Sireh Leaves
Piper betle L.
Piperaceae
DEFINITION
Sireh leaves consist of the whole mature leaves of a vine of P. betle L. (Piperaceae)
SYNONYM
Chavica betle (L.) Miquel, Piper pinguispicum C. DC. & Koord.
VERNACULAR NAMES
Betel pepper, betelvine (English), sirih (Malay), sirih, suruh, seureuh (Indonesian), Philippines: ikmo (Tagalog), buyo (Bikol), mamon (Bisaya), mlu (Cambodia), ph’u (Laos), phlu (Thailand), tr[aaf]u, tr[aaf]u kh[oo]ng (Vietnam), bétel (France).
CHARACTER
| Colour | Bright greenish, yellowish to dark green leaves |
| Odour | Specific strong pungent smell |
| Taste | Distinct aromatic flavour |
IDENTIFICATION
Plant Morphology
P. betle/betel plant/sireh is an evergreen and perennial dioecious, semi woody stem creeper, producing orthotropic (vegetative) and plagiotropic (reproductive) branches, with glossy heart shaped leaves and white catkin [ 1 ]; under normal cultivation, P. betle grows up to height of 2-4 m in one year period. Stem stout with pinkish-stripe along, node dilated and rooting, stem has a conspicuous stout node with strictly one simple leaf on each node. Roots short adventitious arise from each node that aid in fixing the plant in host tree. Leaves simple, alternate, ovate, cordate, acuminate or acute, entire and bright greenish, yellowish to deep green, 5-7 veins arising from the base to the tips, leaves and leaf blade fleshy coriaceous, glabrous have specific strong pungent aromatic and acrid flavor; upper leaves are usually oblong-elliptic, oblong-ovate, broadly-ovate or ovate, 6-18 cm long, 3.5-11 cm wide, mostly 7-plinerved, smooth on both surfaces; mature leaves dark green, glabrous with entire margin, apex is cuminate, base cordate, often unequal base, veins 7-9, elevating beneath, two or three pairs basal, one pair arising from midrib, petiole is stout, 2-6.5 cm long; leaves have multiple epidermis in both the adaxial and abaxial surfaces with glandular, tector trichomes respectively; the cuticle is thick on the upper epidermis and thin on the lower epidermis; the cells of the outer epidermal layers on both sides of the leaf are small, that possess tannins and oils; the sub epidermal cells on the abaxial side are enlarged and they store water; crystal and oil reserves are found in the sub epidermal cells on both sides [ 2 ]. The inflorescence spike develops on the node of the branching stem opposite the leaf and is erect or pendulous, monoecious or dioecious; male spikes/flowers dense, cylindrical, subpendulous, pendulous, slender, 3-13.5 cm long, and 0.5-3.5 mm in diameter, peduncle 2-3 cm long, rachis is hairy, bract orbicular, peltate, stamens 2, stalked, 0.75-1 mm long and the anthers reniform; female spikes long, cylindrical, pendulus having single ovary, fleshy, oblong to elongated oblong, 2.5-8 cm long, and 0.5-1 cm thick/diameter, peduncle 2-3 cm long, bract orbicular, peltate, stigmas 4-6, pubescent, rachis is hairy, and the bracts stalkless, peltate, with a smooth disk, transversely oblong to suborbicular, and about 1 cm wide; female spike is red when mature. Flowers are very small with absent sepal and petal, orbicular bracts, and the stalk is sessile or connected to the rachis; the stamen and stigma number 2-6 and the stigma may be covered with very short hair; the ovary is inferior and sessile or with a short pedicel. Fruit is fleshy, sessile or with a pedicel, and globose or ellipsoid, coalescing with fruiting spike 3-5 cm long, drupe fully embedded in the pulp and concrescent with the rachis, fruits are rarely produced, often sunk in the fleshy spike, forming nodule like structure. Seeds are smooth, oblong to globose-obovoid, 2.25-2.6 mm long, and about 2 mm in diameter [ 3 , 4 ]
Microscopy
Microscopic characters of P. betle leaves powder consist of fragments of epidermis cells and stomata, vessel cells, fragments of epidermis cells and secretory cells, fragments of secondary wall of tracheary elements and simple unicell and multicell trichomes.
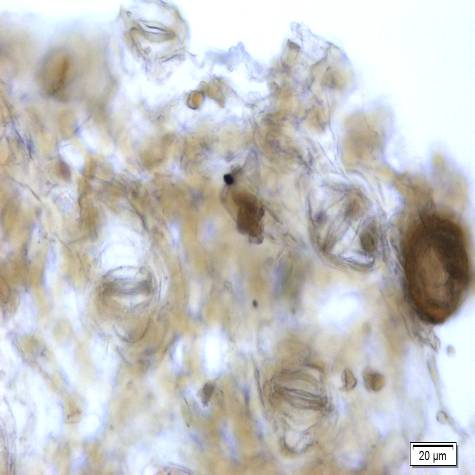


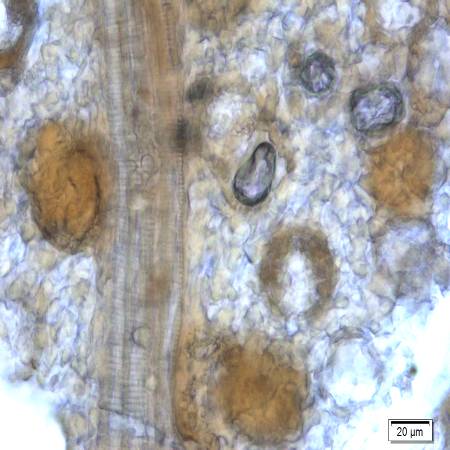
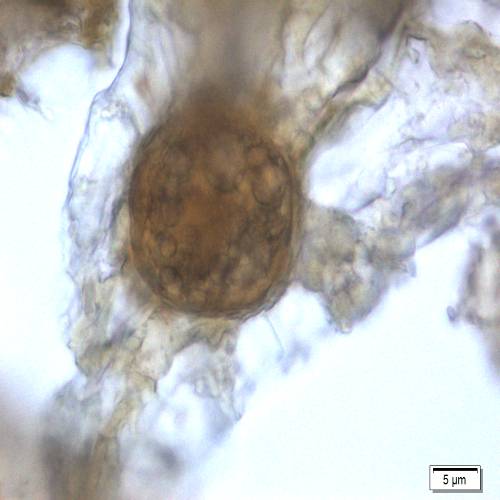
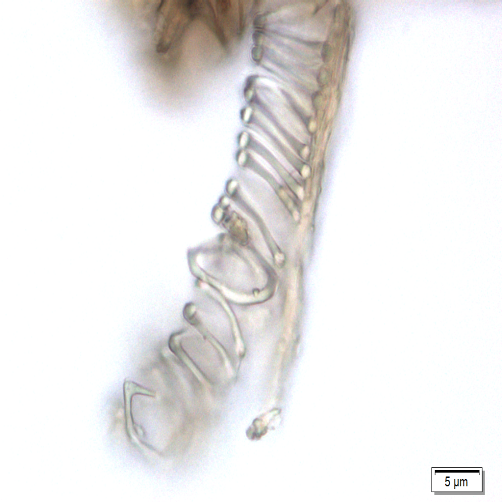
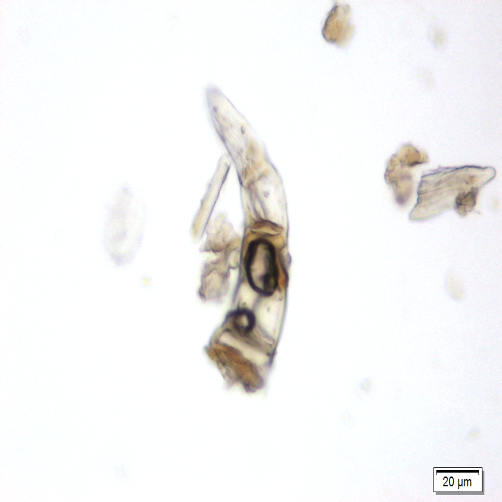
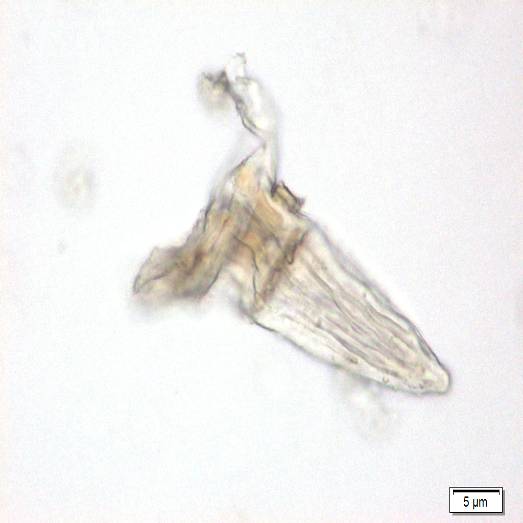
Figure 2 : Microscopic characters of P. betle leaves powder. (a) Fragments of epidermis cells and stomata; (b-c) vessel cells; (d) fragments of epidermis cells and secretory cells; (e) secretory cells; (f) fragments of secondary wall of tracheary elements; (g-h) simple multicell and unicell trichomes. [Scale bars: a, d, g= 20 µm; b, c, e, f, h= 5 µm]
Colour Tests
Observed colour of solution after treatment with various reagents:
| HCl (conc.) | Green |
| 5% NaOH | Brown |
| 5% FeCl3 | Green |
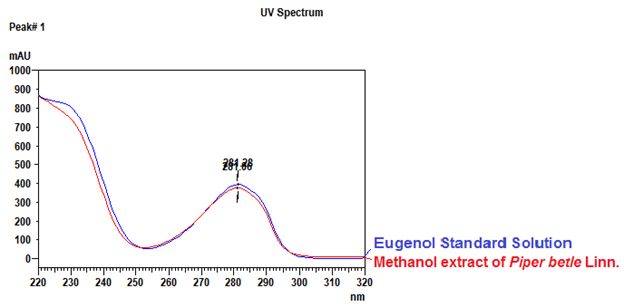
Thin Layer Chromatography (TLC)
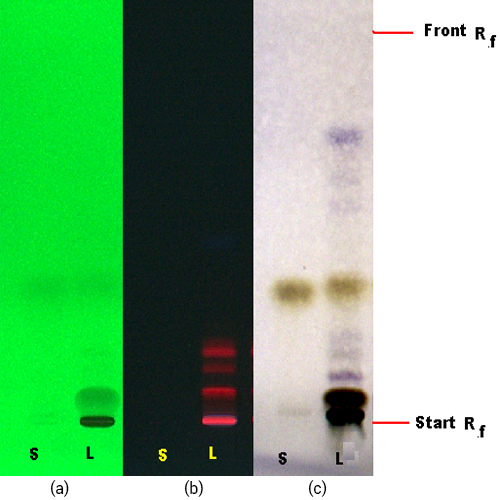
Figure 3 : TLC profiles of eugenol (S) and methanol extract of P. betle dried leaf powder (L) observed under (a) UV at 254 nm and (b) UV at 366 nm before spraying and (c) Visible light after spraying with vanillin-sulphuric acid reagent.
| Test Solutions | Weigh about 0.5 g of P. betle dried leaf powder in a 50 mL screw-capped conical flask and add 10 mL of methanol. Macerate the mixture on water bath for 1 hour at 60°C. Cool the mixture to room temperature and filter. Use the filtrate as test solution. |
| Standard solution | Dissolve 5.0 µL of eugenol standard [CAS no.: 97-53-0] in 10 mL methanol to produce 0.5 µL/mL solution. |
| Stationary Phase | HPTLC Silica gel 60 F254, 10 x 10 cm |
| Mobile phase | Toluenev: ethyl acetate; (48:2) (v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
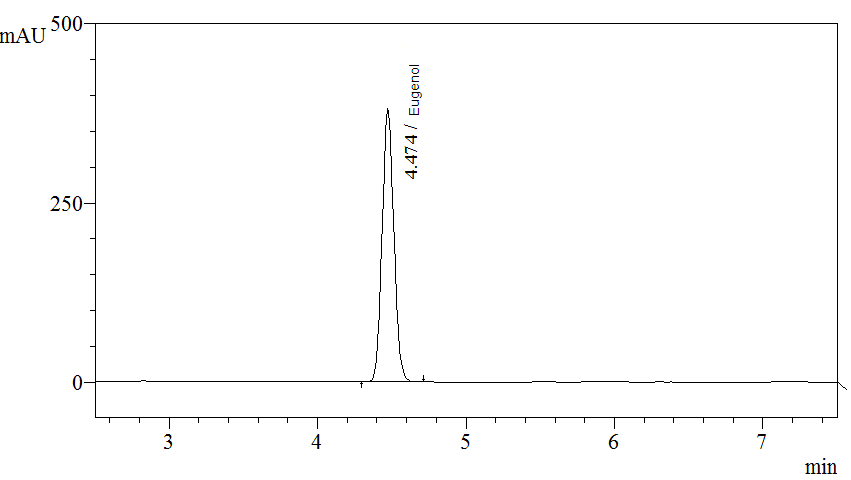
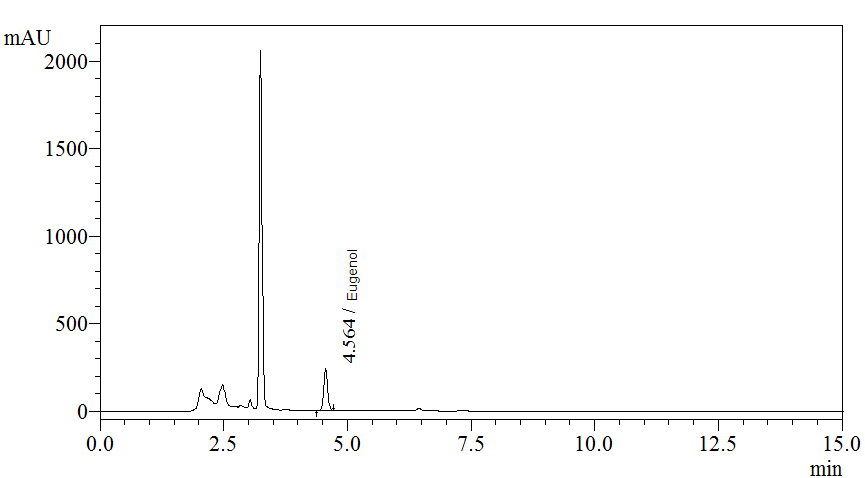
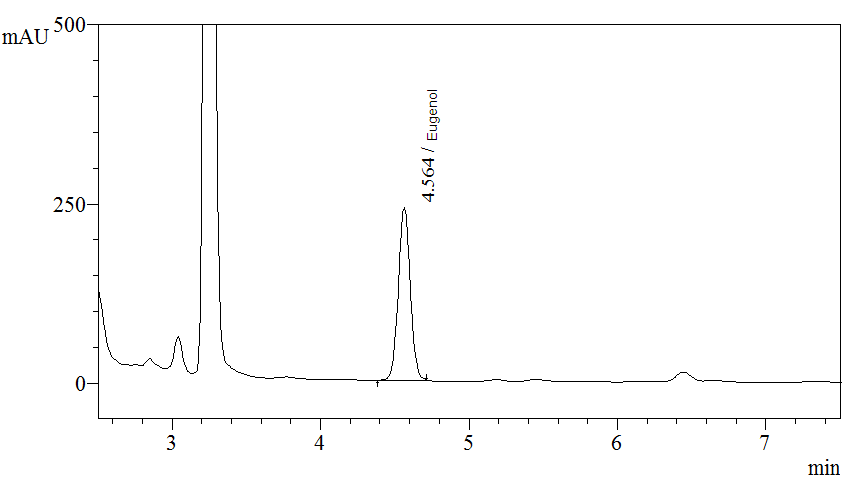
High Performance Liquid Chromatography (HPLC)
| Test solution | Extract about 0.1 g of P. betle dried leaf powder with 10 mL of methanol. Macerate the mixture in water bath for 1 hour at 60°C. Cool the mixture to room temperature and filter the solution with 0.45 µm syringe filter into a vial. Use the filtrate as test solution. | ||||||||||||||||||
| Standard solution | Dissolve 5.0 µL of eugenol standard [CAS no.: 97-53-0] in 10 mL of methanol to produce 0.5 µL/mL solution. | ||||||||||||||||||
| Chromatographic system |
Detector: UV 280 nm |
||||||||||||||||||
| Mobile Phase (gradient mode) |
|
||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the standard solution (0.5 µL/mL). The requirements of the system suitability parameters are as follow:
|
||||||||||||||||||
| Acceptance criteria |
|
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 14% |
| Acid-insoluble ash | Not more than 2% |
| Loss on Drying |
| Not more than 11% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 18% |
| Cold method | Not less than 14% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 11% |
| Cold method | Not less than 8% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Essential oil of the leaf has been found to contain monoterpenes (e.g. α-pinene, β-myrcene, L-limonene, cis-ocimene, trans-ocimene, chavicol, eugenol, β-elemene, methyl-eugenol, acetyleugenol and trans-β-ocimene), sesquiterpenes (e.g. trans-caryophyllene, α-humulene, germacrene D, germacrene B, globulol, γ-cadinene and cis-caryophyllene), and others (e.g. camphene, phenyl acetylaldehyde, linalyl acetate, decanal, cyclohexene,4-methyl-, undecanal, bicyclo[4.1.0]hept-3-en-, γ-muurolene, ledene, 4-allyl-1,2-diacetoxybenzene, ionene, 5-(2-propenyl)-1,3-benzodioxole, 2-methoxy-4-(2-propenyl) acetate-phenol, safrole, chavibitol acetate, β-phellandrene, allylpyrocatechol diacetate and eugenyl acetate) [ 5 ,
6 , 7 , 8 ].
Aqueous extract of the leaf has been found to contain monoterpenes (e.g. hydroxychavicol), organic acids (e.g. stearic, palmitic and hydroxybenzeneacetic) and esters (e.g. hydroxyl ester of stearic, palmitic and myristic) [ 9 ].
MEDICINAL USES
Uses described in folk medicine,not supported by experimental or clinical data
The chewing of P. betle made into a betel-quid is used for ‘pirai’ (i.e gouty arthritis) disease. The juice of P. betle is used as ear-drop or eye-drops and for wounds. The decoction is applied as lotion to the women after childbirth. The leaves of P. betle when heated are applied onto the chest to relieve cough, and the breast to arrest secretion of milk. The poultice of P. betle leaves are applied to ulcer [ 10 ]
One glass of boiled P. betle leaves is gargled and another to drink twice a day for relief of stomach ache and to reduce body and mouth odor [ 11 ].
A mixture containing the juice of P. betle leaves is taken for gonorrhoea [10]. P. betle is chewed together with areca nut to be used for shingles [ 11 ]. P. betle leaves combined with Carica papaya is used as cough remedy while the combination with rhizome of Curcuma domestica has been used to maintain youthfulness [ 12 ].
Biological and pharmacological activities supported by experimental data
Antileishmanial activity
Ethanol extract of P. betle leaves (0-20 µg/mL) reduced the viability of promastigotes (IC50 9.8 μg/mL) in a dose-dependent manner by inhibition of parasitic growth using (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)-phenazine methosulfate (MTS-PMS) assay [ 13 ].
Ethanol extract of P. betle leaves (0-20 µg/mL) reduced the infection of amastigotes (IC50 = 5.45 μg/mL) ) in a dose-dependent manner by inhibition of parasitic load in peritoneal macrophages isolated from BALB/c mice [ 13 ].
Antibacterial activity
Methanol extract of P. betle leaves (500 µg/g) inhibited obligate oral anaerobes using broth (86%) and plate dilution (71%) assays as compared to chlorhexidine where the allylpyrocatechol (APC) was found to be the active constituent [ 14 ].
Ethanol extract of P. betle leaves (1 mg/mL) showed antibacterial activity against Proteus vulgaris with MIC value of 25 µg/mL, Staphylococcus aureus (MIC = 40 µg/mL), Pseudomonas aeruginosa (MIC = 35 µg/mL) and Klebsiella pneumonia (MIC = 25 µg/mL) using disc diffusion assay with ceftriaxone (1 mg/mL) as standard [ 20 ].
Aqueous extract of P. betle leaves reduced the acid production of Streptococcus mutans at concentration of 1 mg/mL (93.53%), 2 mg/mL (88.20%), 5 mg/mL (40.51%) and 10 mg/mL (23.56%) using pH drop assay with chlorhexidine (0.12%) as positive control [ 9 ].
Four different extracts (water, methanol, ethyl acetate and petroleum ether) of P. betle leaves (5, 10, 25, 50 and 100 mg/mL) showed antibacterial activity against four bacterial strains (Streptococcus pyogens, Staphylococcus aureus, Proteus vulgaris, Escherichia coli) using agar diffusion method with lower activity compared to gentamycin (1 mg/ mL)
[ 25 ].
Antifungal & antinematodes activity
Allypyrocatechol isolated from chloroform extract of P. betlePhytium ultimum with effective dose at 50% growth (ED50) of 30.5 µg/mL compared to eugenol (ED50 = 33 µg/mL) and catechol (ED50 22.5 µg/mL) after 24-36 hours inoculation using Phytium bioassay. The isolates (chavicol, chavibetol, allylpyrocatechol, chavibetol acetate and allypyrocatechol diacetate) from the extracts (200 µg/mL each) also showed significant nematocidal activity against Caenorhabditis elegans using M-9 buffer [ 21 ].
Antifertility activity
Ethanol (90%) extract of P. betle leaves (500 mg/kg) dissolved in olive oil was administered orally to male Swiss albino mice for 30 days followed by an increased dose of 1000 mg/kg for the next 30 days. The result showed significant decrease in the weight of reproductive organs (p < 0.01), sperm count (p < 0.001) and sperm motility (p < 0.001) [ 15 ].
Anti-oxidant activity
Ethanol: water (1:1) extract of P. betle leaves (1, 5 and 10 mg/kg) administered orally to albino Swiss mice (7-8 weeks old) for a duration of two weeks significantly increased the glutathione content (p < 0.05) at 10 mg/kg, enhanced the superoxide dismutase activity in dose dependent manner (p < 0.001) at 5 mg/kg but decreased the catalase activity ( p < 0.001) at 5 mg/kg compared to control [ 16 ].
Aqueous extract of P. betle leaves (100, 200, or 300 mg/kg body weight) administered through intragastric tube to adult albino Wistar rats 30 days after intoxication of ethanol (20% v/v) significantly (p < 0.05) reduced the TBARS and lipid hydroperoxides elevation. The extract also significantly (p < 0.05) restored the glutathione (GSH), vitamin C and vitamin E levels comparable to silymarin drug and increased the superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) enzyme activities [ 17 ]
Aqueous extracts of the three local Indian varieties of P. betle (Kauri, Ghanagete and Bagerhati) leaves inhibited the DPPH free radical with IC50 value of 62.6-271.5 µg/mL using DPPH assay compared to tea leaves (CTCHGH). The aqueous extracts of the three varieties also inhibited the hydroxyl (OH) radical with IC50 value of 685-1424 µg/mL using benzoic acid hydroxylation assay, the superoxide radical (O2-) radical with IC50 value of 73.8-279 µg/mL using nitroblue tetrazolium (NBT) assay, the lipid peroxidation with IC50 value of 14.8-81.5 µg/mL using thiobarbituric acid reactive species (TBARS) assay compared to tea leaves (CTCHGH). The aqueous extracts of the three varieties also exhibited total antioxidant capacity at 0.192 mg/mg and 0.5 mg/mg (Kauri), 0.076 mg/mg and 0.021 mg/mg (Ghanagete) & 0.083 mg/mg and 0.022 mg/mg (Bagerhati) using thiobarbituric acid reactive species (TBARS) assay compared to ascorbic acid and gallic acid respectively [ 18 ]
Four different extracts (aqueous, methanol, ethyl acetate and petroleum ether) of P. betle leaves (0.1 mg/mL) showed reduction in lipid peroxidation (3.43-6.54%) when red blood cells are treated with extracts alone or together with hydrogen peroxide toxicant compared to ascorbic acid (5 mg/mL; 2.56% lipid peroxidation activity) [ 25 ].
Antidiabetic activity
Hot water extract and cold ethanolic extract of P. betle leaves when administered orally to adult cross-bred male albino rats significantly (p < 0.05) reduce fasting blood glucose in a dose-dependent manner (100 mg/kg, 200 mg/kg and 300 mg/kg) compared to tolbutamide (22.5 mg/kg) [14]. The extracts also significantly (p < 0.05) reduced glucose concentration from control as compared to tolbutamide (22.5 mg/kg) [ 22 ].
Hot water extract of P. betle leaves (200mg/kg) administered orally to streptozocin-induced diabetic adult cross-bred male albino rats significantly (p < 0.05) reduced the hyperglycaemia [ 22 ].
Antihistaminic activity
Ethanol extract and essential oil (100 & 200 mg/kg) of P. betle leaves administered orally to Dunkin-Hartley Guinea pigs significantly (p < 0.001) protected them against histamine-induced bronchospasm producing progressive dyspnea leading to convulsions (64.5-77.48%) as compared to chlorpheniramine maleate (91.10%) [ 23 ].
Hepatoprotective activity
Aqueous extract of P. betle leaves (100, 200, or 300 mg/kg body weight) administered through intragastric tube to adult albino Wistar rats significantly (p < 0.05) reduced the aspartate aminotransferase (AST) and alanine aminotransferase (ALT) elevation due to ethanol intoxication compared to silymarin (25 mg/kg) [ 17 ].
Aqueous extract of P. betle leaves (10 & 25 mg/kg) administered orally for four weeks to male Wistar rats with liver fibrosis induced by carbon tetrachloride (CCl4) significantly (p < 0.001) diminished the induction effect of CCl4 on the liver enzymes (ALT and AST) in a dose-dependent manner with no significant effect on cholesterol and triglycerides. Liver histology also showed that the P. betle extract reduced the pathological damages [ 24 ]
Antihemolytic activity
Four different extracts (aqueous, methanol, ethyl acetate and petroleum ether) of P. betle leaves (0.1 mg/mL) showed reduction in haemolysis (8.26 – 11.14%) when red blood cells are treated with extracts alone or together with hydrogen peroxide toxicant compared to ascorbic acid (5 mg/mL; 7.83% for haemolytic activity) [ 25 ].
Radioprotective activity
Ethanol:water (1:1) extract of P. betle leaves (0.1 and 1.0 g/mL) significantly (p < 0.001) inhibited oxidative damage of microsomes prepared from liver induced by gamma rays (0–300 Gy) after 6 hours post irradiation time using thiobarbituric acid-reactive substances (TBARS) and 4-hydroxynonenol (4-HNE) in vitro assays [ 16 ].
Ethanol extract of P. betle leaves (1-100 µg/mL) when tested on liver mitochondria of male Wistar rat with g-ray induced peroxidation showed significant (p < 0.001) inhibition of thiobarbituric acid reactive substrates (TBARS) using lipid peroxidation in vitro assay when compared to tetraethoxypropane [ 27 ].
Neuroprotective activity
Aqueous extract of P. betle leaves (100, 200 and 300 mg/kg) administered through intragastric tube to alcohol-treated female albino Wistar rats significantly (p < 0.05) decreased the level of lipids (free fatty acids, cholesterol and phospholipids), concentration of TBARS and hydroperoxides as well as increased non-enzymatic antioxidants (GSH, vitamin C and vitamin B) and enzymatic antioxidants (SOD, CAT and GPx) in the brain tissue [ 28 ].
Anti-ulcerogenic activity
Ethanol extract of P. betle leaves (100-250mg/kg) administered orally to Charles-Foster rats with gastric lesions induced by indomethacin significantly (p < 0.001 for 100, 150 and 200 mg/kg) (p < 0.01 for 250 mg/kg) reduced the mean ulcer index. The optimum dose of the extract (200 mg/kg) significantly (p < 0.001) reduced the malondialdehyde (MDA) concentration of gastric tissue and carbonyl content as well as increased the hexosamine and mucus content of gastric tissue compared to untreated ulcerated group [ 29 ].
Clinical studies
A clinical study was conducted to evaluate the antioxidant effect of ethanol extract of P. betle leaves (0.5 and 1.0 µg/mL) in the erythrocytes of 30 healthy individuals and 30 HbE-beta thalassemic patients. The extract significantly (p < 0.001) reduced the production of reactive oxygen species by geometric mean fluorescence channel (GMFC) in patients with HbE-beta thalassemia in a dose dependent manner with 37.99% reduction (0.5 µg/mL) and 44.70% (1 µg/mL) compared to healthy donors with 42.21% (0.5 µg/mL) and 47.11% (1 µg/mL) reduction. Further test conducted on the free radical scavenging activity of erythrocytes due to oxidative stress after incubation of hydrogen peroxide (0.5 mM and 1.0 mM) resulted in the extract significantly (p < 0.001) decreased the GMFC in healthy donors (0.5 µg/mL – 47.27% and 48.39% for 0.5 mM) and in HbE-beta thalassemia patients (0.5 µg/mL – 37.92% and 37.90% for 0.5 mM) [ 19 ].
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute toxicity
Oral single dose acute toxicity study on female Sprague Dawley rats (aged between 8 and 12 weeks old) using aqueous mixture of powdered P. betle leaves showed no toxic effect on the parameters observed, which include behaviours, body weight, food and water intakes. All rats were observed for 14 days and no death was found throughout the study period. Half lethal dose (LD50) is more than 2,000 mg/kg body weight [ 26 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Side-effect
Steamed P. betle leaves applied as facial dressing showed erythematous reaction upon first 3 days of application followed by irregular hyperpigmentation in unusual pigmentation patients that maybe caused by the reduction-oxidation reaction of eumelanin [ 30 ].
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- Seetha LB, Naidu KC. Comparative morphoanatomy of Piper betle L. cultivars in India. Annals of Biological Research. 2010;1(2):128-134.
- Cheng Y, Xia N, Gilbert MG. Piperaceae. In flora of China vol.4. Edited by Z. Wu & P.H. Raven. Missouri Botanical Garden, St.Louis, Missouri. 1999;p.110-129.
- Shirish AR, Anjali S, Nikhil K. SPAR profiles for the assessment of genetic diversity between male and female landraces of the dioecious betelvine plant (Piper betle L.). In (Oscar Grillo, ed.) Ecosystem Biodiversity, InTech Shanghai China. 2011;p.464.
- Satish AB, Deepa RV, Rohan VG, Nikhil CT, Yatin YR, Vinodkumar SD, Ashwin T. Phytochemistry, pharmacological profile and therapeutic uses of Piper betle Linn. Journal of Pharmacognosy and Phytochemistry. 2013;1(2):10-19.
- Prakash B, Shukla R, Singh P, Kumar A, Mishra PK, Dubey NK. Efficacy of chemically characterized Piper betle L. essential oil against fungal and aflatoxin contamination of some edible commodities and its antioxidant activity. International Journal of Food Microbiology. 2010;142:114-119.
- Sugumaran M, Gandhi SM, Sankarnarayanan K, Yokesh M, Poornima M, Rajasekhar SR. Chemical composition and antimicrobial activity of vellaikodi variety of Piper betle Linn leaf oil against dental pathogens. International Journal of PharmTech Research. 2011;3(4):2135-2139.
- Arambewela LSR, Arawwawala LDAM, Kumaratunga KG, Dissanayake DS, Ratnasooriya WD, Kumarasingha SP. Investigations on Piper betle grown in Sri Lanka. Pharmacognosy Reviews. 2011;5(10):159-163.
- Mohottalage S, Tabacchi R, Guerin PM. Components from Sri Lankan Piper betle L. leaf oil and their analogues showing toxicity against the housefly, Musca domestica. Flavour and Fragrance Journal. 2006;22(2):130-138.
- Nalina T, Rahim ZHA. The crude aqueous extract of Piper betle L. and its antibacterial effect towards Streptoccus mutans. American Journal of Biochemistry and Biotechnology. 2007;3(1):10-15.
- Burkill IH. A dictionary of the economic products of the Malay Peninsula. Kuala Lumpur: Ministry of Agriculture Malaysia. 1935;p.1440.
- Musa Y, Azimah AK, Zaharah H. Tumbuhan Ubatan Popular Malaysia. Serdang, Kuala Lumpur: MARDI. 2009;p.150.
- Kamarudin MS, Latiff A. Tumbuhan ubatan Malaysia. Kuala Lumpur: UKM. 2002;p.153-156.
- Sarkar A, Sen R, Saha P, Ganguly S, Mandal G, Chatterjee M. An ethanolic extract of leaves of Piper betle (Paan) Linn mediates its antileishmanial activity via apoptosis. Parasitology Research. 2008;102(6):1249-1255.
- Ramji N, Ramji N, Iyer R, Chandrasekaran S. Phenolic antibacterials from Piper betle in the prevention of halitosis. Journal of Ethnopharmacology. 2002;83:149-52.
- Sarkar M, Gangopadhyay P, Basak B, Chakrabarty K, Banerji J, Adhikary P, Chatterjee A. The reversible antifertility effect of Piper betle Linn. on Swiss albino male mice. Contraception. 2000;62(5):271-274.
- Choudhary D, Kale RK. Antioxidant and non-toxic properties of Piper betle leaf extract: in vitro and in vivo studies. Phytotherapy Research. 2002;16(5):461-466.
- Saravanan R, Prakasam A, Ramesh B, Pugalendi KV. Influence of Piper betle on hepatic marker enzymes and tissue antioxidant status in ethanol-treated Wister rats. Journal of Medicinal Food. 2002;5:197-204.
- Dasgupta N, De B. Antioxidant activity of Piper betle L. leaf extract in vitro. Food Chemistry. 2004;88(2):219-224.
- Srimani P, Mandal G, Ganguly S, Saha P, Sen R, De R, Chatterjee A, Bhattacharyya M, Chaudhuri U, Bandyopadhyay SK, Chatterjee M. Antioxidant effect of ethanolic extract of Piper betle Linn. (Paan) on erythrocytes from patients with HbE- beta thalassemia. Indian Journal of Biochemistry and Biophysics. 2009;46:241-246.
- Arani D, Shreya G, Mukesh S. Antimicrobial property of Piper betel leaf against clinical isolates of bacteria. International Journal of Pharma Sciences and Research. 2011;2(3):104-109.
- Evans PH, Bowers WS, Funk EJ. Identification of fungicidal and nematocidal components in the leaves of Piper betle (Piperaceae). Journal of Agricultural and Food Chemistry. 1984;32(6):1254-1256.
- Arambewela LSR, Arawwawala LDAM, Ratnasooriya WD. Antidiabetic activities of aqueous and ethanolic extracts of Piper betle leaves in rats. Journal of Ethnopharmacology. 2005;102(2):239-245.
- Hajare R, Darvhekar VM, Shewale A, Patil V. Evaluation of antihistaminic activity of Piper betle leaf in guinea pig. African Journal of Pharmacy and Pharmacology. 2011;5(2):113-117.
- Young SC, Wang CJ, Lin JJ, Peng PL, Hsu JL, Chou FP. Protection effect of piper betel leaf extract against carbon tetrachloride-induced liver fibrosis in rats. Archives of Toxicology. 2007;81(1):45-55.
- Devjani C, Barkha S. Antimicrobial, antioxidative and antihemolytic activity of Piper betle leaf extracts. International Journal of Pharmacy and Pharmaceutical Sciences. 2011;3(3):192-199.
- Teh BP, Hamzah NF, Rosli SNS, Yahaya MAF, Zakiah I, Murizal Z. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2012. Report No. HMRC 11-045/01/PB/L/J.
- Sayanti B, Mahesh S, Susri R, Ajay KB, Jaya PK, Subrata C and Sandip KB. Radioprotective property of the ethanolic extract of Piper betle leaf. Journal of Radiation Research. 2005;46:165–171.
- Saravanan R, Rajendra PN, Pugalendi KV. Effect of Piper betle leaf extract on alcoholic toxicity in the rat brain. Journal of Medicinal Food. 2003;6(3):261-265.
- Biswajit M, Susri RC, Arun R, Sandip KB. Potent antiulcerogenic activity of ethanol extract of leaf of Piper betle Linn by antioxidative mechanism. Indian Journal of Clinical Biochemistry. 2002;17(1):49-57.
- Liao YL, Chiang YC, Tsai TF, Lee RF, Chan YC, Hsiao CH. Contact leukomelanosis induced by the leaves of Piper betle L. (Piperaceae): a clinical and histopathologic survey. Journal of the American Academy of Dermatology. 1999;40(4):583-589.