Pokok Melaka Leaves
Phyllanthus emblica L.
Phyllanthaceae
DEFINITION
Pokok Melaka leaves consist of the powder of dried leaves of Phyllanthus emblica L. (Phyllanthaceae).
SYNONYM
Emblica officinalis Gaertn.; Emblica grandis Gaertn.; Emblica arborea Raf.; Cicca emblica (L.) Kurz; Diasperus emblica Kuntze; Dichelactina nodicaulis Hance; Phyllanthus mairei H.Lév.; Phyllanthus mimosifolius Salisb.; Phyllanthus taxifolius D.Don [1, 2, 3].
VERNACULAR NAMES
Emblic, emblic myrobalan, indian-gooseberry, (English); melaka, laka, pokok melaka, (Malay); yu gan ye (Chinese); nelli (Tamil) [3, 4, 5, 6, 7].
CHARACTER
IDENTIFICATION
Plant morphology
P. emblica is a small to medium sized tree, up to 36 m tall, deciduous. Bole terete, diameter of a one-metre-high tree, is up to 50 cm, often crooked and with a fluted base. Bark is greyish–brown to orange–brown. Leaves are simple, alternate and distichously arranged, resembling pinnate leaves. Leaf blades are linear–oblong or oblong with truncate, round or obtuse apex, shallowly cordate and slightly oblique base, entire margin, glabrous and often have papery to leathery texture. Petioles are very short; stipules are brown, triangular to ovate. Shoots are yellowish green, occasionally with the tip, tinged red. Inflorescences axillary fascicles, each with several staminate flowers and 1 or 2 pistillate flowers. Flowers are small, greenish-yellow or sometimes pinkish; apetalous; stamens 3, fused at base; styles 3, connate at base, deeply bifid, lobes divided at tip; sepals 6, oblong; pedicels very short. Fruits a drupe, indehiscent, nearly spherical or globose, 1–2 cm diameter, pale green or yellowish green and smooth surface that are often clustered at the base of the leaves. Pulp pale green or yellowish white, sour taste. Endocarp crustaceous, ovoid with six shallow ridges, occasionally with the presence of yellow glands and sessile stellate trichomes, green turning brown when matured. Seeds reddish or dark chesnut–brown, trigonous and smooth [4, 5, 6, 9].
Microscopy
Powdered dried leaves consist of fragments of adaxial and abaxial epidermal cells with slightly wavy anticlinal walls, hypostomatic, paracytic stomata, stomata outline is oval or elliptic; abundant of calcium oxalate crystals (solitary and druse form). Other features observed include the presence of parenchyma and sclerenchyma cells; lignified parenchyma cells wall, fibre fragments, annular and spiral vessels; palisade mesophyll cells; long simple, multicellular trichome, mucilage cells and oil globules [10, 11].
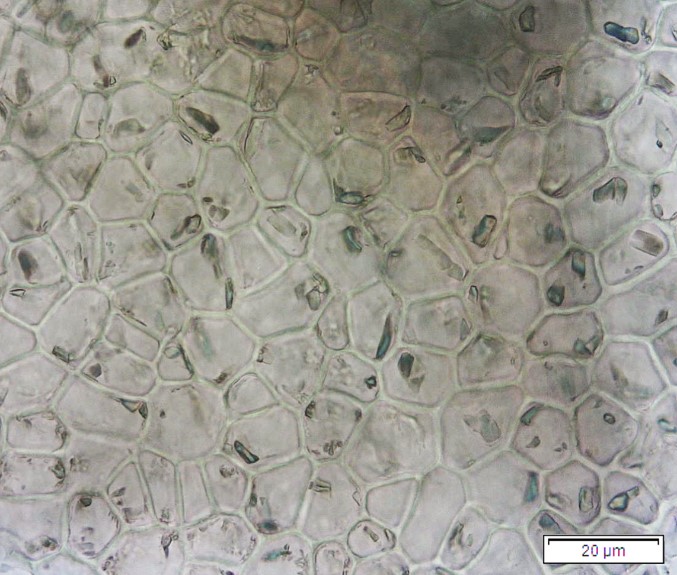
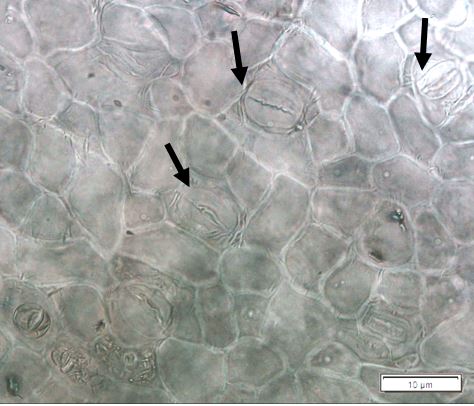


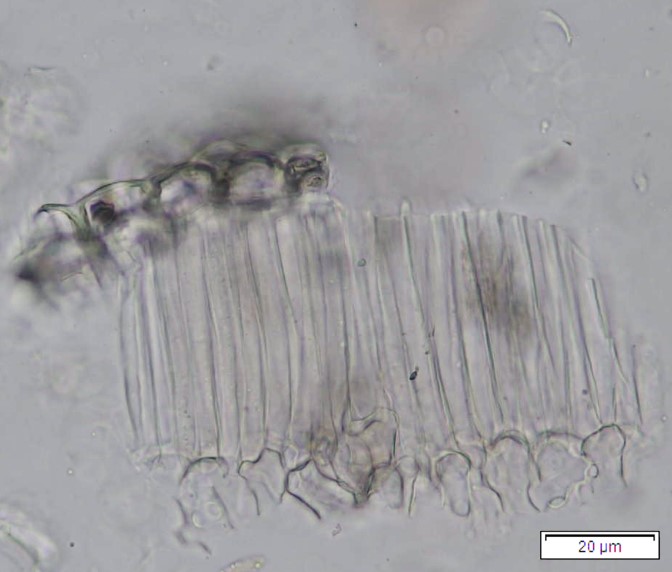

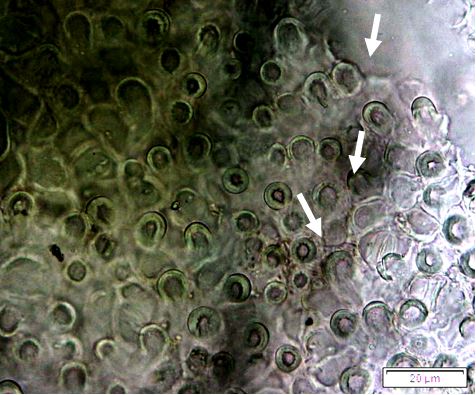
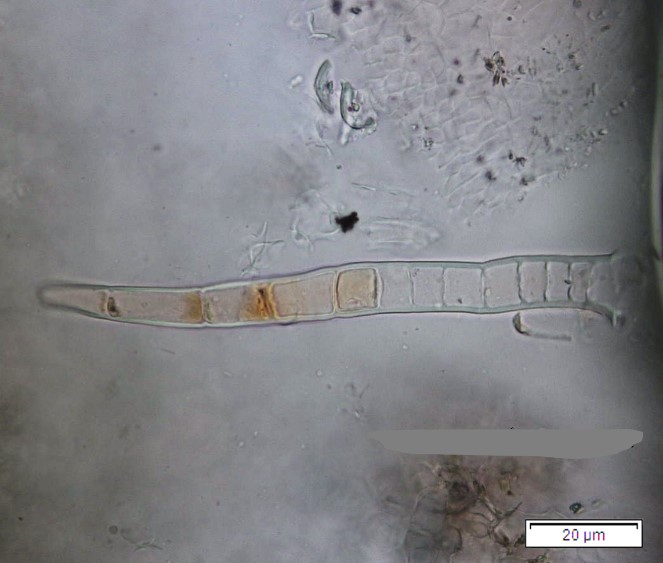




Figure 2 : Microscopic characters of P. emblica dried leaf powder of 0.355 mm size. (a) adaxial epidermis cells, slightly wavy anticlinal walls (magnification 20×); (b) abaxial epidermis cells, slightly wavy anticlinal walls and the presence of paracytic stomata (arrow) (magnification 40×); (c) calcium oxalate crystals under polarised filter (magnification 20×); (d) fibre fragments (magnification 20×); (e) palisade mesophyll cells fragment (magnification 20×); (f) parenchyma cells (magnification 20×). (g) sclerenchyma cells (arrow) (magnification 20×); (h) long simple, multicellular trichome (magnification 20×); (i) annular vessels (arrow) (magnification 40×); (j) spiral vessels (arrow) (magnification 40x); (k) mucilage cells (arrow) (magnification 10×); (l) lignified parenchyma cells walls observed in Sudan red (III) solution (arrow) (magnification 20×). [Scale bars: a, c, d, e, g, h, l = 20 µm; b, f, i, j = 10 µm; k = 50 µm]
Chemical Tests
Observed colour of solution after treatment with various reagents:
| Test for the presence of phenolics | Bluish green |
High Performance Thin Layer Chromatography (HPTLC)
| Test Solution | Weigh about 0.5 g of P. emblica dried leaf powder of 0.355 mm size in a 14 mL screw-capped vial. Add 5 mL of ethanol and sonicate for 15 min at room temperature. Filter the mixture with 0.45 µm syringe filter into HPLC vial. Use the filtrate as test solution. |
| Standard solution | Disssolve chebulagic acid standard [CAS ni.: 23094-71-50], in methanol to produce a standard concentration of 0.5 mg/mL. |
| Stationary phase | HPTLC Silica gel 60 F254, 10 x 10 cm |
| Mobile phase |
Ethyl acetate : formic acid : acetic acid : water (100 : 11 : 11 : 25) 20 mL (v/v/v/v) |
| Application |
|
| Development distance | 8 cm |
|
Drying |
CAMAG TLC plate heater (Temperature: 105°C) |
|
Detection |
|

Figure 3 : HPTLC chromatogram of chebulagic acid (S) and ethanol extract of P. emblica dried leaf powder (L) observed under (a) UV at 254 nm before derivatisation with NP/PEG reagent, (b) UV at 366 nm after derivatisation with NP/PEG reagent and (c) visible light after derivatisation with NP/PEG reagent.
High Performance liquid Chromatography (HPLC)
| Test solution |
Weigh about 0.5 g of P. emblica dried leaf powder of 0.355 mm size in a 14 mL screw-capped vial. Add 5 mL of ethanol and sonicate for 15 min at room temperature. Filter the mixture with 0.45 µm syringe filter into HPLC vial. Use the filtrate as test solution. |
|||||||||||||||
| Standard solution |
Dissolve chebulagic acid standard [CAS no.: 23094-71-5] in methanol to produce a standard concentration of 0.5 mg/mL. |
|||||||||||||||
| Chromatographic system |
Detector: UV 280 nm Column: Phenomenex Luna PFP(2) (250 x 4.6 mm) 5μm Column oven temperature: Ambient Flow rate: 1 mL/min Injection volume:10 µL |
|||||||||||||||
| Mobile Phase (gradient mode) |
|
|||||||||||||||
| System suitability requirements |
Perform at least five replicate injections of the standard solution (0.5 mg/mL). The requirements of the system suitability parameters are as follow:
|
|||||||||||||||
| Acceptance criteria |
|

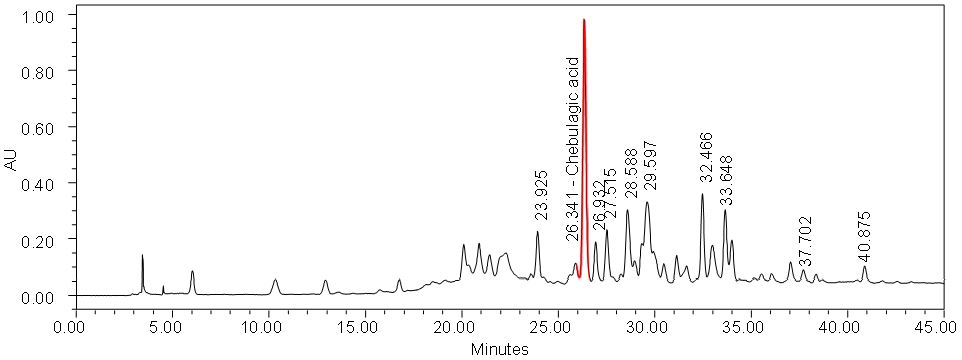
Figure 4 : Whole HPLC chromatogram of (a) chebulagic acid standard solution (0.5 mg/mL) at tr= 26.648 min and (b) ethanol extract of P. emblica dried leaf powder showing a peak corresponding to chebulagic acid at tr = 26.341 min.
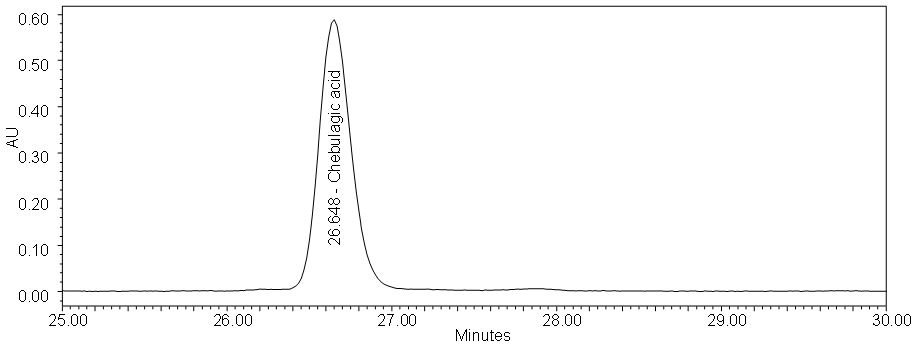
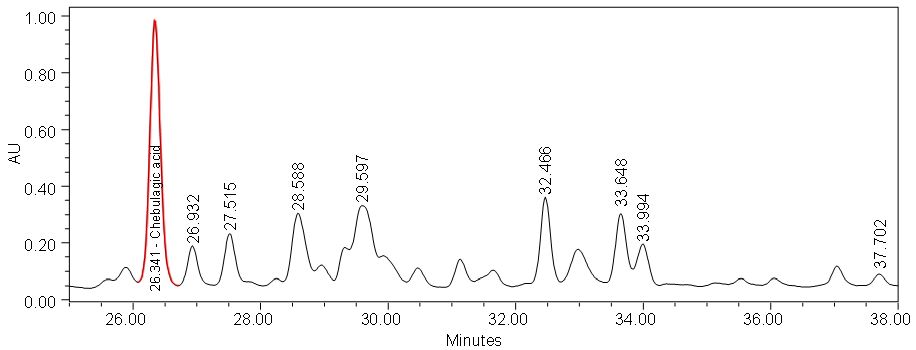
Figure 5 : HPLC chromatogram highlighting the elution region of (a) chebulagic acid standard solution (0.5 mg/mL) at tr = 26.648 min and (b) ethanol extract of P. emblicadried leaf powder showing a peak corresponding to chebulagic acid standard solution at tr = 26.341 min.
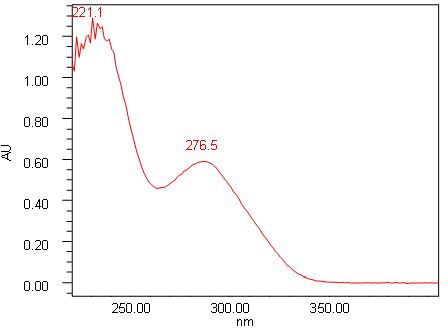

Figure 6 : UV spectra of (a) chebulagic acid standard solution (0.5 mg/mL) and (b) ethanol extract of P. emblica dried leaf powder.
PURITY TESTS
The purity tests are based on P. emblica dried leaf powder of 0.355 mm particle size.
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 5% |
| Acid-insoluble ash | Not more than 0.5% |
| Water soluble ash | Not less than 0.6% |
| Loss on Drying |
| Not more than 12% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 25% |
| Cold method | Not less than 18% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 33% |
| Cold method | Not less than 18% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Methanol extract of P. emblica dried leaves was found to contain fatty acids (e.g. isopropyl palmitate, γ-linolenic acid, 8,11-octadecadienoic acid methyl ester, 2-methylhexadecanoic acid and linolenic acid) and alkaloid (e.g. 1,3-dimethylindole) [12].
Ethanol extract of P. emblica dried leaves was found to contain phenolic acids (e.g. chebulagic acid and 3,6-di-O-galloylglucose); fatty acids (e.g. octadecanoic acid, 9,12-octadecadienoic acid, hexadecenoic acid, 9-hexadecenoic acid, tetradecanoic acid), flavonoids (e.g. (S)-eriodictyol 7-O-(6”-O-trans-p-coumaroyl)-β-D-glucopyranoside, (S)-eriodictyol 7-O-(6”-O-galloyl)-β-D-glucopyranoside); terpenes (e.g. sativen, δ-guaiene, caryophellene); long-chain aldehyde (e.g. octadecanal); phenolic glycoside (e.g. 2-(2-methylbutyryl)phloroglucinol 1-O-(6”-O-β-D-apiofuranosyl)-β-D-glucopyranoside); and alcohols (e.g. 1-hexadecanol, 11-tetradecen-1-ol, 2-furanmethanol) [13, 14, 15].
n-butanol and ethyl acetate extracts of P. emblica dried leaves were found to contain phenolic acids (e.g. gallic acid, chlorogenic acid, caffeic acid, syringic acid, ellagic acid, coumaric acid, ferulic acid, cinnamic acid, vanillin, propyl gallate); phenolic acids (e.g. vanillin, propyl gallate); flavonoids (e.g. catechin, rutin, naringenin, quercetin, kaempferol-3-O-β-D-glucoside) and phytosterol (e.g. stigmast-4-en-3-one) [16].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally, a decoction of the leaves is used to treat fever [17].
Biological and pharmacological activities supported by experimental data
Antioxidant activity
Aqueous extract of P. emblica leaves exhibited 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity with 50% inhibition concentration (IC50) value of 7.72 ± 0.25 µg/mL compared to ascorbic acid (IC50 of 8.06 ± 0.01 µg/mL) using DPPH assay [18].
Antibacterial activity
Petroleum ether extract of P. emblica leaves (1000 µg/mL) showed antibacterial activity with inhibition zones against Staphylococcus aureus (11 mm), Escherichia coli (11 mm), Salmonella typhi (9 mm), Bacillus subtillus (8 mm), and Enterococcus faecalis (0 mm) compared to streptomycin (10 µg/mL) with inhibition zones againt S. aureus (16 mm), E. coli (15 mm), S. typhi (15 mm), B. subtillus (15 mm), and E. faecalis (14 mm) using agar disc diffusion method [19].
Anti-psoriatic activity
Methanol extract of P. emblica leaves (50 mg/kg BW) was applied topically as a cream on the irradiated tail of male Wistar rats (250 g body weight) once daily for 2 weeks. The irradiated tail undergoes orthokeratotis (OrthKer) whereby there is a portion of the tail where no nucleus in the surrounding cells. Healing was indicated by the increase in relative ratio of orthokeratotis in the mouse tail epidermis. Based on OrthKer ratio, the extract exhibited anti-psoriatic activity with 17.5 mm width of orthokeratotic layer and 26.5 mm in keratinocyte layer giving OrthKer ratio of 0.6603, compared to standard Retino-A (0.025%) cream of 0.75 OrthKer ratio [20].
Anti-proliferative activity
n-butanol extract of P. emblica leaves showed cytotoxic effect on hepatocellular carcinoma (HepG-2) with an IC50 of 25.63 ± 2.10 mg/mL compared to doxorubicin IC50 (4.17 ± 0.20 mg/mL) using 2,5-diphenyl-2H-tetrazolium bromide (MTT) assay [21].
n-butanol extracts of P. emblica leaves showed cytotoxic effect on mammary gland breast cancer (MCF-7) with an IC50 of 22.80 ± 1.90 mg/mL compared to doxorubicin IC50 (4.50 ± 0.20 mg/mL) using 2,5-diphenyl-2H-tetrazolium bromide (MTT) assay [21].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
14 days acute toxicity
In acute toxicity 14-day study, 500, 1000 and 2000 mg/kg BW doses of the petroleum ether extract of P. emblica leaves was orally administered to male Wistar rats (180–200 g) were observed for signs of toxicity for two weeks. A single administration up to a dose of 2000 mg/kg showed no mortality and behavioral changes. Hence, the lethal dose, 50% (LD50) was estimated to be greater than 2000 mg/kg [22].
Others (Adverse reactions, contraindications, side effects, warning, precautions)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The International Plant Names Index and World Checklist of Vascular Plants. [Internet]. Phyllanthus emblica L.; 2023. [cited on 15th September 2023]. Available from: http://www.ipni.org/ and https://powo.science.kew.org/
- Global Tree Search online database. Botanic Gardens Conservation International. Richmond, U.K. [Internet]. Phyllanthus emblica L.; 2022. [cited on 15th September 2023]. Available from: DOI: 10.13140/RG.2.2.34206.61761
- Van HFL. 1999. Phyllanthus emblica L. In: de Padua LS, Bunyapraphatsara N, Lemmens RHMJ. (Editors). Plant Resources of South-East Asia. No 12(1): Medicinal and poisonous plants. Vol. 1. PROSEA Foundation, Bogor, Indonesia.
- Malaysia Biodiversity Information System (MyBIS) Database. [Internet]. Phyllanthus emblica L.; 2010. [cited on 15th September 2023]. Available from: https://www.mybis.gov.my/sp/11393
- Flora of China @ eFloras. [Internet]. Phyllanthus emblica L.; 2008. [cited on 15th September 2023]. Available from: http://www.efloras.org/flora_page.aspx?flora_id=2
- Flora of India eFloraofIndia. [Internet]. Phyllanthus emblica L.; 2022. [cited on 15th September 2023]. Available from: http://www.flowersofindia.net/catalog/slides/Amla.html
- Pushpendra KJ, Debajyoti D. Pharmacognostic comparison of Bacopa monnieri, Cyperus rotundus and Emblica officinalis. Innovative Journal of Ayurvedic Sciences. 2016; 4(4): 16–26.
- Radhika B, Akshitha K, Dharani M, Karthikeya P. Pharmacognostic and phytochemical evaluation of Emblica officinalis leaves (Amla). International Journal of Creative Research Thoughts. 2023; 11(3): 2320–2882.
- Thai Herbal Pharmacopoeia. [Internet]. Phyllanthus emblica L.; 2021. [cited on 15th September 2023]. Available from: https://bdn.go.th/thp/member/login
- Dhale DA. Pharmacognostic evaluation of Phyllanthus emblica Linn (Euphorbiaceae). International Journal of Pharmacognocy Biological Science. 2012; 3(3): 210–217.
- Khatijah H, Ruzi AR. Malaysian Medicinal Plants. Vol. 5. Bangi, Selangor: Universiti Kebangsaan Malaysia. 2009; p. 112–115.
- Abdel-Hady H, Morsi EA, El-Wakil EA. In-vitro antimicrobial potentialities of Phyllanthus emblica leaf extract against some human pathogens. Egyptian Journal of Chemistry. 2022; 65(7): 701–707.
- Zhang YJ, Abe T, Tanaka T, Yang CR, Kouno I. Phyllanemblinins A−F, New Ellagitannins from Phyllanthus emblica. Journal of Natural Products. 2001; 64(12): 1527–1532.
- Asmilia N, Yudha F, Abrar M, Rinidar R. Chemical compounds of Malacca leaf (Phyllanthus emblica) after triple extraction with N-hexane, ethyl acetate and ethanol. Hindawi. 2020. Open Access: https://doi.org/10.1155/2020/2739056
- Mohamed TS, Refahya LA, Romeihb MH, Hameda MM, Ahmedb HO, El-Hashash MA. Chromatographic isolation and characterization of certain bioactive chemical ingredients of Phyllanthus emblica extracts and assessment of their potentials as antiviral and anticancer agents. Egyptian Journal of Chemistry. 2022; 65(1): 179–192.
- Zhang YJ, Abe T, Tanaka T, Yang CR, Kouno I. Two new acylated flavanone glycosides from the leaves and branches of Phyllanthus emblica. Chemical and Pharmaceutical Bulletin. 2002; 50(6): 841–843.
- Burkill IH. A Dictionary of the Economic Products of the Malay Peninsular. Vol. 2. Kuala Lumpur: Ministry of Agriculture and Cooperatives. 1966; p. 936.
- Iamsaard S, Arun S, Burawat J, Sukhorum W, Wattanathorn J, Nualkaew S, Sripanidkulcha B. Phenolic contents and antioxidant capacities of Thai-Makham Pom (Phyllanthus emblica L.) aqueous extracts. Journal of Zhejiang University-Science B. 2014; 15(4): 405–408.
- Elangovan MN, Dhanarajan MS, Elangovan I. Evaluation of antibacterial and antifungal activity of Phyllanthus emblica leaf extract. International Research Journal of Pharmaceutical and Biosciences. 2015; 2(2): 59–66.
- Meena P, Hrishikesh V, Ravindra M. Evaluation of anti-psoriatic activity of Cyperus rotundus and Phyllanthus emblica. International Journal of Biotechnology. 2012; 5(2): 28–34.
- Mohamed TS, Refahya LA, Romeihb MH, Hameda MM, Ahmedb HO, El-Hashash MA. Chromatographic isolation and characterization of certain bioactive chemical ingredients of Phyllanthus emblica extracts and assessment of their potentials as antiviral and anticancer agents. Egyptian Journal of Chemistry. 2022; 65(1): 179–192.
- Elangovan MN, Dhanarajan MS, Elangovan I. Evaluation of hepatoprotective activity of leaf extract of Phyllanthus emblica and Moringa oleifera on carbon tetrachloride induced hepatic damage in albino rats. World Journal of Pharmaceutical Research. 2018; 7(13): 977–990.








