Dukung anak Shoot
Phyllanthus amarus Schumach. & Thonn.
Phyllanthaceae
DEFINITION
Dukung anak shoot consists of the powder of dried shoot of Phyllanthus amarus Schumach. & Thonn. (Phyllanthaceae).
SYNONYM
Diasperus nanus (Hook.f.) Kuntze, Phyllanthus amarus subsp. amarus, Phyllanthus amarus var. baronianus Leandri, Phyllanthus nanus Hook.f., Phyllanthus niruri var. amarus (Schumach. & Thonn.) Leandri, Phyllanthus niruri var. genuinus Beille, Phyllanthus niruri var. scabrellus (Webb) Müll.Arg., Phyllanthus scabrellus Webb, Phyllanthus swartzii Kostel
[ 1 ].
VERNACULAR NAMES
Carry me seed, black catnip, stone breaker, seed on the leaf, child-pick-a-back (English); dukung anak, meniran, rami buah (Malay); zen chu cao, ye xia zhu (China); keela nelli, kilanelli (Tamil) [ 2 , 3 , 4 , 5 ].
CHARACTER
| Colour | Brownish-green |
| Odour | Slight odour |
| Taste | Bitter taste |
IDENTIFICATION
Plant Morphology
Phyllanthus amarus is a herb and seen in moist deciduous, in plains and forest plantations. Leaves are membranous; usually glaucous beneath; usually broadly obtuse at apex; very variable in size, but usually under 0.5 inches long; elliptic-obovate or oblong; prominently distichous so that the branchlets resemble pinnate leaves; stipules not peltate, lanceolate and oblong, 1 mm, subsessile, scarious. Flowers are monoecious, male and female flowers occur solitarily or in clusters of 2 or 3 in axils of lower leaves of a branch, bracteate, subsessile, actinomorphic, hypogynous, cyclic, minute, green; petals 5, ovate, valvate, a glandular disk is present within the perianth, each lobe has a distinct green midrib; stamens 5, exerted, monadelphous, filaments connate in a column with three bithecous anther lobe borne at its tip, anthers sessile, introse, dehiscing by slits; carpel 3, syncarpous, ovary superior, trilocular, each loculus with two ovules, placentation axile, ovary globular; styles 3, short, not dilated, bilobed, at maturity ovary becomes hexalocular. Fruits are capsule dry, dehiscent, more or less verrucose, and glandular. Seeds are regular lines of very minute tubercles joined by minute crossbars, muriculate, triquetrous [ 3 ].
Microscopy
Powdered material consists of rod calcium oxalate crystals, spiral vessels, straight to curve anticlinal wall on adaxial epidermis, sinuous anticlinal wall on abaxial epidermis, anomocytic stomata, collenchyma cells, mesophyll sponge with air spaces, pollen grains, macrosclereids, starch grains, druses, brachysclereids, idioblast cells and tanniferous idioblast cells.
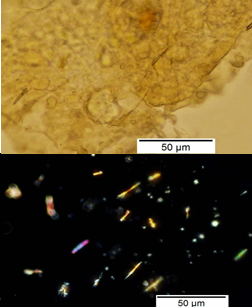
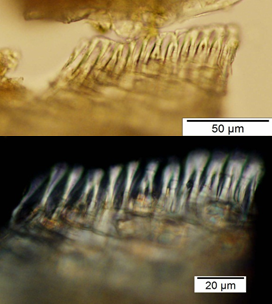
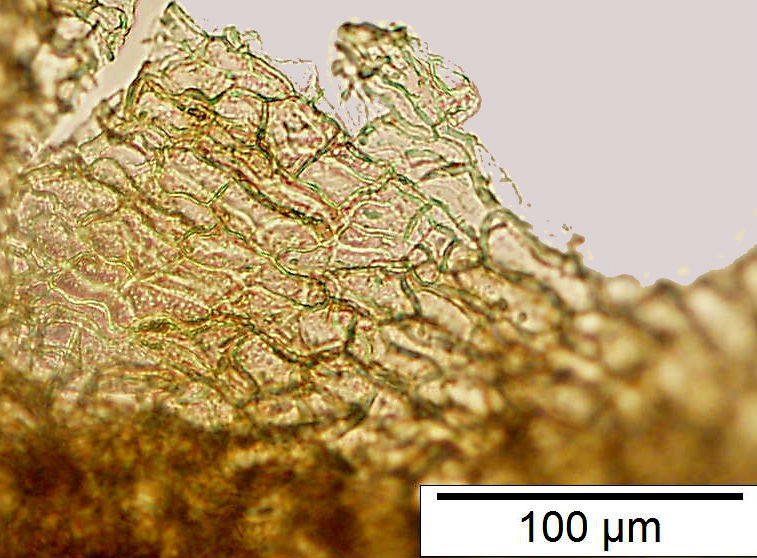

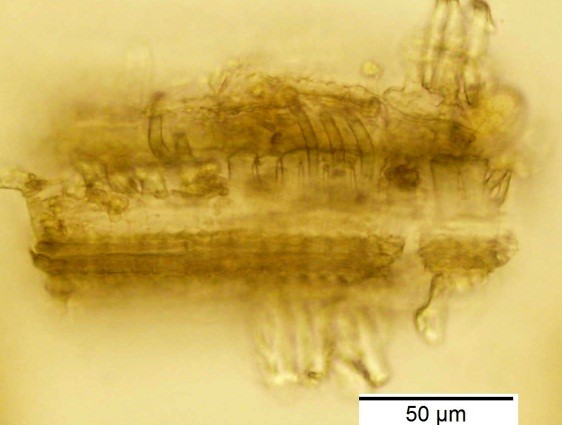
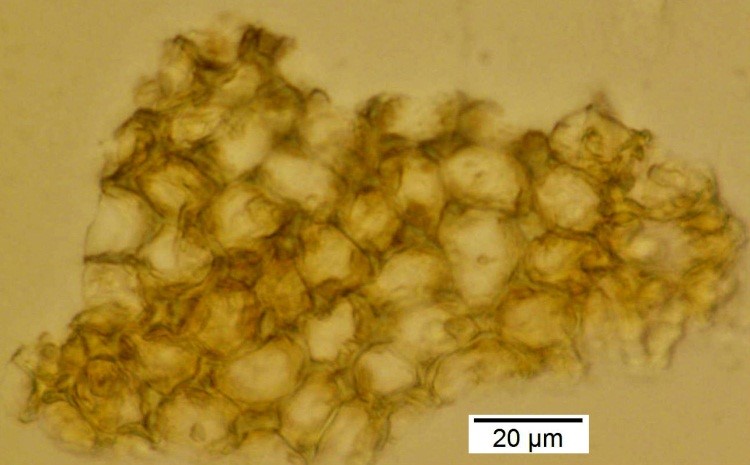

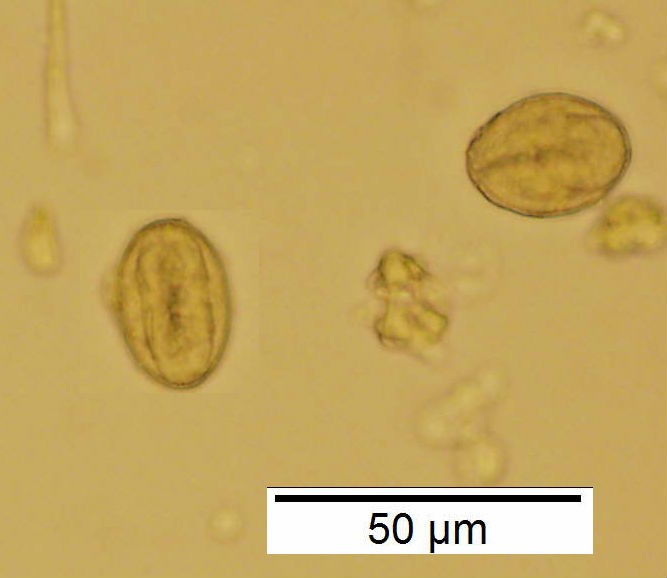





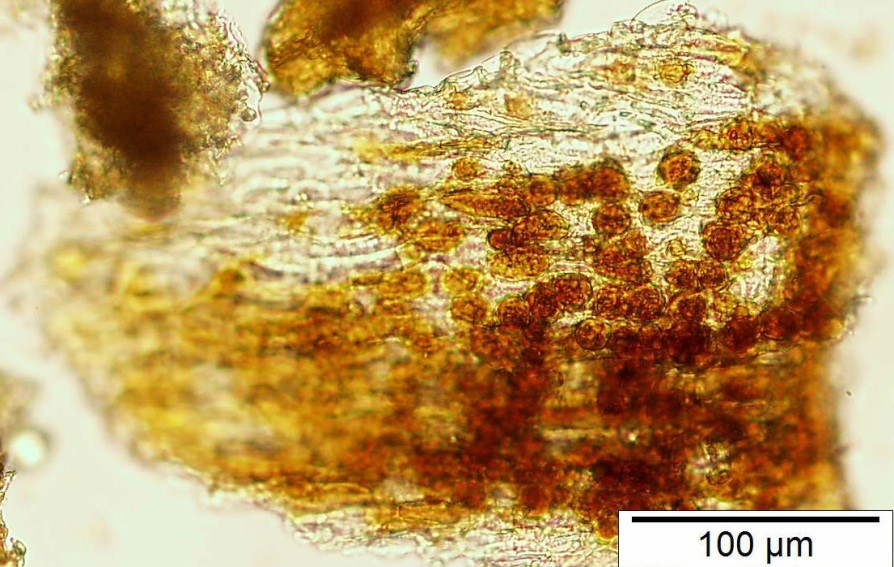
Figure 2 : Microscopic characters of dried P. amarus shoot powder of 0.106 mm size. (a) Rod calcium oxalate crystals and under polarized filter (magnification 400x); (b) spiral vessel (magnification 400x and 600x); (c) straight to curve anticlinal wall on adaxial epidermis (magnification 200x); (d) sinuous anticinal wall on abaxial epidermis and anomocytic stomata (magnification 400x); (e) spiral vessel (magnification 400x); (f) collenchyma cell (magnification 600x); (g) mesophyll sponge with air spaces (magnification 200x); (h) pollen grain (magnification 400x); (i) macrosclereid (magnification 400x); (j) leaf fragment with clusters of starch grain (magnification 200x); (k) druses (magnification 400x); (l) brachysclereid (magnification 600x); (m) idioblast cell (magnification 400x); (n) tanniferous idioblast cells (magnification 200x). [Scale bars: b, f, l = 20 µm; a, b, d, e, h, i, k, m = 50 µm; c, g, j, n = 100 µm].
Chemical Tests
Observation of solution after treatment with various reagents:
| Test for the presence of steroids | Bluish green |
| Test for presence of phenolics (tannins) | Bluish green |
Thin Layer Chromatography (TLC)
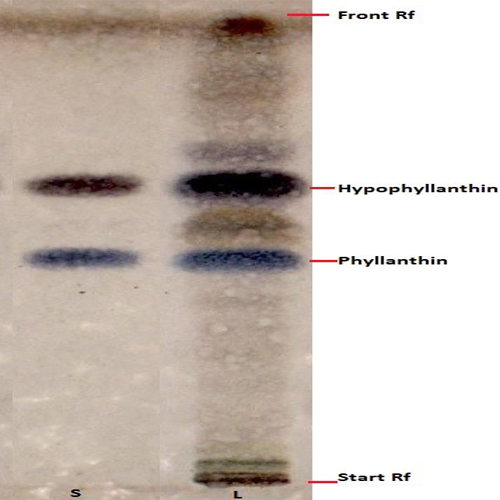
Figure 3 : HPTLC chromatogram mixture of phyllanthin and hypophyllanthin (S) and ethanol extract of dried P. amarus shoot powder (L) observed under visible light after derivatisation.
| Test solution | Weigh about 5.0 g of dried P. amarus shoot powder in a screw-capped conical flask. Add 50 mL of ethanol into the flask and shake the mixture for 30 min and filter. Evaporate the filtrate to dryness over a water bath. Reconstitute with 5 mL of methanol. Filter the mixture through a 0.45 μm syringe filter and use the filtrate as the test solution. |
| Standard solution | Dissolve mixture of phyllanthin and hypophyllanthin standard in methanol to produce phyllanthin and hypophyllanthin standard concentration 1.0 mg/mL. |
| Stationary phase | HPTLC glass silica gel 60 F254, 20 x 10 cm |
| Mobile phase | Toluene : ethyl acetate; (2 : 1) (v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
High Performance Liquid Chromatography (HPLC)
| Test solution | Weigh about 5.0 g of dried P. amarus shoot powder in a screw-capped conical flask. Add 50 mL of ethanol into the flask and shake the mixture for 30 min and filter. Evaporate the filtrate to dryness over a water bath. Reconstitute with 5 mL of methanol. Filter the mixture through a 0.45 μm syringe filter and inject into HPLC column. |
| Standard solution | Dissolve mixture of phyllanthin and hypophyllanthin standard in methanol to produce phyllanthin and hypophyllanthin standard solution at concentration of 0.5 mg/mL each. |
| Chromatographic system | Detector : UV 230 nm
Column : C8 4.6 X 150 mm, 5 µm (SP95) (Zorbax Eclipse Plus, if necessary) Column oven temperature : Ambient Flow rate : 1 mL/min Injection volume : 20 µL |
| Mobile phase (isocratic mode) | Acetonitrile : water; (45 :55) (v/v) |
| Run time | 25 min |
| System suitability requirements |
Perform at least five injections of mixture of phyllanthin (0.5 mg/mL) and hypophyllanthin (0.5 mg/mL) standard solution. The requirements of the system suitability parameters are as follow:
|
| Acceptance criteria |
|
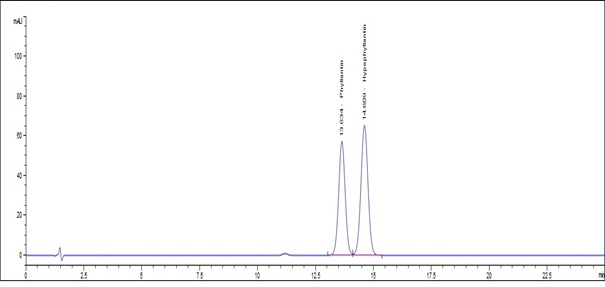
(a)
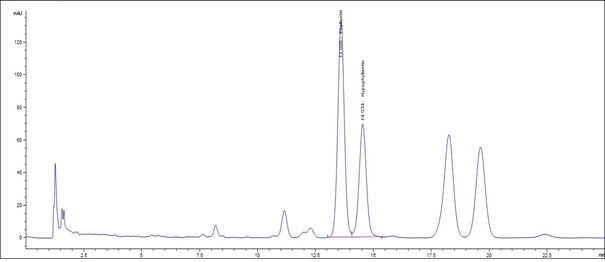
(b)
Figure 4 : Whole HPLC chromatogram of (a) a mixture of phyllanthin and hypophyllanthin standard solution (0.5 mg/mL) at tr = 13.634 min for phyllanthin and tr = 14.609 min for hypophyllanthin and (b) ethanol extract of dried P. amarus shoot powder showing peak corresponding to the mixture standard solution at tr = 13.595 min for phyllanthin and tr = 14.534 min for hypophyllanthin.
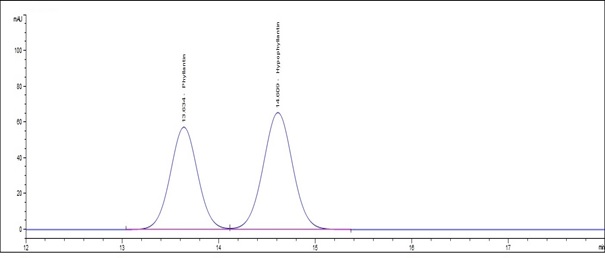
(a)
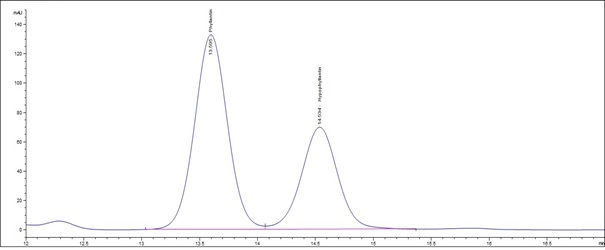
(b)
Figure 5 : HPLC chromatogram highlighting the elution region of phyllanthin and hypophyllanthin in (a) a mixture of phyllanthin and hypophyllanthin standard solution (0.5 mg/mL) at tr = 13.634 min for phyllanthin and tr = 14.609 min for hypophyllanthin and (b) ethanol extract of dried P. amarus shoot powder showing peak corresponding to the mixture of standard solution at tr = 13.595 min for phyllanthin and tr = 14.534 min for hypophyllanthin.
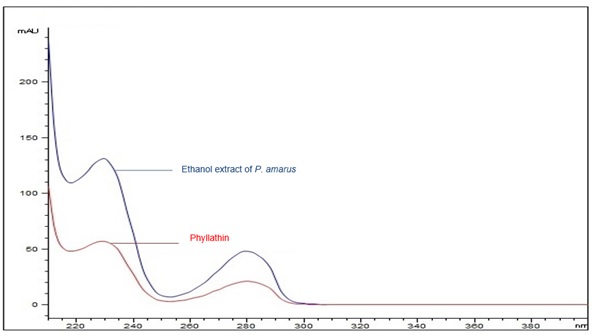
Figure 6 : UV spectrum of phyllanthin standard solution (0.5 mg/mL) and ethanol extract of dried P. amarus shoot powder.
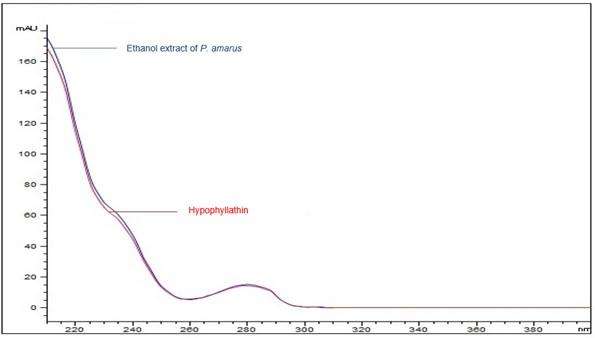
Figure 7 : UV spectrum of hypophyllanthin standard solution (0.5 mg/mL) and ethanol extract of dried P. amarus shoot powder.
PURITY TESTS
The purity tests except foreign matter test are based on P. amarus dried shoot powder of 0.106 mm particle size.
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 10% |
| Acid-insoluble ash | Not more than 2% |
| Water soluble ash | Not less than 2% |
| Loss on Drying |
| Not more than 17% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 12% |
| Cold method | Not less than 11% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 1% |
| Cold method | Not less than 1% |
SAFETY TESTS
The safety tests are based on P. amarus dried shoot powder of 0.106 mm particle size.
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 1.0mg/kg Not more than 0.3mg/kg* *in finished product |
| Microbial Limits | |
| Total bacterial count | Not more than 105cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Methanol extract of dried and fresh P. amarus whole plant was found to contain lignans (eg. phyllanthin and hypophyllanthin) [ 6 , 7 , 8 , 9 ].
Ethanol (99%) extract of dried P. amarus leaves was found to contain phenol (eg. 3,5-di-t-butylphenol), esters (eg. methyl,14-methyl pentadecanoate, 10-octadecanoate, glycerol 1,3-dipalmitate, dioctyl ester, heptanoic acid (9-dece-1-yl ester)), fatty acid (eg. palmitic acid), aldehyde (eg. 9-hexadecenal) and fatty alcohol (eg. 2,13-octadecadiene-1-ol) [ 10 ].
Ethanol extract of dried P. amarus aerial part was found to contain lignan (eg. phyllanthin) [ 11 ].
Ethanol extract of dried P. amarus whole plant was found to contain alicyclic hydrocarbon (eg. pentyl-cyclopentane), monoamine alkaloids (eg. 2-methoxyphenanthylamine, 2-methoxy-α-methyl-4,5-(methylenedioxy)-phenethylamine) and others (eg. 3-[3-[1-aziridinyl]propoxyl]-2,5-dimethylpyrazine, 3-(cyclopropylamino) propionitrile, 1,2-dimethoxy-4-[[(4-methylphenyl)sulfonyl]methyl]-benzene, 4-(5-hydroxy methylfuffur-2-ylidenamino)-1,5-dimethyl-2-phenyl pyrazol-3(2H)-one) [ 12 ].
Acetone extract of dried P. amarus whole plant was found to contain amine (eg. cycloheptylamine), α-amino acid (eg. phenylalanine), monoamine alkaloid (eg. 2-methoxy-α-methyl-4,5-(methlenedioxy)-phenethylamine) and others (eg. hexahydro 2(3H)-cyclopent(e)-1,3-oxazin-2-one, cyclooctanamine, dihydro-4,4-dimethyl-2H-pyran-2,6(3H)-dione, hex-5-enylamine, 5,8,11,14-satetrayonic acid, 4-methoxy-N2-(2-bromo-5-(2-propynyloxy)benzylideno) benzhydrazine) [ 12 ].
Essential oil of fresh P. amarus shrubs was found to contain monoterpenes (eg. myrcene, p-cymene, nerol), cyclic terpene (eg. limonene), monoterpenoids (eg. 1,8-cineole, geraniol), aldehydes (eg. (Z)-3-hexenal, octanal, nonanal, benzaldehyde), terpene (eg. γ-terpinene), alkane hydrocarbon (eg. tridecane), fatty alcohols (eg. (Z)-3-hexenol, octanol, nonanol, tetradecanol), alkane hydrocarbons (eg. pentadecane, hexadecane, heptadecane, octadecane, tetracosane), terpenoid (eg. camphor), terpene alcohol (eg. linalool), monoterpene alcohol (eg. α-terpineol), bicyclic sesquiterpene (eg. β-caryophyllene), polycyclic hydrocarbons (eg. naphthalene, 1-methylnaphthalene, 2-methylnaphthalene, dimethyl naphthalene), sesquiterpene (eg. (E)-nerolidol), acyclic alkane (eg. heneicosane), sesquiterpenoid alcohols (eg. t-cadinol, α-cadinol), monoterpene phenol (eg. thymol), fatty acid esters (eg. methyl hexadecanoate, methyl octadecanoate), tricyclic diterpene (eg. pimaradiene), acyclic diterpene alcohol (eg. phytol), alkylphenol (eg. nonyl phenol isomer) and others (eg. 1,2,4-trimethylbenzene, 2,2,6-trimethylcyclohexanone, 2,5-dimethylpyrazine, trans-linalool oxide, 1-octen-3-ol, cis-linalool oxide, β-cyclocitral, neoisomenthol, safranal, salicylaldehyde, β-chamigrene, 1-heptadecene, α-muurolene, 1,2-dihydro-1,1,6-trimethyl naphthalene, trans-linalool oxide, β-bazzanene, α-ionone, isoamyl benzoate, neophytadiene isomer I, (E)-β-ionone, neophytadiene isomer II, benzothiazole, 1-ethylnaphthalene, neophytadiene isomer III, (E)-β-ionone-5,6-epoxide, eicosane, pentadecanal, α-corocalene, hexyl benzoate, hexahydrofarnesyl acetone, (Z)-3-hexenyl benzoate, 3,4-dimethyl-5-pentylidene-2(5H)-furanone, 2,6-diisopropylnaphthalene, docosane, isophytol, tricosane, farnesyl acetone, pentacosane, heptacosane, octacosane, nonacosane) [ 13 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally, the infusion of P. amarus is used as tonic and considered diuretic. The infusion of the stem and leaves is also used as eye drops to treat eye infection. Meanwhile, the decoction of the aerial parts is used to treat gonorrhea, diarrhea, dysentery, stomach-ache, pain in the sides, haemorrhoids, absence of menstruation and externally applied to treat skin problems. Leaf sap is applied as ear drops to treat inflammation of the ear and applied to boils, sores and wounds. Plant decoction is taken to facilitate childbirth, to treat oedema, fever, sore throat and jaundice [ 14 ].
Biological and pharmacological activities supported by experimental data
Antinociceptive activity
Hydroalcoholic (50% ethanol-water) extract of P. amarus aerial (0.3, 3 and 30 mg/kg) was administered intraperitoneally to male Swiss mice (25 – 35 g) 30 min before induction of acetic acid to induce abdominal constriction. The extracts showed significant (p < 0.01) reduction in the number of abdominal constrictions in dose dependent manner from (0.3 mg/kg: 25.2 ± 1.7; 3 mg/kg: 7.2 ± 1.9; 30 mg/kg: 1.0 ± 0.8) [ 15 ].
Hydroalcoholic (50% ethanol-water) extract of P. amarus aerial (30 mg/kg) was administered intraperitoneally to male Swiss mice (25 – 35 g) 30 min before induction of formalin. After 15 min, the extract showed significant (p < 0.01) inhibition in licking activity (27.5 ± 1.1) using formalin test [ 15 ].
Anticonvulsant activity
Aqueous extract of P. amarus aerial powder (70 mg/kg) was administered orally to Wistar albino rats of either sex (150 – 200 g) daily for 10 days and one hour before induction of convulsions (at 10th day). No hind limb extension (0 ± 0.000 sec) was recorded compared 6to normal saline treated group (14.441 ± 0.643 sec) and phenytoin treated group (0 ± 0.000 sec) using maximal electroshock (MES) seizure [ 16 ].
Aqueous extract of P. amarus aerial powder (70 mg/kg) was administered orally to Wistar albino rats of either sex (150 – 200 g) daily for 10 days and one hour before induction of convulsions (at 10th day). The extract showed significant (p < 0.001) decreased in the mean duration of convulsions (1.01 ± 0.81 sec) compared to normal saline treated group (12.161 ± 0.55 sec) and sodium valproate treated group (1 ± 0.65 sec) using pentylene tetrazole (PTZ) induced convulsion [ 16 ].
Antidiabetic activity
Aqueous extract of P. amarus dried whole plant (500 and 1000 mg/kg) was administered orally by gastric intubation to male alloxan-induced diabetic Wistar rats (200 – 250 g) for duration of 15 days. After 15 days, the extracts showed significant decreased in the blood glucose level from day 1 (500 mg/kg: from 337.14 ± 33 mg/dL to 210.28 ± 39 mg/dL; 1000 mg/mL: from 341.57 ± 35.30 mg/dL to 264.5 ± 35.6 mg/dL) compared to metformin treated group (from 299.5 ± 18.34 mg/dL to 225 ± 35.67 mg/dL) [ 17 ].
Hydroalcoholic extract of P. amarus dried whole plant (1000 mg/kg) was administered orally by gastric intubation to male alloxan-induced diabetic Wistar rats (200 – 250 g) for duration of 15 days. After 15 days, the extract showed significant decreased in the blood glucose level from day 1 (from 341.57 ± 35.30 mg/dL to 264.5 ± 35.6 mg/dL) compared to metformin treated group (from 299.5 ± 18.34 mg/dL to 225 ± 35.67 mg/dL) [ 17 ].
Aqueous extract of P. amarus dried aerial powder (150, 300 and 600 mg/kg) was administered daily to male Swiss mice (25 – 30 g) for duration of 21 days. The extracts showed decreased in fasting plasma glucose level (150 mg/kg: 4 mmol/L; 300 mg/kg: 3 mmol/L; 600 mg/kg: 1.5 mmol/L) compared to normal saline treated group (~6.5 mmol/L) [ 18 ].
Anti-inflammatory activity
Hexane extract of P. amarus whole plant powder (300 mg/kg) was administered orally to male Swiss mice (25 – 35 g) one hour before induction of paw edema using carrageenan. After 120 min, the extract inhibited the paw edema volume (42 ± 7%) compared to aspirin (47 ± 5%) [ 19 ].
Hepatoprotective effect
Aqueous extract of P. amarus dried aerial (50 and 75 mg/kg) was administered orally to male Wistar rats (180 – 200 g; aged 6-8 weeks old) 24 hours before induction of toxicity using ethanol. The extracts showed decreased in the aspartate aminotransferase (AST) level (50 mg/kg: 51.9 ± 1.3 U/I; 75 mg/kg: 50.0 ± 2.9 U/I) compared to silymarin treated group (52.4 ± 2.4 U/I). The extracts also showed decreased in the alanine aminotransferase (ALT) level at the highest dose with 19.1 ± 0.7 U/I comparable with silymarin treated group (19.9 ± 0.7 U/I) [ 20 ].
Aqueous extract of P. amarus dried aerial (75 mg/kg) was administered orally to male Wistar rats daily for seven days after 21 days of ethanol toxicity. The extract showed decreased in the AST level (47.3 ± 2.3 U/I) and ALT level (19.7 ± 0.9 U/I) compared to silymarin treated group (AST: 44.3 ± 1.8 U/I ; ALT: 18.7 ± 1.0 U/I) [ 20 ].
Antioxidant activity
Hot water extract of P. amarus oven-dried aerial powder showed antioxidant activity with 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical with inhibition of concentration at 50% (IC50) of 0.169 ± 0.005 mg/mL compared to fresh aerial powder (0.154 ± 0.012 mg/mL) using DPPH assay [ 21 ].
Hot water extract of P. amarus sun-dried aerial powder showed antioxidant activity with 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical with inhibition of concentration at 50% (IC50) of 0.141 ± 0.009 mg/mL compared to fresh aerial powder (0.154 ± 0.012 mg/mL) using DPPH assay [ 21 ].
Antimicrobial activity
Ethanol (80%) extract of P. amarus dried aerial showed inhibitory activity against Gram-postive, Bacilus subtilis with minimum inhibitory concentration of 1 mg/mL, Enterococcus faecalis (0.25 mg/mL) and Staphylococcus epidermidis (1 mg/mL) comparable with ciproflocaxin (B. subtilis: 2 mg/mL, E. faecalis and S. epidermidis: 1 mg/mL) using microdilution method [ 22 ].
Ethanol extract of P. amarus dried leaves showed inhibitory activity against Salmonella typhi with inhibition zone of 8.3 mm comparable with standard ciprofloxacin (9.0 mm) using disc diffusion method [ 23 ].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
24-hr toxicity study
Aqueous extract of P. amarus aerial powder administered orally at a single dose to Wistar albino rats of either sex (150 – 200 g) showed no toxicity effect and mortality for a period of 24 hours with lethal dose at 50% (LD50) > 2000 mg/kg [ 24 ].
48-hr toxicity study
Aqueous extract of P. amarus dried leaves powdered (2000, 4000 and 8000 mg/kg) administered orally at a single dose to female Albino rats (70 – 230 g) showed no signs or mortality after 48 hours with LD50 > 8000 mg/kg [ 25 ].
28-days toxicity study
Aqueous extract of P. amarus dried leaves powdered (2000 and 4000 mg/kg) was administered once in three days for 28 days. The rats were sacrificed at the end of 28th day. No significant changes in blood levels of alanine aminotransferase and aspartate aminotransferase was recorded. No adverse clinical signs and mortality was observed in all rats during 28 days of treatment [ 25 ].
Others (Adverse reactions, contraindications, side effects, warning, precautions)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The Plant List 2013. [Internet] Phyllanthus amarus Schumach. & Thonn; 2012 [cited on 24 January 2017]. Available from: http://www.theplantlist.org/tpl1.1/record/kew-153315.
- Malaysian Herbal Monograph. Vol. 2. Kuala Lumpur: Forest Research Institute Malaysia (FRIM). 2009; 106-113.
- Ramadasan Kuttan, K. B. Harikumar. Traditional Herbal Medicines for Modern Times Phyllanthus Species Scientific Evaluation and Medicinal Applications. Boca Raton. Taylor & Francis Group. 2012; 25.
- Musa Y, Zaharah A, Wan Zaki WM. Dukung Anak (Phyllanthus amarus). In: Penanaman Tumbuhan Ubatan dan Beraroma. (Musa Y, Muhammad Ghawas M, Mansor P. ed.) Serdang: MARDI. 2005: p.14-20.
- Multilingual Multiscript Plant Name Database. [Internet]. Phyllanthus amarus Schumach. & Thonn; 2012 [cited on 24 January 2017]. Available from: http://www.plantnames.unimelb.edu.au/Sorting/Phyllanthus.html.
- Srivastava P, Raut HN, Puntambekar HM, Desai AC. HPLC analysis of Phyllanthus amarus samples stored in stability chambers under different conditions and study of the effect on quantification of the phytomarkers phyllanthin and hypophyllanthin. Acta Chromatographica. 2015;27(1):147–156.
- Tripathi AK, Verma RK, Gupta AK, Gupta MM, Khanuja SPS. Quantitative determination of phyllanthin and hypophyllanthin in Phyllanthus species by high-performance thin layer chromatography. Phytochemical Analysis. 2006;17:394-397.
- Nayak PS, Upadhyay A, Dwivedi SK, Rao S. Quantitative determination of phyllanthin in Phyllanthus amarus by high performance thin layer chromatography. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas. 2010;9(5):353-358.
- Yuandani, Ilangkovan M, Jantan I, Mohamad HF, Husain K, Abdul-Razak AF. Inhibitory effects of standardized extracts of Phyllanthus amarus and Phyllanthus urinaria and their marker compounds on phagocytic activity of human neutrophils. Evidence-Based Complementary and Alternative Medicine. 2013;2013.
- Mamza UT, Sodipo OA, Khan IZ. Gas chromatography-mass spectrometry (GC-MS) analysis of bioactive components of Phyllanthus amarus International Research Journal of Plant Science. 2012;3(10): 208-215.
- Alvari A, Rafsanjani MSO, Ahmed FJ, Hejazi MS, Abdin MZ. Rapid RP-HPLC technique for the determination of phyllanthin as bulk and its quantification in Phyllanthus amarus International Journal of Phytomedicine. 2011;3:115-119.
- Arun T, Senthilkumar B, Purushothaman K, Aarthy A. GC-MS determination of bioactive components of Phyllanthus amarus (L.) and its antibacterial activity. Journal of Pharmacy Research. 2012;5(9):4767-4771.
- Moronkola DO, Ogunwande IA, Oyewole IO, Başer KHC, Ozek T, Ozek G. Studies on the volatile oils of Momordica charantia (Cucurbitaceae) and Phyllanthus amarus Sch. et. Thonn. (Euphorbiaceae). Journal of Essential Oil Research. 2009;21:393-399.
- Phyllanthus amarus Schumach. & Thonn.[Internet]. 2008 [cited on 13th June 2018]. Available from: http://database.prota.org/PROTAhtml/Phyllanthus%20amarus_En.htm.
- Santos ARS, Rafael OP, Campos D, Miguel OG, Filho VC, Siani AC, Yunes RA, Calixto JB. Antinociceptive properties of extracts of new species of plants of the genus Phyllanthus (Euphorbiaceae). Journal of Ethnopharmacology. 2000;72:229–238.
- Manikkoth S, Deepa B, Joy AE, Rao S. Anticonvulsant activity of Phyllanthus amarus in experimental animal models. International Journal of Applied Biology and Pharmaceutical Technology. 2011; 4:144-149.
- Lawson-Evi P, Eklu-Gadegbeku K, Agbonon A, Aklikokou K, Creppy E, Gbeassor M. Antidiabetic activity of Phyllanthus amarus Schum and Thonn on alloxan induced diabetes in male Wistar rats. Journal of Applied Sciences. 2011;11(16):2968-2973.
- Adeneye AA, Amole OO, Adeneye AK. Hypoglycemic and hypocholesterolemic activities of the aqueous leaf and seed extract of Phyllanthus amarus in mice. Fitoterapia. 2006;77(7-8):511-514.
- Kassuya UA, Leite DF, de Melo LV, Rehder VL, Calixto JB. Anti-inflammatory properties of extracts, fractions and lignans isolated from Phyllanthus amarus. Planta Medica. 2005;71:721-726.
- Pramyothin P, Ngamtin C, Poungshompoo S, Chaichantipyuth C. Hepatoprotective activity of Phyllanthus amarus et. Thonn. extract in ethanol treated rats: In vitro and in vivo studies. Journal of Ethnopharmacology. 2007;114(2):169-173.
- Lim YY, Murtijaya J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. Swiss Society of Food Science and Technology. 2007; 40:1664–1669.
- Kloucek P, Polesny Z, Svobodova B, Vlkova E, Kokoska L. Antibacterial screening of some Peruvian medicinal plants used in Calleria district. Journal of Ethnopharmacology. 2005;99:309-312.
- Oluwafemi F, Debiri F. Antimicrobial Effect of Phyllanthus amarus and Parquetina nigrescens on Salmonella typhi. African Journal of Biomedical Research. 2008;11: 215 – 219.
- Manikkoth S, Deepa B, Joy AE, Rao S. Anticonvulsant activity of Phyllanthus amarus in experimental animal models. International Journal of Applied Biology and Pharmaceutical Technology. 2011; 4:144-149.
- Adomi PO, Owhe-Ureghe UB, Asagba SO. Evaluation of the toxicity of Phyllanthus amarus in Wistar albino rats. African Journal of Cellular Pathology. 2017;8(5):27-35.






