathery, with prominent, complete rib and with 2-3 partial and less prominent ribs.
The seed is oblong-ellipsoid, somewhat laterally compressed, measuring about 15 mm x 10 mm x 5 mm and with variable colour. It is light or pinkish-brown with dark brown mosaic, mottled with grey, purple or black background, almost entirely black, grey, greyish-black or white. The hilum is oblong, lateral, eccentric, measures about 4 mm long, surrounded by a prominent, cream-coloured aril and with scale-like extension at the rim. Seedling is with hypogeal germination.
The roots are numerous, 7-10 m long and with many lateral taproots. [2]
Cultivation
Soil Suitability and Climate Requirement
Mucuna pruriens tolerates a wide range of annual rainfall from 400-3000 mm, but is not drought resistant because of its shallow root system. Only Mauritius bean shows better drought tolerance. M. pruriens grows best at an average annual temperature of 19-27°C. Plants are sensitive to frost and exposure to a temperature below 5°C for more than 24 hours is fatal even for cultivars from Florida. A night temperature of over 21°C is said to stimulate flowering. M. pruriens requires a high light intensity and yields poorly when intercropped with cassava or maize. [2]
It grows best on well-drained sand and clay soils and on ultisols with a pH of 5-6.5, but also grows vigorously on acidic sandy soils. It does not tolerate waterlogging. In soils with a fertile topsoil and an acidic subsoil, the latter being low in P and high in AI, root growth is concentrated in the topsoil. If a fertile topsoil is absent, an extensive root system develops even in acidic soils [2]
Field Preparation
Production of Planting Materials
Propogation is mostly by seed. Seed requires no scarification, but dry seed requires soaking in water for 24 hours. The germination rate of fresh seed is 90-100%, declining with time. Seed is stored in a cool dry place remained viable for about 2 years, but seed stored in a sealed jar for 3 months lost its viability. Germination takes 4-7 day. [2]
Field Planting
In South East Asia, sowing is done from January to May, at the onset of rainy season. Seed is placed 2 cm deep with 2-4 seeds per hole, For cover crops in rubber plantations in Indonesia and Malaysia, a spacing of 2m x 1m or 1.5m is recommended, requiring about 15 kg seed per ha. [2]
Field maintenance
Fertilisation
When grown as a cover crop in tree plantations, velvet bean is mostly grown in combination with other cover crops, because of tis short life span. Species commonly used in such combinations are Calopogonium mucunoides Desv. and C. caeruleum (Benth.) Sauv., Centrosema pubescens Benth. and Pueraria phaseoloides (Roxb.) Benth. [2]
Velvet bean is currently being tested in a number of cropping systems, mainly in combination with maize. It is either intercropped, relay planted 15-40 days after sowing of the maize, or grown in rotation. When grown as a group manure crop, velvet bean tends to become weedy when the seed is left to mature. In tests in Nigeria, mowing velvet bean before maturation of the seed followed by zero tillage planting of maize effectively solved this problem. [2]
Weed Control
After sowing, velvet bean requires 1-2 weedings. Hand weeding is most common, but both pre-and post emergence herbicides are applied effectively. [2]
Pest and Disease Control
Velvet bean is little affected by diseases, although in Zimbabwe, it is very susceptible to a vine rot of unknown cause that can wipe out the crop. It is resistant but not immune to root-know nematodes and is attacked by several other Meloidogyne spp. Very few insect and small mammals attack velvet bean possibly because of its high L-dopa content. The velvet bean caterpillar (Anticarsia gemmatalis) in Florida is one of the few insects reported to cause damage. In Malaysia, green bugs (Brachyplatys spp.) feed on the leaves. Striga gesneriodes (Willd.) Vatke parasites the velvet bean. [2]
Harvesting
The optimum time for harvesting velvet bean for green manure is at flower initiation, attained 55-145 days after sowing, depending on the cultivar. Plants are pulled up by hand or by hoe and buried in the soil. Grown for forage in the United States, it may be harvested 90-120 days after sowing. A cutiing interval of 5 weeks and cutting at a height of 30cm provide a reasonable yield of forage of adequate qulity. Harvesting for pod production ca start as soon as the pods start changing colour from green to dark brown or black: in Malaysia this is possible at 215-255 days after sowing. Pods are harvested by hand. When intercropped with maize, cutting velvet bean below the level of the maturing maize cobs facilitates harvesting the latter. [2]
Postharvest handling
Green manure should be buries in the soil immediately after the harvesting. If left todry above the ground, he nitrogen content may be reduces by as much as 50%. Dried pods are threshed with a regular grain thresher or by hand. In Java and Africa, threshing is done by beating the pods put n sacks. Only the best seed is used to make ‘temp’. It is washed and boiled for 2-3 hours. After cooling seeds are dehulled and soaked in ample water for 1-2 days, changing the water 2-3 times a day to ensure that all toxic substances have been removed. The cotyledons are then chopped into smaller pieces and steamed. When cool the beans are sieved and inoculated evenly with the fungus Rhizopus arrhizus or R. oryzae, flattened and wrapped in banana leaves or a similar material for 24-40 hours at 31degree Celcius. The product is a cake covered with mats of mycelium. It is consumed fried, or mixed with vegetables in a soup. [2]
Estimated cost of production
No documentation
Chemical Constituent
M. pruriens has been reported to contain L-dihydroxyphenylalanine (L-dopa, approximately 40 mg/g seed), alkaloids mucunine, mucunadine, mucuadinine, pruriendine and nicotine, β-sitosterol, glutathione, lecithin oils, venolic and gallic acids, D-chiro inositol, tryptamine, alkylamines, steroids, flavonoids, coumarins, cardenolides. [3-8]
Plant Part Used
Bean/Seed [7]
Traditional Use
In Central America, M. pruriens is used to make a beverage by grinding and roasting in a process similar to that of making coffee. It is also used as a food and cooked as a vegetable. The medicinal uses range broadly depending upon the region and tribal traditions. These applications include preparations to treat nervous disorders, parasites, intestinal disturbances and impotence [9]. Other reported uses include asthma, Parkinson’s disease, menstrual complaints pleurisy and ringworm [10].
Preclinical Data
Pharmacology
In laboratory animal studies, M. pruriens has been reported to possess antiparkinson and neuroprotective effects in Parkinson’s disease [11] M. pruriens contains L-dopa, and L-dopa is used to make dopamine, the neurochemical involved in behavior and cognition, voluntary movement, motivation and reward, inhibition of prolactin, sleep, mood, attention and learning. M. pruriens also has antioxidant properties, which can lead to neuroprotection [12][13]. A laboratory study found that M. pruriens chelates divalent copper ions in a dose-dependent manner, with the copper chelating property probably one of the mechanisms by which M. pruriens exerts its neuroprotective effects [14].
M. pruriens cotyledon powder (MPCP) has shown antiparkinson and neuroprotective effects in animal models of Parkinson’s disease that is superior to synthetic levodopa. In the present study two different doses of MPCP protected both plasmid DNA and genomic DNA against levodopa and divalent copper-induced DNA strand scission and damage. [11][14]
One of the traditional uses of M. pruriens is in diabetes and blood sugar imbalances. Laboratory animal studies support the use in blood sugar regulation through improving plasma glucose levels, helping prevent polyuria and helping decrease urinary albumin levels [15][16][17]. High levels of trace elements like manganese and zinc along with lecithin are found in the seeds and may be a possible link to the ability of M. pruriens to regulated blood sugar [18]. A study found that M. pruriens contains D-chiro-inositol, which also may explain the hypoglycemic effects of M. pruriens seeds [7].
M. pruriens is also used traditionally for sexual health and as an aphrodisiac. Laboratory animal studies support this claim, with M. pruriens improving sexual desire [19].
M. pruriens has been reported traditionally to be used in snake bites and is supported in these uses by laboratory studies. Several laboratory animal studies report that pretreatment of rats with M. pruriens seed extract helps protect them against snake venom poisoning, including vipers and cobras. Potential uses for M. pruriens in this area include the production of anti-Velvet bean antibodies that could be used in the antiserum therapy of various poisonous snake bites. [20][21].
Clinical Data
Clinical findings
A human study of 60 individuals with Parkinson’s disease found that administration of M. pruriens significantly reduced symptoms of the disease [22]. Another study in 8 individuals with Parkinson’s disease found that administration of 30grams M. pruriens (not standardized) decreased dyskinesias (involuntary movements) faster than the synthetic drug levodopa [23].
Human studies also support the use of M. pruriens for sexual health and vitality. A study in infertile men found that treatment with M. pruriens significantly inhibited lipid peroxidation, elevated spermatogenesis, and improved sperm motility. Treatment also recovered the levels of total lipids, triglycerides, cholesterol, phospholipids, and vitamin A, C, and E and corrected fructose in seminal plasma of infertile men. The authors concluded that M. pruriens may play a role as a restorative and invigorating agent for infertile men. [24]
Another study of 60 men in a fertility study suffering from psychological stress found that M. pruriens (5 g daily of seed powder) significantly decreased psychological stress and seminal plasma lipid peroxide levels along with improved sperm count and motility. Treatment with M. pruriens also restored the levels of SOD (serum oxide dismutase), catalase, GSH (glutathione) and ascorbic acid in seminal plasma of infertile men. The authors concluded that M. pruriens may improve sexual health in infertile men under chronic stress by reactivating the anti-oxidant defense system, improving semen quality and by helping in the management of chronic stress. [25]
A study of 75 healthy, fertile men compared to 75 men undergoing fertility screening treatment with M. pruriens significantly improved testosterone levels, luteinizing hormone (LH), dopamine, adrenaline, and noradrenaline levels in infertile men and reduced levels of follicle stimulating hormone (FSH) and prolactin. M. pruriens also improved sperm count and sperm motility in infertile men [26].
Mechanisms of action in sexual health include anti-oxidant support, nutritional value and activity on the hypothalamus-pituitary-gonadal axis (including inhibition of prolactin secretion) [26][27].
Precautions
Use with caution in individuals with an increased risk of prostate cancer or those having prostate disease [26].
Side effects
Discontinue if allergy occurs. Hairs on M. pruriens flowers and pods have been reported to cause severe pruritus (itching) [28].
Avoid in individuals with psychosis or schizophrenia, as M. pruriens has been reported to cause acute toxic psychosis [29].
Pregnancy/Breast Feeding
Avoid in pregnant or breastfeeding as M. pruriens may inhibit prolactin secretion [27].
Interaction & Depletion
Interaction with drug
Based on laboratory studies, use only under the supervision of a doctor if taking anticoagulant medications, such as aspirin or warfarin (Coumadin). [30]
Poisonous Management
No documentation
Line Drawing
References
- The Plant List. Ver1.1. Mucuna pruriens (L.) DC. c2013 [updated 2010 July 14; cited 2016 May 3]. Available from: http://www.theplantlist.org/tpl1.1/record/ild-2863
- Wulijarni-Soetjipto N, Maligalig RF. Mucuna pruriens (L.) DC. cv. group Utilis. In: Faridah Hanum I, van der Maesen LJG, editors. Plant Resources of South-East Asia No. 11: Auxiliary plants. Leiden, Netherlands, Backhuys Publisher, 1997; p. 199-203
- Ghosal S, Singh S, Bhattacharya SK. Alkaloids of Mucuna pruries chemistry and pharmacology. Planta Med. 1971;19(3):280-284.
- Panikkar KR, Majella VL, Pillai PM. Lecithin from Mucuna pruriens. Planta Med. 1987;53(5):503.
- Prakash D, Niranjan A, Tewari SK. Some nutritional properties of the seeds of three Mucuna species. Int J Food Sci Nutr. 2001;52(1):79-82.
- Rajyalakshmi P, Geervani P. Nutritive value of the foods cultivated and consumed by the tribals of south India. Plant Foods Hum Nutr. 1994;46(1):53-61.
- Donati D, Lampariello LR, Pagani R, Guerranti R, Cinci G, Marinello E. Antidiabetic oligocyclitols in seeds of Mucuna pruriens. Phytother Res. 2005;19(12):1057-1060.
- Vadivel V, Janardhanan K. Nutritional and anti-nutritional composition of velvet bean: An under-utilized food legume in south India. Int J Food Sci Nutr. 2000;51(4):279-287.
- Taylor L. The healing power of rainforest herbs: A guide to understanding and using herbal medicinals. New York: Square One Publishers, 2005; p. 344.
- Duke JA. Medicinal plants of Latin America. New York: Taylor and Francis, 2009; p. 413.
- Manyam BV, Dhanasekaran M, Hare TA. Neuroprotective effects of the antiparkinson drug Mucuna pruriens. Phytother Res. 2004;18(9):706-712.
- Dhanasekaran M, Tharakan B, Manyam BV. Antiparkinson drug–Mucuna pruriens shows antioxidant and metal chelating activity.Phytother Res. 2008;22(1):6-11.
- Tripathi YB, Upadhyay AK. Effect of the alcohol extract of the seeds of Mucuna pruriens on free radicals and oxidative stress in albino rats. Phytother Res. 2002;16(6):534-538.
- Tharakan B, Dhanasekaran M, Mize-Berge J, Manyam BV. Anti-Parkinson botanical Mucuna pruriens prevents levodopa induced plasmid and genomic DNA damage. Phytother Res. 2007;21(12):1124-1126.
- Rathi SS, Grover JK, Vats V. The effect of Momordica charantia and Mucuna pruriens in experimental diabetes and their effect on key metabolic enzymes involved in carbohydrate metabolism. Phytother Res. 2002;16(3):236-243.
- Grover JK, Vats V, Rathi SS, Dawar R. Traditional Indian anti-diabetic plants attenuate progression of renal damage in streptozotocin induced diabetic mice. J Ethnopharmacol. 2001;76(3):233-238.
- Bhaskar A, Vidhya VG, Ramya M. Hypoglycemic effect of Mucuna pruriens seed extract on normal and streptozotocin-diabetic rats. Fitoterapia. 2008;79(7-8):539-543.
- Akhtar MS, Qureshi AQ, Iqbal J. Antidiabetic evaluation of Mucuna pruriens, Linn seeds. J Pak Med Assoc. 1990;40(7):147-150.
- Suresh S, Prithiviraj E, Prakash S. Dose- and time-dependent effects of ethanolic extract of Mucuna pruriens Linn. seed on sexual behaviour of normal male rats. J Ethnopharmacol. 2009;122(3):497-501.
- Aguiyi JC, Guerranti R, Pagani R, Marinello E. Blood chemistry of rats pretreated with Mucuna pruriens seed aqueous extract MP101UJ after Echis carinatus venom challenge. Phytother Res. 2001;15(8):712-714.
- Tan NH. The protective effect of Mucuna pruriens seeds against snake venom poisoning. J Ethnopharmacol. 2009;123(2):356-358.
- An alternative medicine treatment for Parkinson’s disease: Results of a multicenter clinical trial. HP-200 in parkinson’s disease study group. J Altern Complement Med. 1995;1(3):249-255.
- Katzenschlager R, Evans A, Manson A, et al. Mucuna pruriens in Parkinson’s disease: A double blind clinical and pharmacological study. J Neurol Neurosurg Psychiatry. 2004;75(12):1672-1677.
- Ahmad MK, Mahdi AA, Shukla KK, Islam N, Jaiswar SP, Ahmad S. Effect of Mucuna pruriens on semen profile and biochemical parameters in seminal plasma of infertile men. Fertil Steril. 2008; 90(3):627-635.
- Shukla KK, Mahdi AA, Ahmad MK, et al. Mucuna pruriens reduces stress and improves the quality of semen in infertile men. Evid Based Complement Alternat Med. 2010;7(1):137-144.
- Shukla KK, Mahdi AA, Ahmad MK, et al. Mucuna pruriens improves male fertility by its action on the hypothalamus-pituitary-gonadal axis. Fertil Steril. 2009;92(6):1934-1940.
- Vaidya RA, Aloorkar SD, Sheth AR, Pandya SK. Activity of bromoergocryptine, Mucuna pruriens and L-dopa in the control of hyperprolactinaemia. Neurol India. 1978;26(4):179-182
- Davidson S. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27(37):10007-10014.
- Infante ME. Outbreak of acute toxic psychosis attributed to Mucuna pruriens. Lancet. 1990;336(8723):1129.
- Aguiyi JC, Guerranti R, Pagani R, Marinello E. Blood chemistry of rats pretreated with Mucuna pruriens seed aqueous extract MP101UJ after Echis carinatus venom challenge. Phytother Res. 2001;15(8):712-714.


