Scientific Name
Piper nigrum L.
Synonyms
Muldera multinervis Miq. [1]
Vernacular Name
| Malaysia | Lada hitam, lada puteh, lada padi [2], lada sulah [3], lada [4] |
| English | Pepper, black pepper, white paper [2], black peppercorns, common pepper, green peppercorns, Madagascar pepper, Malabar black peppercorns, pepper plant, pepper vine, round pepper, Tellicherry peppercorns, whole black pepper [4] |
| China | Hu jiao (hu chiao), hei hu jiao, bai hu jiao, woo jiu [2], hu chiao [4] |
| India | Milagoo, milaagu [2], aguttam, cavyam, kalinai, kami, kantanakuli, kari, kola, kurumilagu, miri, miricam, miriyalu, muntan, shudha, shyama, repam, ruksha, suvrtta, uciram, utanam, vara, vellija, yavanapriya, zira siyah [4] |
| Indonesia | Lada, marica hitam [1], pedes (Sundanese); lado ketek, lado kobon (Sumatran); maricha, micha (Javanese) [3]; maritja, merica [4] |
| Philippines | Paminta [2], malisa, pamienta, paminta-luso [4] |
| Myanmar | Nayukon, nga youk kuan [2] |
| Cambodia | Mréch, morech [2] |
| Laos | Mak phik noi, phi noi, phik noy, phik Thai [2] |
| Thailand | Phrik-thai (Central) [2]; phrik-noi [4] |
| Vietnam | Ti[ee]u, h[oof] ti[ee]u, trieu, hat trieu [2], may loi, hat tieu [4] |
| French | Poivre commun, poivre blanc, poivre noir [2] |
| Tibet | Na le sa ma, na le sam, na-le-sham, nale sham, pho-ba-ri, pho(ba)ris [4] |
| Papua New Guinea | Daka [4] |
| Tanzania | Pilipili manga [4] |
| Madagascar | Tsimahalatsaka [4]. |
Geographical Distributions
Piper nigrum is native to India and Sri Lanka. It is now widely cultivated in the tropics worldwide. The most suitable climate for P. nigrum is per-humid tropical requires direct sunlight and tolerates little shade with a well-distributed annual rainfall of 2000-4000 mm associated with a mean air temperature of 25-30°C and a relative humidity of 65-95%. [3]
Botanical Description
P. nigrum is a member of the family Piperaceae. It is a perennial woody climber that can reach up to 10 m long or more. They are 3-4 m tall and measuring 1.25 m in diametre. The mature plants that grow on supports may also appear as bushy columns. [5]
The leaves are arranged alternately, simple, hairless, coriaceous and petiolate. The petiole is 2-5 cm long. The blade is ovate, measuring 8-20 cm x 4-12 cm, entire, oblique to rounded at the base, with tip acuminate, shiny dark green above, pale and densely glandular-dotted beneath with 5-7 veins. [5]
The inflorescence is with a spike, appeared opposite the leaves on the plagiotropic branches and measures 3-15 cm long with 50-150 flowers. The flowers are unisexual or bisexual (cultivars usually up to 90% bisexual flowers) and without perianth. There are 2-4 stamens and with 3-5 lobes stigma. [5]
The fruit is a spherical drupe, measuring 4-6 mm in diametre, sessile, with pulpy mesocarp and red when mature. The seed is spherical and measuring 3-4 mm in diametre. [5]
The root system is with 5-20 main roots, measure 4 m or more deep and with feeder roots in the upper soil of 60 cm which form an extensive dense mat. The orthotropic stems climb and remaining vegetative, adhering to support with short adventitious roots that present at the nodes. The internodes are 5-12 cm long and measuring 4-6 cm in diametre. The plagiotropic branches are generative, without adventitious roots, measure 4-6 cm long of internodes and measuring 1-1.5 cm in diametre and produce higher-order of branches as well as the inflorescences. [5]


Cultivation
Soil Suitability and Climate Requirement
P. nigrum vines prefer moist, hot, tropical climates for optimum growth. It requires evenly distributed annual rainfall of about 2,500 mm and grows best on flat or gently sloping land from sea level up to approximately 600 m. Maximum production occurs on deep soils rich in organic matter and medium texture, pH of about 5.5 to 6.0, well drained and good aeration. The plant can tolerate some shade. [6]
Field Preparation
Land Preparation
Prior to planting of P. nigrum, normal field operations such as land clearing, disc ploughing and rotovation are to be conducted. The disc ploughing is done to prepare the area for liming activity if the pH of the area is low (acid soil). The second ploughing by using rotovator must be carried out after application of the liming material to ensure the material is well incorporated into the soil. Field drainage system should also be established in areas that are easily waterlogged. [6]
Production of Planting Materials
P. nigrum is normally propagated vegetatively by using stolon cuttings selected from the vigorous, healthy and high yielding mother vines. The young stolon obtained from the upper part is preferred. These stolons are firstly sowed into the polybags and kept under shade for about 8 weeks. The seedlings are ready for field planting when the new shoots grow to about 10 cm long. [6]

Field Planting
The recommended planting distance for monocrops of P. nigrum is 2.5 m between rows and 2.0 m between plants in the row. This will give the population density of about 2,000 plants/ha. The long planting poles of about 4 m or compatible trees are required as the support for growth. Several cuttings may be planted adjacent to each support. Occasional pruning are carried out to encourage lateral branching and keeps the plant to the required height. [6][7]
Field maintenance
Fertilisation
Both the organic and inorganic fertilisers are usually applied in pepper cultivation. The organic manure (chicken dung) at the rate of 2-4 t/ha is incorporated into the planting hole about 7 days before planting. The recommended inorganic fertiliser for the established crop is 300 kg N, 100 P2O5 and 300 K2O ha/year. Both fertilisers (organic and inorganic) are divided into 4 equal portions and given at 3-month intervals. Half of these rates are given for the immature plants. [6][8]
Weed Control
The weeds are normally controlled manually by using grass cutters during the initial stages of crop growth. The organic mulch such as dry grasses is given around the planting points to control weeds. The mulch also helps to conserve moisture and reduce the soil temperature, especially during the drier months. Very minimal weed problem is observed when the plants are fully developed. [6]
Water management
P. nigrum requires constant moist soil, but not water logged for maximum vines growth and yield. Hence, supplementary irrigations should be given during the dry months. The drip irrigation is recommended since it is easier to manage, cheaper and requires less water. Proper drainage systems should be established to avoid flash floods. [6]
Pest and Disease Control
The primary disease problem with P. nigrum cultivation is root rot and foot rot caused by Phytophthora capsici and stem rot and wilts disease caused Fusarium solani. Symptoms of these diseases include the wilting of leaves and discolouration of stems near the soil line. The main insect pests are stem borer (Lophobaris piperis) and thrips (Leothrips crassipes). Good phytosanitary measures and the proper use of proper pesticides and fungicides could help to control these diseases and pests. [6][9]
Harvesting
First harvest can be done at 3 years after planting. The full potential is realised at 7-8 year from planting and remained most productive until about 20 years of age. The potential yield of fresh berries from fully-grown crop is about 11,200 kg/ha (or 3,100 kg/ha dried). Ripe berries may be picked about 9 months after flowering. Berries ripen over a period of 2–6 months depending on climate. Berries are usually harvested every 7–14 days during the harvesting period. [6]

Postharvest handling
The P. nigrum berries are harvested when the colour is greenish yellow. It is then dipped in boiling water for about 10 minutes. This provides a surface disinfestation and starts the fermentation process, which turns the berries black. Berries are dried in the sun after the hot water treatment. Depending on the weather, it takes about 14 days of sun drying in order to reduce the moisture to about 12%. Good postharvest handling will help to produce a uniform (brown to black) berries having pungent smell and free from fungal infestations. [6]
Estimated cost of production
The estimated total cost of production for pepper is RM 22000 per hectare. The cost covers the both the cost of initial development (RM6500) and crop maintenance (RM 15500). At the production level of about 3,200 kg/ha, the cost of production for a kilogram of black pepper (dried berries) from the established crop is about RM 4.80 / kg. The cost of production was estimated based on the current inputs cost during writing of this article. [6]
Chemical Constituent
Methanol extract of P. nigrum fruits (black pepper) has been reported to contain alkamides (e.g. retrofractamide A, pipercide, piperchabamide D, pellitorin, dehydroretrofractamide C, dehydropipernonaline, and guineensine). [10][11]
Ethanol extracts of P. nigrum fruits has been reported to contain amide alkaloids (e.g. pipericine, piperine, [(2E,4E)-octadienoyl]-N-isobutylamide, [(2E)-hexadecanoyl]pyrrolidine, [(2E,4E)-octadecadienoyl]-N-isobutylamide, (2E,4E)-eicosadienoyl-N-isobutylamide, [(2E,4E)-octadecadienoyl]piperidine, pipercallosine, tricholein, trichostachine, 1-piperettylpyrrolidine, ∆α,β-dihydrowisanidine, retrofractamide A, retrofractamide D, piperettine, pipwaqarine, piperamide-C5:1(2E), piperamide-C7:1(6E), piperamide-C7:2(2E,6E), piperamide-C9(8E), piperamide-C9:2(2E,8E), piperamide-C9:3(2E,4E,8E), 1-[(2E,4E)-2,4-decadienoyl]pyrrolidine, 1-[(2E,4E)-2,4-dodecadienoyl]pyrrolidine, piperylin, pipercide, guineensine and piperolein B and others (e.g. 30,40-methylenedioxycinnamaldehyde, β-caryophyllene, β-elemene and octadecanoic acid). [12][13][14]
Acetone extract of P. nigrum fruits has been reported to contain amides (e.g. (E,E)-N-(2-methylpropyl)- 2,4-decadienamide, (E,E,E)-13(1,3-benzodioxol-5-yl)-N(2-methylpropyl)-2,4,12-tridecatrienamide and (E,E,E)-11-(1,3-benzodioxol-5-yl)-N-(2-methylpropyl)-2,4,10-undecatrienamide). [15]
Chloroform extracts of P. nigrum fruits has been reported to contain alkaloids (e.g. pipercyclobutanamides A and B) and sesquiterpene (e.g.β-caryophyllene). [16][17]
Petroleum ether (60-80°) extract of P. nigrum fruits has been reported to contain alkamide (e.g. isopiperolein B). [18]
Petroleum ether extracts of P. nigrum fruits has been reported to contain amides (e.g.2E,4E,8Z–N-isobutyleicosatrienamide, pellitorine, trachyone, pergumidiene, isopiperoleine B, pipnoohine, pipyahyine, [(2E,4E)-octadienoyl]-N-isobutylamide, sarmentine, kalecide, [(2E,4E)-dodecadienoyl]pyrrolidine, pellitorine, hexadecanoylpyrrolidine, [(2E)-octadecanoyl]pyrrolidine, 1-[(2E,4E,12Z)-octadecatrienoyl]-N-isobutylamide, piptaline and 1-[7-(3,4-methylenedioxyphenyl)-(2E,4E)-heptadienoyl]-N-isobutylamide) and others (e.g. stigmastanol, β-sitosterol, stigmasterol, stigmastanol 3-O–β–D-glucopyranoside, β-sitosterol 3-O–β–D-glucopyranoside, hexadecanoic ethyl ester, octadecanoic acid and 1-(3,4-methylenedioxyphenyl)-(1E)-tetradecene). [19][20]
Hexane extract of P. nigrum fruits has been reported to containamides (e.g. piperine, piperolein B, pediculicide and (2E,4E)-N-isobutyl-2,4-decadienamide). [21]
Diethyl ether extract of P. nigrum fruits has been reported to contain alkaloid (e.g. piperine or 1-piperoylpiperidine (C17H19N03). [22]
Ethanol and ethyl acetate oleoresins of P. nigrum fruits has been reported to contain β-caryophyllene, α-humulene, β-selinene, α-selinene, α-muurolene, β-bisabolene, δ-cadinene, elemol, caryophyllene oxide, torreyol, pellitorin, 2,4-decadienoic acid piperidide, plasticizer, piperanine, N-isobutyl-(2E,4E,12E)-octadecatrienamide, N-isobutyl-(2E,4E)-octadecadienamide, retrofractamide B, piperine, 4,5-dihydropiperettine, N-isobutyl-(2E,4E,14Z)-eicosatrienamide, piperamide C 9:1 (8E), piperolein B, and dehydropipernonaline. Meanwhile other components (e.g. α-pinene, α-thujene, sabinene, β-pinene, 3-carene, ρ-cymene, limonene, β-phellandrene, δ-elemene, α-copaene, β-elemene, trans-nerolidol, dill-apiole, 1-octadecene, octadecane, nonadecane, palmitic acid, 1-eicosene, eicosane, 1-cinnamoylpiperidine, heneicosane, docosane, and guineensine) was found only in ethyl acetate oleoresins. [23]
Essential oil of P. nigrum fruits (black pepper) has been reported to contain monoterpenoids (e.g. α-pinene, camphene, β-pinene, myrcene, 3-carene, δ-carene, β-carene, α-phellandrene, β-phellandrene, α-pinene oxide, limonene oxide, safrole, limonen-6-ol, limonene, γ-terpinene, ρ-cymene, terpinolene, α-thujene, citronellol, α-terpinene, δ-limonene, ρ-phellandrene, ocimene, 3-methylbutanal and methylpropanal, 1,8-cineol, linalool, α-terpineol, 1-terpinen-4-ol, 1-terpinen-5-ol, ρ-cymene-8-ol, ρ-cymen-8-ol-methyl ether, trans-carveol, cis-carveol, trans-pinocarveol, dihydrocarveol, borneol, dihyrocarvone, nerol, carvacrol, (E)-β-ocimene, camphor, sabinene, cis– and trans-sabinene hydrate, m-mentha-3(8),6-diene (isosylveterpinolene) and carvone), sesquiterpenoids (e.g. β-farnesene, trans-β-farnesene, (E)(E)-β-farnesene, α-humulene, nerolidol, (E)-nerolidol, δ-gurjunene, α-gurjunene, β-gurjunene, α-cububene, calarene, thujopsene, γ-muurolene, α-muurolol, β-cubebene, γ-cadinene, δ-cadinene, cuparene, α-copaene, copaene, δ-guaiol, calamenene, cadinol, elemol, β-eudesmol and farnesol (Z,E)), bisabolane-type sesquiterpenoids (e.g. β-bisabolene, curcumene, ar-curcumene and α-curcumene), guaiane-type sesquiterpenoids (e.g. δ-guaiene and α-guaiene), elemane-type sesquiterpenoids (e.g. β-elemene, γ-elemene and δ-elemene), germacrane type (e.g. germacrene D, germacrene B and bicyclogermacrene) santalene-type sesquiterpenoid (e.g. α-santalene), bergamotene-type sesquiterpenoids (e.g. α-cis-bergamotene and α-trans-bergamotene), caryophyllane-type sesquiterpenoids (e.g. β-caryophyllene alcohol, isocaryophyllene, caryophyllene oxide, β-caryophyllene, epoxydihydrocaryophyllene, and trans-caryophyllene), sesquisabinane-type sesquiterpenoid (e.g. sesquisarbene), selinene-type sesquiterpenoids (e.g. α-selinene and β-selinene), cedrane-type sesquitepenoid (e.g. cedrene) and others (e.g. cadina-1,4-diene, cis-piperitol, cyclosativene, tricyclene, calamine, phenylacetic acid, cis-ρ.menthen-1-ol, cis-ρ.-2,8-methandien-1-ol, 2-undecanone, myrtenal, carvetonacetone, myrtenol, 1(7),2-p-methadien-6-ol, 4,10,10-trimethyl-7-methylene bicycle [6.2.0]-decane-4-carboxaldehyde, caryophylla-3(12),7(15)-dien-4β-ol, caryophylla-2,7(15)-dien-4β-ol, caryophylla-2,7(15)-dien-4α-ol, cis-ρ-2- methen-1-ol, cis-ρ-2,8-menthadien-1-ol, 2-isopropyl-3-methoxypyrazine, 2,3-diethyl-5-methylpyrazine, 1-formylpiperidine, 1-acetylpiperidine, 2,3,5,6-tetramethylpyrazine, pyrazine, pyridine, cyclohexane, 1-methyl-4-(1-methylethylidene), 3-cyclohexen-1-ol, 4-methyl-1-(1-methylethyl), pivalate, (E)-3(10)-caren-4-ol, bicyclo[3.1.0]hexane, 4-methylene-1-(1-methylethyl), bufa-20,22-dienolide,14-hydroxy-3-oxo-(5á), 2-cyclohexen-1-ol, 1-methyl-4-(1-methylethyl)-, eudesma-4(14),11-diene, trans-9-octadecenoic acid, trimethylsilyl ester, 1,2-dihydropyridine, 1-(1-oxobutyl),1-chloroeicosane, β-pinone, 1,1,4-trimethylcyclohepta-2,4-dien-6-one, 3,8(9)-ρ-menthadien-1-ol, N-formyl piperidine, 1(7),2-p-methadien-4-ol, 5,10(15)-cadiene-4-ol, cryptone, methyl eugenol,myristicin, cis-β-ocimene, (E)-β-caryophyllene, ∆-3-carene, hedycaryol, α-terpineneol, α-cubebene, α-quaiene, (E)-β-farnesen, zingiberene, α-farnesen, caryophyllenol, ledol, δ-cadinol, T-muurolol, α-cadinol, T-cadinol, α-farnesol, and α-bisabolol). [23–45]
Ethyl acetate extract of P. nigrum kernels has been reported to contain piperamide (e.g. pellitorine, piperylin, 4,5-dihydropiperlonguminine, piperlonguminine, 4,5-dihydropiperine, piperine, and pipercide). [46]
Aqueous extract of P. nigrum seeds (black pepper) has been reported to contain choline and acetylcholine. [47]
Ethanol extract of P. nigrum seeds has been reported to contain alkamides (e.g. guineensine, retrofracamide C, (2E,4Z,8E)-N-[9-(3,4-methylenedioxyphenyl)-2,4,8-nonatrienoyl]piperidine, pipernonaline, piperrolein B, piperchabamide D, pellitorin and pipkirine), piperine, piperonal and pipercide) and others (e.g. stigmastanol, stigmasterol, stigmastanol glucoside, stigmasterol glucoside, cinnamylideneacetone, methylenedioxyphenylpropiophenone and 2-hydroxy-4,5-methylenedioxypropiophenone). [48][49]
Acetone extract of P. nigrum seeds has been reported to contain amide alkaloids (e.g. piperylin, piperine, N–trans-feruloyltyramine, retrofractamide A, piperamide, piperolein, piperettine and guineensine), lignin (e.g. hinokinin) and sesquiterpenes (e.g. torreyol, α-copaene,β-caryophyllene, β-bisabolene, δ-cadinene, spathulenol and caryophyllene oxide). [24]
Petroleum ether extract of P. nigrum seeds has been reported to contain amides (e.g. piptigrine, piperine, wisanine, pipsaeedine, pipbinine, (E,E)-N-(2-methylpropyl)dodeca-2,4-dienamide, (E,E)-N-(2-methylpropyl)hexadeca-2,4-dienamide, piptaline, piperanine, ∆,α,β-dihydrowisanidine, ∆,α,β-dihydrowisanine, (E,E,E)-1-{[9-[3,4-(methylenedioxy)phenyl]nona-2,4,8-trienoyl}pyrrolidine, (E,E,E)-1-{11-[3,4-(methylenedioxy) phenyl]undeca-2,4,10-trienoyl}piperidine, 1-piperettylpyrrolidine and piperettine) and fatty acids (e.g. capric, lauric, myristic, palmitic, stearic, oleic, linoleic, malvalic, sterculic and vernolic). [50][51][52]
Essential oil of P. nigrum roots has been reported to contain a major component of trans-caryophyllene. [53]
Plant Part Used
Fruit (peppercorns), leaves, stem and roots [54]
Traditional Use
P. nigrum has been used in India for centuries as traditional remedies in Ayurvedic, Unani and Siddha medicines. It has been used for illnesses such as constipation, diarrhoea, earache, gangrene, heart disease, hernia, indigestion, insect bites, insomnia, joint pain, lung diseases, liver problems, tooth decay, toothache and treatment of eye problems by applying pepper salves or poultices directly onto the eyes. [2]
P. nigrum has also been used as one of the ingredients of jamu tonic, indigestion mixture with ginger, electuary or paste mixed with honey and preparation for after childbirth. It has been used externally as a counter-irritant, as poultices for colic, rheumatism and headache, and by smearing on the body for after childbirth. P. nigrum oil could be used for cholera. High dose of P. nigrum with wild bamboo shoots may be used for abortion. Malay women have used P. nigrum pills with honey and ginger as an abortifacient. [55]
P. nigrum L. is acrid and is easily recognizable when applied to the tongue. Upon swallowing it creates a sensation of warmth in the stomach assisting digestive functions. It is a stimulant of the secreting system. It is given in cases of vomiting, abdominal pain, diarrhoea and anorexia. The Japanese use pepper to treat acute gastroenteritis. In Chinese Traditional Medicine the roots and stems are used to treat vomiting and abdominal pain by giving a decoction of it orally. [54]
P. nigrum is being used in the treatment of inflammatory diseases like gout and rheumatism. In these cases large doses of the drug is being prescribed [56]. Dioscorides recommend its use in infective inflammatory process like tonsillitis and scrofulous tumour [57].
Preclinical Data
Pharmacology
Antibacterial activity
The isolated amide alkaloids compounds (i.e. 2E, 4E, 8Z-N-isobutyleicosatrienamide, trachyone, and pergumidiene) from the petroleum ether extract of the P. nigrum berries showed antibacterial activity against Gram positive i.e. Bacillus subtilis (MIC value of 34, 30 and 58 μM, respectively), Bacillus sphaericus (17, 30 and 29 μM, respectively), and Staphylococcus aureus (34, 30 and 29 μM, respectively) as well as Gram negative bacterial strains i.e. Klebsiella aerogenes and Chromobacterium violaceum with MIC values of 70, 60, 58 μM with respect to the three compounds. [19]
Ethanol extract of P. nigrum ripe fruit showed antimicrobial activity by effectively inhibited the growth of S. aureus by the agar-well diffusion method compared to cephazolin as a standard antibiotic. [58]
Volatile oil of P. nigrum showed strong antimicrobial activity towards Aeromonas hydrophila with zones of growth inhibition more than 90 mm and mild activity toward Acinetobacter calcoacetica (12.3±0.3 mm), Beneckea natriegens (10.8±0.7 mm), Brevibacterium linens (15.9±1.0 mm), Citrobacter freundii (12.0±1.6 mm), Erwinia carotovora (12.9±1.0 mm), Flavobacterium suaveolens (10.1±0.1 mm), Leuconostoc cremoris (16.3±0.8 mm), Micrococcus luteus (12.4±0.1 mm), S. aureus (14.5±0.3 mm), and Yersinia enterocolitica (11.7±2.2 mm). [59]
Antifungal activity
Acetone extract and essential oil from P. nigrum fruits (black pepper) were tested for their antifungal activity against various pathogenic fungi by inverted petriplate and food-poisoning techniques. In the food-poisoning technique, the essential oil (6 µL dose) possessed more than 80% activity in controlling the mycelial growth of Fusarium graminearum, Penicillium viridicatum and Curvularia lunata, and the extract (6 µL dose) inhibited 100% mycelial growth of P. viridicatum and Aspergillus ochraceus. In inverted petriplate technique, only the essential oil (6 µL dose) was found to be 100% effective in controlling the mycelial growth of F. graminearum and more than 70% effective against Aspergillus niger, Aspergillus oryzae and Penicillium madriti. [24]
Antioxidant activity
Water and ethanol extracts of P. nigrum (75 mg/mL) significantly (p < 0.01) decreased DPPH (2,2′-diphenyl-1-picrylhydrazyl) free radical scavenging capacity at 55% and 48%, respectively, in comparison with positive controls; butylated hydroxyl anisole (79%), butylated hydroxytoluene (76%) and α-tocopherol (78%). [60]
Ethanol extract of P. nigrum fruit (250 μg/mL) exhibited a 74.61 ± 0.02% DPPH free radical scavenging capacity with (inhibitory concentration at 50% (IC50))value of 14.51 μg/mg. [61]
Two fractions (R2 and R3) of petroleum ether extract of P. nigrum fruits showed significant (p < 0.05) antioxidant activities via different mechanisms as compared to the respective positive controls including inhibition of lipid peroxidation in a linoleic acid emulsion (500 μg/mL; R2 = 58.79%, R3 = 60.48%, α-tocopherol = 76.47%), DPPH free radicals (250 μg/mL; R2 = 61.24%, R3 = 61.11%, butylated hydroxyl anisole = 82.59%), nitric oxide free radical generation (100 μg/mL; R2 = 40.23%, R3 = 55.68%, curcumin = 84.27%), superoxide anion radicals (100 μg/mL; R2 = 62.23%, R3 = 70.22%, butylated hydroxyl toluene = 81.54%) and hydroxyl radicals (1000 μg/mL; R2 = 61.04%, R3 = 63.56%, catechin = 70.95%). [62]
Essential oil and oleoresin extract of P. nigrum showed antioxidant activity by exhibit strong free radical scavenging activity when evaluated against mustard oil by peroxide, p-anisidine, and thiobarbituric acid compared to butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) but lower than that of propyl gallate (PG). The extract also showed inhibition and scavenging activiy towards DPPH radical. [23]
Essential oil of P. nigrum fruits (50 , 20, 10, and 5 g/ L) showed moderate antioxidant activity evidenced with its radical scavenging activity towards DPPH (61%, 37%, 22%, and 14% inhibition, respectively), ferric reducing antioxidant power (FRAP) (11 mmol/L, 3 mmol/L, 2 mmol/L, and 1 mmol/L, respectively), thiobarbituric acid reactive species (TBARS) assay using antioxident index (AI) (36 %, 36 %, 27 %, and 16 %, respectively), and the determination of oxidative stability of fat for P. nigrum showed antioxidant activity index (AAI) of 0.9. [63]
Water and ethanol extracts of P. nigrum (75 µg/mL) showed strong antioxidant and radical scavenging activities as proven by their series of assays including total antioxidant activity, reducing power, DPPH free radical scavenging, superoxide anion radical scavenging, hydrogen peroxide scavenging, and metal chelating activities. The extracts showed 95.5% and 93.3% inhibition on peroxidation of linoleic acid emulsion, respectively, comparable to standard antioxidant butylated hydroxyanisole (BHA) (92.1%), butylated hydroxytoluene (BHT) (95.0%) and α-tocopherol (70.4%). In addition the total phenolics content of the extracts (1 mg) were determined to be 54.3 and 42.8 µg gallic acid equivalent of phenols when detected by the Folin-Ciocalteu procedure. [64]
P. nigrum showed antioxidant activity in high fat diet-induced oxidative stress in male Wistar rats (95-115 g) compared to control rats for duration of 10 weeks. It was reported that supplementing the diet with black pepper and/or piperine significantly reduced TBARS, conjugated dienes (CD) and maintained the activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione-S-transferase (GST) and glutathione (GSH) in the liver, heart, kidney, intestine and aorta. [65]
P. nigrum as part of salt-spice-herbal mixture namely Amrita Bindu (4 g/kg of feed) fed to rats for duraton of 3 weeks showed the highest antioxidant activity among 5 plants species against the free radical 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) compared to normal fed rats. The rats pretreated with Amrita Bindu significantly lowered the levels of free radicals, lipid peroxidation and protein carbonyls along with significantly higher levels of antioxidants when compared with rats without Amrita Bindu after post injection with phenylhydrazine (PHZ). [66]
Anti-inflammatory activity
Piperine isolated from P. nigrum (5-40 mg/kg) administered orally to male albino Hindustan Antibiotics strain rats (100-125 g) showed a significant anti-inflammatory activity on carageenan-induced paw edema (45-240 min), except for the dose of 5 mg/kg BW at 45 min, with values in the range of 12 to 99.2% compared to the normal control (38.3-121.2%) and positive control, oxyphenyl butazone (OPB, 50 mg/kg: 17.2-48.7%) groups. In histamine and formaline-induced paw edema experiments, piperine gave 28.3% and 32.8% anti-inflammatory activity, respectively, compared to OPB group (32.8 and 38.1%, respectively). Piperine also exhibited anti-inflammatory activity in rats induced with cotton pellet granuloma with cotton pellets weight of 20.4 mg compared to normal control (36.21 mg) and OPB (22.60 mg) groups. The weight of granuloma of croton oil-induced inflammatory assay of rated treated with P. nigrum was 0.339 mg compared to normal control (0.846 mg) and OPB (0.321 mg) groups. [67] Another study showed piperine also reduced liver lipid peroxidation, acid phosphatase, and edema in carrageenin induced rat paw oedema, comparable to standard oxyphenylbutazone. [68]
Intercellular cell adhesion molecules binding inhibitory activity
Isolated compounds ((2E,4Z,8E)-N-[9-(3,4-methylenedioxyphenyl)-2,4,8-nonatrienoyl]piperidine, pipernonaline, piperrolein B, pellitorin and dehydropipernonaline) from P. nigrum fruits inhibited direct binding between sICAM-1 (intercellular cell adhesion molecules) and LFA-1 (lymphocyte function associated antigen-1) of THP-1 cells (human monocytic leukaemia cell line) with IC50 values of 10.7, 8.8, 13.4, 13.5 and 6.0 μg/mL, respectively. Inhibitors of LFA-1/ICAM-1 mediated cell adhesion are potentially useful for the treatment of inflammatory diseases. [48]
Immunomodultary activity
Polysaccharides, PN-Ib and PN-IIa, isolated from P. nigrum fruits showed significant (p < 0.05) anti-complementary activity with 50% inhibition of total complement hemolysis (ITCH50) values of 96.5 ± 2.2% and 98.7 ± 1.9%, respectively, compared to the positive control, polysaccharide-K (60.2 ± 1.5%). [69]
Aqueous extract of P. nigrum fruits significantly enhance proliferation of T helper (Th)1 but suppress (Th)2 cytokine release by splenocytes dose-dependently. [70]
Anticancer activity
Anticarcinogenic activity
Piperine isolated from P. nigrum was investigated for inhibition or reduction of the oxidative changes induced by chemical carcinogens in rat intestinal model. A segment of intestinal luminal of Charles foster male rats (150-200 g) was ligated and carcinogenesis-induced with 7,12-dimethyl benzanthracene (DMBA, 50 μg/mL), dimethyl amino-methyl azobenzene (DMAMAB, 20 mg/mL) and 3-methyl cholenthrene (3-MC, 3 mg/mL). Aqueous suspension of piperine in 1% carboxy methylcellulose equivalent to optimum concentration of 3.5 mM was exposed to the ligated segment. Piperine treatment with carcinogens significantly inhibited thiobarbituric reactive substances (TBARS) compared to DMBA-treated (p < 0.05), DMAMAB-treated (p < 0.01) and 3-MC-treated (p < 0.01) rat intestines. The carcinogens decreased levels of non-protein thiols (N-SH) in intestinal mucosa but exposure of piperine significantly (p < 0.01) decreased the levels. Piperine was also found to significantly reduce elevation of enzymes activities of γ-glutamyl transpeptidase (γ-GT) caused by DMAMAB and 3-MC (p < 0.001), as well as Na+-K+-ATPase induced by DMAMAB (p < 0.001), DMBA (p < 0.01) and 3-MC (p < 0.001). The study suggested a protective role of piperine against chemical carcinogen-induced oxidative stress. [71]
Dried P. nigrum fruit (black pepper) powder (0.5% w/w mixed with the diet and 20% peanut oil) was administered orally daily for 30 weeks to male Wistar rats (100-120 g) with 1,2-dimethylhydrazine (DMH)-induced colon cancer. DMH-induced group treated with P. nigrum had smaller tumour size (0.5 cm) compared to DMH-induced control (2 cm). Presence of vascular granulation in the former animal group might indicate protective mechanism towards tumour development to deeper layers. Excretion of fecal bile acids and neutral sterols in 24-hour fecal samples was significantly (p < 0.05) increased in DMH-induced group treated with P. nigrum. P. nigrum given to DMH-induced group had lower (p < 0.05) cholesterol/phospholipid ratio (0.500-0.919) and increased 3-hydroxy-3-methylglutaryl (HMG-CoA) reductase activity (1.08-2.14) in the colon, intestine and liver compared to the DMH-induced control (0.818-1.567 and 0.85-1.68, respectively). The results showed that supplementation of P. nigrum prevented accumulation of lipids in tissues and optimized the excretion of fecal sterols and bile acids, suggesting the risk reduction of colon cancer in the presence of the procarcinogen DMH. [72]
A pepper terpenoids of P. nigrum namely d-limonene force-fed to mice for a long duration reduced carcinogenic activity of the safrole, tannic acid and carcinogenic control substance methylcholanthrene (MCA)-induced tumours in mice. MCA showed stronger carcinogens compared to safrole and tannic acid. [73]
Antimetastasis activity
Piperine administered to B16F-10 melanoma cells-induced lung metastasis in C57BL/6 mice significantly reduced tumour nodule formation about 95.2%, reduced the lung collagen hydroxyproline (2.59 µg/mg protein) of the elevated metastasized lungs mice (22.37 µg/mg protein) compared to control (0.95 µg/mg protein), reduced the uronic acid (65 µg/100 mg tissue) from the high amount in the metastasized control mice (355.83 µg/100 mg tissue), reduced Lung hexosamine content was (0.98 mg/100 mg lyophilized tissue) compared to the untreated tumor-bearing animals (4.2 mg/100 mg lyophilized tissue), reduced the elevated levels of serum sialic acid and serum gamma glutamyl transpeptidase activity in the untreated control animals, and survived the 90 days of experiments. [74]
Antimutagenic activity
Aqueous extract of P. nigrum significantly (p < 0.05) reduced mutational events induced by promutagen agent ethyl carbamate using the wing somatic mutation and recombination test (SMART) in Drosophila melanogaster. The extract reduced 38% (1% w/v) and 46% (2% w/v) of total number of spots. [75]
Melanocyte proliferative activity
Aqueous extract of P. nigrum fruits (black pepper) (0.1 mg/mL) stimulated almost 300% growth of mouse melanocyte cell line, melan-a, in vitro in 8 days of experiment (p < 0.01) compared to piperine. [76]
Chloroform extract of dried P. nigrum seeds (black pepper) (containing equivalent concentration of 1 μM piperine) stimulated proliferation of copiously pigmented non-tumorigenic mouse melanocyte cells (melan-a) in vitro greater than the pure piperine (1 µM piperine), suggesting that other phytochemical compounds were also responsible for the activity. Thus, piperine (p < 0.01), guineensine (p < 0.05), pipericide (p < 0.05), piperettine (p < 0.01) and piperlonguminine (p < 0.01) isolated from the chloroform extract, also stimulated proliferation of melanocytes at a concentration range of 0.1-10 µM, compared with the positive control 12-O-tetradecanoylphorbol-13-acetate (20 and 200 nM). The results suggested the potential use in vitiligo. [77]
Melanogenesis stimulatory activity
Methanol extract of P. nigrum leaves and its two isolated lignans (i.e. (-)-cubebin (1) and (-)-3,4-dimethoxy-3,4-desmethylenedioxycubebin) showed significant stimulatory activity of melanogenesis in murine B16 melanoma cells without any significant effects on cell proliferation. [78]
Gastrointestinal activity
Food digestion
Piperine (20 mg %) included in the spice diet administered to albino rats aid the food digestion by stimulating the secretion of digestive enzymes such as pancreatic amylase, trypsin, chymotrypsin and lipase. However, piperine did not appear to have these enzymes activity when administered only by a single dose. [79]
Antispasmolytic
Methanol (80%) extract of P. nigrum (1-10 mg/mL) possessed a spasmodic effect in guinea pig ileum at a concentration dependent manner of acetylcholine (Ach, 0.3 µM)-induced contractions with IC50 value of 0.7 mg/mL, compared to positive control loperamide and nifedipine with IC50 values of 5.9 µM and 0.3 µM, respectively. When tested against potassium (K+, 80 mM)-induced contractions, P. nigrum inhibited contraction with IC50 value of 0.6 mg/mL, compared to loperamide and nifedipine with respective IC50 values of 0.9 µM and 0.04 µM. P. nigrum extract (1000 mg/kg) produced 12.4% wet feces, compared to positive control, carbachol (47.1%). P. nigrum (500 and 1000 mg/kg) reduced castor oil-induced fluid accumulation with respective (Pi/Pm)x1000 values of 143 and 90, compared to loperamide (78). P. nigrum extract (1000 mg/kg) exhibited 80% protective activity of castor oil-induced wet feces, compared to loperamide (10 mg/kg) as a positive control (p < 0.01). [80]
Effect of aqueous extract of P. nigrum fruits on utero-spasmolytic activity was studied. Adult female Wistar rats (240-320 g) were injected subcutaneously with estradiol valerate (0.5 mg/kg) 24 hours prior to experiment. The rats were sacrificed and a piece (1-1.5 cm) of cervical portion of the uterus was dissected and mounted in an organ bath. The extract (0.125-2 mg/mL) dose-dependently (p < 0.01-0.001) reduced uterus contractions of KCl-induced (60 nM) greater than that of oxytocin-induced (10 mU/mL). The spasmolytic effect of extract on the KCl-induced contractions was not reduced by L-NAME (nitric oxide synthase inhibitor, 100 μM), phentolamine (α-adrenoceptor antagonist, 1 μM) and naloxone (opoid receptor antagonist, 1 μM), however the extract spasmolytic effect was reduced in the presence of propranolol (β-adrenoceptor antagonist, 1 μM) (p < 0.01-0.0001). The extract (0.0312-0.25 mg/mL) also reduced calcium chloride-induced uterus contraction dose-dependently. The study indicated that utero-spasmolytic effect of aqueous extract of P. nigrum was mediated via voltage dependent calcium channels and by inhibition of β-adrenoceptors. [81]
Hot water extract of P. nigrum fruit was observed to be able to inhibit spasm of isolated rat ileum from KCl-induced contraction in male adult Wistar rats (21.3±5.6 g). Study suggested that the spasmolytic effects was possibly mediated via Ca2+ influx. [82]
Anticonvulsant activity
Piperine administered intraperitoneally to kainate-induced convulsions in mice showed significant inhibition of clonic convulsion (1 nmol) with an ED50 (and 95% confidence interval) of 46 (25-86) mg/kg, but did not appear to act as a kainate receptor antagonist. Piperine did not have or had only slight effects on seizure activity induced by L-glutamate, N-methyl-D-aspartate or guanidinosuccinate. [83]
Antidiabetic activity
Aqueous extract (0.5 mL/day) of P. nigrum seeds was administered orally to alloxan-induced diabetic on male albino Wistar rats once a day. After 4 weeks of experiment, blood glucose levels by treatment with the extract-treated group were 129 mg/100 mL compared to the insulin-treated (120 mg/100 mL), diabetic control (270 mg/100 mL) and normal control (102 mg/100 mL) groups. Total cholesterol (TC), low-density lipoprotein (LDL-C), high-density lipoprotein (HDL-C) and triglycerides (TG) values of the extract-treated group were 172.0, 90.0, 58.7 and 130 mg/100 mL, respectively, comparable to the insulin-treated group (165.5, 95.2, 48.2 and 138.6 mg/100 mL, respectively). Similarly, the activity of liver anti-oxidative stress enzymes, such as catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx) of the extract-treated group were 0.145 U/mg protein x 103, 15.68 U/mg protein and 0.131 U/mg protein, respectively, comparable to the insulin-treated group (0.138 U/mg protein x 103, 15.10 U/mg protein and 0.131 U/mg protein). The study suggested potential hypoglycemic effect of aqueous extract of P. nigrum seeds via antioxidant property. [84]
Four alkamides, (2E,4Z,8E)-N-[9-(3,4-methylenedioxyphenyl)2,4,8-nonatrienoyl]piperidine, pipernonaline, piperrolein B and dehydropipernonaline, isolated from chloroform extract of P. nigrum fruits inhibited acyl CoA:diacylglycerol acyltransferase (DGAT) in vitro with IC50 values of 29.8, 37.2, 20.1 and 21.2 µM, respectively. Inhibition of DGAT is associated with improved insulin sensitivity in vivo. [85]
Hypotensive and vasomodulator activity
Piperine administered intravenously to normotensive anaesthetized Sprague-Dawley (SD) male rats (200 to 250 g) resulted in a decrease in the Mean Arterial Pressure (MAP) in a dose-dependent manner. It also caused a partial inhibition of force and rate of ventricular contractions and coronary flow. Piperine inhibited high K+ (80 mM) pre-contractions and partially inhibited phenylephrine indicating it has a Ca2+ channel blockade activity. In a Ca2+ free medium piperine exhibited vasoconstrictor effect. There is endothelial-independent vasodilator effect more potent against high K+ percontractions than phenylephrine. [86]
Hepatoprotective activity
P. nigrum powder (0.5, 1 and 2% w/w mixed with diet) was fed to random-bred Swiss albino mice of either sex (aged eight weeks old) for 10 and 20 days each. Hepatic glutathione S-transferase (GST) was significantly and dose-dependently increased in both sex groups (1 and 2% w/w for 10 days: 1.22-1.38 µM 1-chloro-2-4-dinitrobenzene (CDNB)-GSH conjugate formed/min/mg protein (p < 0.05) vs. normal control, 1.12-1.13 µM CDNB-GSH conjugate formed/min/mg protein; 0.5-2% w/w for 20 days: 1.35-1.74 µM CDNB-GSH conjugate formed/min/mg protein (p < 0.05) vs. normal control, 1.26-1.31 µM CDNB-GSH conjugate formed/min/mg protein); similarly with the estimated content of acid-soluble sulfhydryl (–SH) (1 and 2% w/w for 10 days: 5.05-5.57 µM/g tissue (p < 0.05) vs. normal control, 5.03-5.24 µM/g tissue; 1 and 2% w/w for 20 days: 6.79-7.20 µM/g tissue (p < 0.05) vs. normal control, 6.48-6.49 µM/g tissue). Cytochromes b5 and P-450 were also significantly (p < 0.05) and dose-dependently elevated for all doses and both time periods and sex groups with values in the range of 0.40-0.70 (cyt. b5) and 0.65-0.96 (cyt. P-450) nM/mg protein compared to the normal control (0.35-0.36 and 0.50-0.56 nM/mg protein for the respective cytochromes). Significant reduction (p < 0.05) of lipid peroxidation (expressed in terms of malondialdehyde) was only observed for 2% w/w P. nigrum treated groups for 20 days. The study suggested that P. nigrum could be a potential inducer of hepatic detoxication system. [87]
Piperine showed antihepatotoxic activity evidenved by exerting a significant protection against tert-butyl hydroperoxide and carbon tetrachloride hepatotoxicity by reducing both in vitro and in vivo lipid peroxidation, enzymatic leakage of GPT and AP, and by preventing the depletion of GSH and total thiols in the intoxicated mice. However it showed lower hepatoprotective potency than standard silymarin. [88]
Hydrocholagoguic activity
Suspension containing P. nigrum (black pepper) powder (250 and 500 mg/kg BW) in 1 mL aqueous dispersion of soluble starch was administered by force-fed gavage to male Wistar albino rats (330-390 g) for 4 weeks. Intragastric administration of P. nigrum (250 mg/kg) significantly (p < 0.05) increased bile solids content of 2.99% compared to normal control rats (2.77%). Feeding of P. nigrum for 4 weeks significantly increased bile flow (250 mg/kg: 0.87 mL/h, p < 0.001; 500 mg/kg: 0.74 mL/h, p < 0.05 compared to normal control: 0.54 mL/h), phospholipid (250 mg/kg: 1.78 μmol/h, p < 0.02 compared to normal control: 1.12 μmol/h) and total uronic acid (250 mg/kg: 2.80 μmol/h, p < 0.001; 500 mg/kg: 2.27 μmol/h, p < 0.05 compared to normal control: 1.68 μmol/h), but reduced bile solid content (250 mg/kg: 2.21%, p < 0.001; 500 mg/kg: 2.38%, p < 0.05 compared to normal control: 2.77%). Hydrocholagoguic effect was obtained due to increased bile flow with concomitant decrease in bile solids. [89]
Antianderogenic activity
Ethanol (50%) extract of P. nigrum fruits (2 mg/mL) inhibited testosterone 5-alpha-reductase in vitro with a percentage inhibition of 63.0% compared to ethinylestradiol (1 mM, 63.3% inhibition), whereas piperine isolated from the fruits had IC50 value of 0.48 mM compared to ethinylestradiol (IC50 = 0.81 mM). [90]
Antispermatogenic activity
Piperine (10 mg/kg body weight) administered orally to mature male albino Wistar rats for duration of 30 days showed significant reduction in the weights of testis and accessory sex organs, caused severe damage to the seminiferous tubule, decrease in seminiferous tubular and Leydig cell nuclear diameter, desquamation of spermatocytes and spermatids, increase in serum gonadotropins and a decrease in intratesticular testosterone concentration, despite normal serum testosterone titres. [91]
Bioavailability activity
Piperine, an alkaloid isolated from P. nigrumadministered together with β-lactam enhanced the bioavailability of antibiotics amoxicillin trihydrate and cefoxamine sodium in rats. This is reflected in various pharmacokinetic parameters namely tmax, Cmax, t(1/2) and AUC of these antibiotics. [92]
Hypnotics activity
Piperine also significantly potentiated pentobarbitone sleeping time effect on pentobarbitone induced hypnosis in rats compared to control. The effect of the extract also included higher blood and brain levels of phenobarbitone. The mechanism was probably because the piperine inhibits liver microsomal enzyme system and thereby potentiates the pentobarbitone sleeping time. [93]
Insecticidal activity
A study of insecticidal properties of P. nigrum fruit extracts and essential oils against tobacco army worm, Spodoptera litura has been done using topical application bioassay on uniform weighted second instar larvae in the laboratory. The result showed that, the hexane extract was the highest toxicity at 48 h after treatment and most effective in killing the larvae. Toxicity of extracts decreased in the order of hexane (LD50 1.8 mg/g) > acetone (LD50 18.8 mg/g) > chloroform (LD50 NA, the toxicity was very low) > essential oil (no mortality). Insect development and growth index observations showed that the hexane extract had antifeedant properties resulting in severe growth inhibition of S. litura. [94]
Petroleum ether extract of P. nigrum fruits (black pepper) was active against rice moth, Corcyra cephalonica (Stainton) 3rd instar larvae with median lethal concentration (LC50) value of 12.52 μL/mL. The result also showed that the extract had strong inhibition on egg hatchabilities and adult emergence of C. cephalonica at the lowest concentration. [95]
Ethanol extract of P. nigrum (black pepper) (0.3 g) resulted in 100% mortality of millipede Orthoporus fuscipes after 4 days of exposure. However, only 70% mortality was achieved after 4 days of exposure to piperine (0.3 g). [96]
Ethanol extract of P. nigrum fruits (black pepper) had LC50 values of 0.405 ppm and 0.016 ppm against fourth and third instar larvae of A. aegypti, respectively. Hexane extract of P. nigrum was found to be more toxic against fourth larvae of A. aegypti with LC50 0.007 ppm. [97][98]
Isobutylmide alkaloids isolated from P. nigrum fruits were found to have insecticidal activity against third instar larvae of Culex pipiens pallens (i.e. pipercide (LC50 0.004 ppm), retrofractamide A (0.028 ppm), guineensine (0.17 ppm) and pellitorine (0.86 ppm)), Aedes aegypti (i.e. retrofractamide A (0.039 ppm), pipercide (0.1 ppm), guineensine (0.89 ppm) and pellitorine (0.92 ppm)) and Aedes togoi (i.e. retrofractamide A (0.01 ppm), pipercide (0.26 ppm), pellitorine (0.71 ppm), and guineensine (0.75 ppm)). Piperine was the least toxic with LC50 values of 3.21, 5.1 and 4.6 ppm, respectively. [11]
Piptigrine, pipsaeedine, pipbinine, pipnoohine, pipyahyine and pipwaqarine isolated from P. nigrum fruits exhibited toxicity against fourth instar larvae of A. aegypti with LC50 values of 15.0, 45.0, 40.0, 35.0, 30.0 and 30.0 ppm, respectively. [20][50][51]
Insecticidal activity against female C. pipiens pallens was in the following order: pellitorine (LD50 values of 0.4 μg/female), guineensine (1.9), retrofractamide A (2.4) and pipercide (3.2) compared to chlorpyrifos (0.03 μg/female); whereas activity against A. aegypti was in the order of pellitorine (0.17), retrofractamide A (1.5), guineensine (1.7) and pipercide (2.0) compared to chlorpyrifos (0.0014 μg/female). [99]
Ethanol extract, piperolein-A and piperine isolated from P. nigrum fruits were found to be toxic against pyrethroid-resistant A. aegypti larvae with LC50 values of 0.98, 1.46 and 1.53 ppm, respectively. [100]
Toxicity
Carcinogenicity
P. nigrum extract (2mg) administered orally and topically to mice for duration of 3 days a week for 3 months showed the increased in the number of tumor-bearing mice. The effect could be countered by similar administration of 5 or 10 mg of vitamin A-palmitate twice weekly for duration of 3 months during and subsequent to application of pepper extract. On the other hand, the black pepper powder administered in the diet (50g/3kg food) had no impact on carcinogenesis. [101]
Milled P. nigrum extract (black pepper) (2 mg) force-fed as a suspension in amphibian saline or injected subcutaneously in the dorsal lymph sac as an ethanol extract to Egyptian toad Bufo regularis 3 times a week for duration of 5 months induced primary tumours in the liver and secondary tumours in kidney and spleen. Topical administration of the ethanol extract of P. nigrum extract (black pepper) to the skin induced primary tumours in the liver and secondary tumours in the ileum and stomach. The first tumors appeared after two months of the administration. Tumors of the liver diagnosed as hepatocellular carcinomas, lymphosarcomas and fibrosarcomas, and those of the other organs, spleen, kidney, ovary, as metastases of the primary liver tumors. One or more constituents of P. nigrum was speculated to be responsible for the tumor induction in the organs. [102]
Piperine has been reported gave LD50 of 15, 43, 200, 330, and 400 mg/kg by single dose intravenous, intraperitoneal, subcutaneous, intragastrical, and intramuscular administratierion to male mice, with death by respiratory paralysis within 3-17 minutes. Hemorrhagic necrosis and edema were seen in the gastrointestinal and urinary tracts in subacute cases. [103]
Piperine also has shown some evidence of being mutagenic and potentially carcinogenic under some circumstances. It has reportedly given rise to mutagenic products on reaction with nitrites. This causes concern since nitrites and piperine may be consumed simultaneously.Risk might increase with high-dose piperine supplementation. [104]
Clinical Data
Clinical findings
P. nigrum fruit powder (25 and 100 mg/kg BW) was administered orally to male Parkes strain mice daily for 20 and 90 days. Administration of both doses of P. nigrum for 90 days significantly (p < 0.05) reduced weight of sex organs, such as testis (25 mg/kg: 213.04 mg/100 g BW; 100 mg/kg: 183.01 mg/100 g BW compared to vehicle control: 314.49 mg/100 g BW), epididymis (25 mg/kg: 82.97 mg/100 g BW; 100 mg/kg: 77.21 mg/100 g BW compared to vehicle control: 105.22 mg/100 g BW) and seminal vesicle (25 mg/kg: 179.05 mg/100 g BW; 100 mg/kg: 171.76 mg/100 g BW compared to vehicle control: 323.69 mg/100 g BW). Mice treated with 100 mg/kg BW dose for 90 days showed degenerative changes in all seminiferous tubules, including intraepithelial vacuolation, loosening of germinal epithelium, occurrence of giant cells, and mixing of spermatids of different stages of spermatogenesis; in severe cases, the tubules were lined by mainly a layer of Sertoli cells. Treatment for 90 days also caused detectable alteration in the duct, as well as adverse effects on sperm parameters, levels of sialic acid in epididymis (54.87 μmole/100 g tissue vs. control = 128.69 μmole/100 g tissue, p < 0.05) and fructose in seminal vesicle (55.07 μmole/100 g tissue vs. control = 222.21 μmole/100 g tissue, p < 0.05). [105]
In a double-blind crossover study, piperine form P. nigrum (5 mg) administered orally daily for duration of 14 days during supplementation with β-carotene resulted in a significant increase (p<0.0001) in serum β-carotene levels (49.8±9.6μg/dL vs. 30.9±5.4μg/dL) and 60% greater increase in area under the serum β-carotene curve (AUC) compared to β-carotene plus placebo. [106]
A randomized control trial were investigated among 48 cigarette smokers for duration of 3 hours session conducted after overnight deprivation from smoking to study the effect of P. nigrum vapour inhaled to reduce the smoking withdrawal symptoms. Subjects were randomly assigned either to one of the goups: smokers puffed on a device that delivered a vapor from essential oil of P. nigrum; smokers puffed on the device with a mint/menthol cartridge; and a third group used a device containing an empty cartridge. The negative effects and somatic symptoms of anxiety were alleviated in the P. nigrum condition relative to the unflavored placebo. The intensity of sensations in the chest was also significantly higher for the pepper condition. The results support the view that respiratory tract sensations are important in alleviating smoking withdrawal symptoms, thus suggested its application in smoking cessation treatment. [107]
Piperine from P. nigrum fruit extract was clinically studied using double-blind design to healthy adult male volunteers with (presupplementation) fasting coenzyme Q10 values between 0.30 and 0.60 mg/L on its capability to increase the bioavailability of coenzyme Q10. Piperine (5 mg) or placebo administered with 90 mg and 120 mg of coenzyme Q10 to study its relative bioavailability in a single-dose experiment or in separate experiments for 14 and 21 days was determined by comparing measured changes in plasma concentration. The results of the single-dose study and the 14-day study showed smaller, but not significant, increases in plasma concentrations of coenzyme Q10 in the control group compared with the group receiving coenzyme Q10 with a supplement of piperine. While supplementation of 120 mg coenzyme Q10 with piperine for 21 days produced a significant (p = 0.0348), approximately 30% greater, area under the plasma curve than was observed during supplementation with coenzyme Q10 plus placebo. It was suggested that piperine acted as a thermo-nutrient and increased the absorption of coenzyme Q10 from the gastrointestinal tract by producing a local thermogenic action. The same dose of piperine produced similar results in another study involving coenzyme Q10. [108]
Piperine (20 mg) administered in healthy human volunteers given curcumin (2 g) has increased the absorption bioavailability of curcumin from 0.25 to 1 h post drug (p < 0.01 at 0.25 and 0.5 h; p < 0.001 at 1 h) with the bioavailability increase rate was 2000%. The level of curcumin in human serum was very low to undetectable after one hour post-administration. The study showed that in the dosages used, piperine could promote the serum concentration and extent the absorption and bioavailability of curcumin with no adverse effects. [109]
Precautions
Piperine applied to the skin or in the storage of piperine product should avoid the exposure to sunlight. It also suggested that if UVA incorporated in the treatment of vitiligo using piperine, then irradiation and application of piperine should be staggered in order to effectively induce pigmentation. [110]
Side effects
In a region of Northern Iran, where esophageal cancer was higher among women than men, and the main food during pregnancy contained strong black pepper and sharp crushed pomegranate seeds, caused irritation to the esophagus. [111]
Pregnancy/Breast Feeding
This essential oil should not be used during pregnancy or breastfeeding. [112]
Age limitation
No documentation.
Adverse reaction
No documentation.
Interaction & Depletion
No documentation.
Interaction with drug
No documentation.
Interaction with other Herbs
No documentation.
Contraindications
Peptic ulcer patients should avoid taking P. nigrum (black pepper) as it may cause discomfort and produce dyspepsia. [113]
Case Report
No documentation.
Dosage
Dosage Range
In Ayurvedic practice, the recommended dosage is 250 mg-1 g of P. nigrum powder. [114]
Most Common Dosage
No documentation.
Standardization
The herb is standardized to its piperine extract. [115]
Poisonous
No documentation.
Line drawing
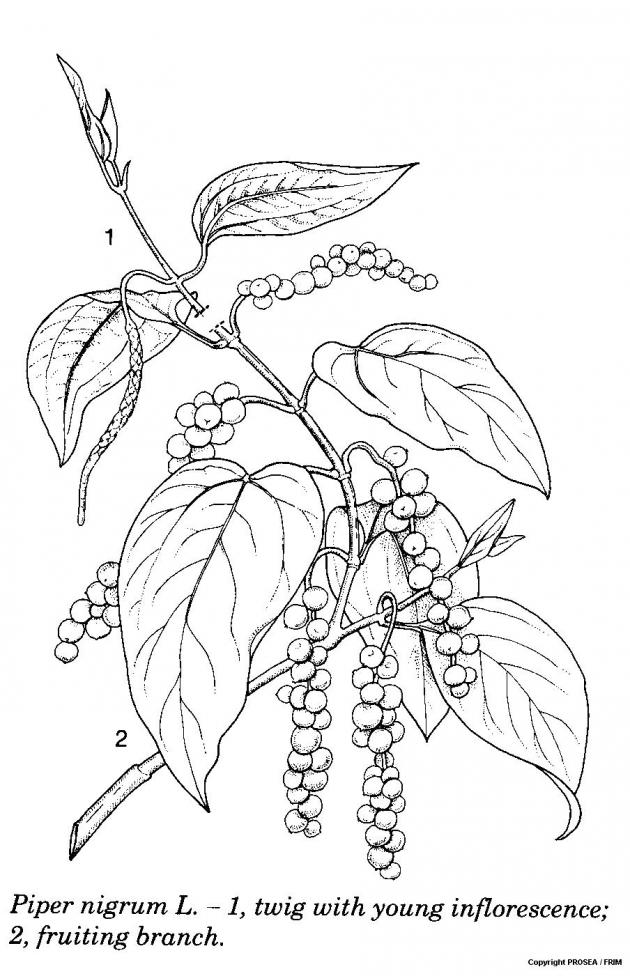
References
- The Plant List. Ver 1.1. Piper Nigrum L. [homepage on the Internet]. c2013 [updated 2012 Mar 23; cited 2014 Oct 9] Available from: http://www.theplantlist.org/tpl1.1/record/kew-2569664
- Lim TK. Edible medicinal and non-medicinal plants: Volume 4, Fruits. Netherlands: Springer 2012; p. 322-323, 345-346.
- Herbal Medicine Research Centre, Institute for Medical Research. Compendium of medicinal plants used in Malaysia. Volume. 1. Kuala Lumpur: HMRC-IMR: 2002. p. 229.
- Quattrocchi U. CRC World dictionary of medicinal and poisonous plants: Common names, scientific names, eponyms, synonyms and etymology; Volume IV M-Q. Boca Raton, Florida: CRC Press; 2012. Pp 592-593
- de Waard PWF, Anunciado IS. Piper nigrum L. In: de Guzman CC, Siemonsma JS, editors. Plant Resources of South-East Asia No. 13: Spices. Leiden, Netherlands: Backhuys Publisher, 1999; p. 189-194.
- Abd. Rahman Azmil, I. 2005. Manual Penanaman Lada Menggunakan Pokok Derdap. MARDI : Serdang.
- Abd. Rahman Azmil, I. 1996. Studies on Black pepper Support Systems in Peninsular Malaysia. Journal of Plantation Crops Vol. 24; 1996. Proc. International Symposium on Plantation Crops (PLACROSYM-XI) 30 Nov.-3 Dis. 1994, Calicut, Kerala, India. Kasaragad, Kerala: Indian Society for Plantation Crops
- Abd. Rahman Azmil, I. dan Shukor, N. 1991. Pembajaan untuk tanaman lada di tanah Siri Rengam di Johor. Teknol. Pelbagai Tanaman, MARDI 7: 37-40
- Mohd. Anuar, A. dan Loh, C.F. 1987. Pests and diseases of pepper, Piper nigrum L. In Johore. (MARDI Report No. 118),20 him. Serdang: MARDI
- Rho M-C, Lee SW, Park HR, et al. ACAT inhibition of alkamides identified in the fruits of Piper nigrum. Phytochemistry. 2007;68:899-903.
- Park IK, Lee SG, Shin SC, Park JD, Ahn YJ. Larvicidal activity of isobutylamides identified in Piper nigrum fruits against three mosquito species. J Agric Food Chem. 2002;50(7):1866-1870.
- Siddiqui BS, Begum S, Gulvar T, Farhat, Noor F. An amide from fruits of Piper nigrum. Phytochemistry. 1997;45(8):1617-1619.
- Siddiqui BS, Gulzar T, Begum S, Afshan F, Sattar FA. Insecticidal amides from fruits of Piper nigrum Linn. Natural Product Research. 2005;19(2):143-150.
- Kiuchi F, Nakamura N, Tsuda Y, Kondo K, Yoshimura H. Studies on crude drugs effective on visceral larva migrans. IV. Isolation and identification of larvicidal principles in pepper. Chemistry and Pharmaceutical Bulletin. 1998;36(7): 2452-2465.
- Su HCF, Horvat R. Isolation, identification and insecticidal of Piper nigrum amides. Journal of Agricultural Food Chemistry. 1981;29(1):115-117.
- Fijiwara Y, Naithaou K, Miyazaki T, Hashimoto K, Mori K, Yamamoto Y. 2001. Two new alkaloids, pipercyclobutanamides A and B from Piper nigrum. Tetrahedron Letters 42: 2497-2499.
- Yamaguchi I, Ozeki S. β-Caryophyllene from black pepper. Bulletin Tokyo Kasei Daigaku 1986;26:95-98.
- Srinivas PV, Rao JM. Isopiperolein B: an alkamide from Piper nigrum. Phytochemistry. 1999;52:957-958.
- Reddy SV, Srinivas PV, Praveen B, et al. Antibacterial constituents from the berries of Piper nigrum. Phytomedicine. 2004;11:697-700.
- Siddiqui BS, Gulzar T, Mahmood A, Begum S, Khan B, Afshan F. New insecticidal amides from petroleum ether extract of dried Piper nigrum L. whole fruits. Chemistry of Pharmaceutical Bulletin. 2004;52(11): 1349-1352.
- Ohigashi H, Nishimuro S, Koshimizu K. Larva-development inhibitors of black pepper. Bulletin of the Institute for Chemical Research, Kyoto University. 1983;61(2):104-108.
- Rathnawathic Nt, Buckle KA. Determination of piperine in pepper (Piper nigrum) using high-performance liquid chromatography. J Chromatogr. 1983;264(2):316-320.
- Kapoor IP, Singh B, Singh G, De Heluani CS, De Lampasona MP, Catalan CA. Chemistry and in vitro antioxidant activity of volatile oil and oleoresins of black pepper (Piper nigrum). J Agric Food Chem. 2009;57(12):5358-5364.
- Singh G, Marimuthu P, Catalan C, deLampasona MP. Chemical, antioxidant and antifungal activities of volatile oil of black pepper and its acetone extract. Journal of the Science of Food and Agriculture. 2004;84:1878-1884.
- Muller CJ, Creveling RK, Jennings WG. Some minor sesquiterpene hydrocarbons of black pepper. Journal of Agricultural and Food Chemistry. 1968;16(1):113-117.
- Muller CJ, Jennings CG. Constituents of black pepper. Some sesquiterpene hydrocarbons. Journal of Agricultural and Food Chemistry. 1967;15(5):762-766.
- Richard HM, Jennings WG. Volatile composition of black pepper. Journal of Food Science. 1971;36(4):584-589.
- Russell GF, Jennings WG. Constituents of black pepper. Oxygenated compounds. Journal of Agricultural and Food Chemistry. 1969;17(5):1107-1112.
- Russell GF, Jennings WG. Occurrence of cis and trans sabinene hydrate in oil of black pepper. Journal of Agricultural and Food Chemistry. 1971;18(4):733.
- Russell GF, Murray WJ, Muller CJ, Jenning WG. α-Bergamotenes in oil of black pepper. Communications. 1968;16(6):1047-1049.
- Wrolstad RE, Jennings WG. Volatile constituents of black pepper. III. The monoterpene hydrocarbon fraction. Journal of Food Science. 1968;30:274-279.
- Debrauwere J, Verzele M. Constituents of peppers. Part VI: The oxygenated fraction of pepper essential oil. Bulletin des Sociѐtѐs Chimiques Belges. 1975;84:167-177.
- Debrauwere J, Verzele M. Constituents of peppers. Part IV: The hydrocarbon of pepper essential oil. Journal of Chromatographic Science. 1976;14:296-298.
- Hasselstrom T. Composition of volatile oil of black pepper, Piper nigrum. Pepper Analysis. 1957;5(1):53-55.
- Kumoro AC, Hasan M, Singh H. Extraction of Sarawak black pepper essential oil using supercritical carbon dioxide. Arabian Journal for Science and Engineering. 2010;35(2B):7-16.
- Sasidharan I, Menon AN. Comparative chemical composition and antimicrobial activity of berry and leaf essential oils of Piper nigrum L. International Journal of Biological and Medical Research. 2010;1(4):215-218.
- Jagella T, Grosch W. Flavour and off-flavour compounds of black and white pepper (Piper nigrum L.). I. Evaluation of potent odorants of black pepper by dilution and concentration techniques. European Food Research and Technology. 1999;209:16-21.
- Jagella T, Grosch W. Flavour and off-flavour compounds of black and white pepper (Piper nigrum L.). III. Desirable and undesirable odorants of white pepper. European Food Research and Technology. 1999;209:27-31.
- Nussbaumer C, Cadalbert R, Kraft P. Identification of m-mentha3(8),6-diene (Isosylveterpinolene) in black pepper oil. Helvetica Chimica Acta. 1999;82(1):53-58.
- Clery RA, Hammond CJ, Wright AC. Nitrogen containing compounds in black pepper oil (Piper nigrum L.). Journal of Essential Oil Research 18(1):1-3.
- Liang R, Shi S, Ma Y. 2010. Analysis of volatile oil composition of the peppers from different production areas. Medicinal Chemistry Research. 2006;19(2):157-165.
- Pino JA, Rodriguez-Feo G, Borges P, Rosado A. Chemical and sensory properties of black pepper oil. Nahrung. 1990;34(6):555-560.
- Turhene SJ, Hogg JW, Bromstein AC, Lawrence BM. Four new sesquiterpene analogs of common monoterpenes. Canadian Journal of Chemistry. 1974;53:3285-3293.
- Jirovetz L, Buchbauer G, Ngassoum MB, Geissler M. Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction-gas chromatography, solid-phase microextraction-gas chromatography-mass spectrometry and olfactometry. J Chromatogr A. 2002;976:265-275.
- Orav A. Effect of storage on the essential oil composition of Piper nigrum L. fruits of different ripening states. J Agric Food Chem. 5May2004;52(9):2582-2586.
- Scott IM, Puniani E, Jensen H, et al. Analysis of Piperaceae Germplasm by HPLC and LCMS: A Method for Isolating and Identifying Unsaturated Amides from Piper spp Extracts. Journal of agricultural and food chemistry. 2005;53(6):1907-13.
- Haranath PSRK, Akther MH, Sharif SI. Acetylcholine and choline in common species. Phytotheraphy Research. 1987;1(2):91-92.
- Lee SW, Kim YK, Kim K, et al. Alkamides from the fruits of Piper longum and Piper nigrum displaying potent cell adhesion inhibition. Bioorganic and Medicinal Chemistry Letters 2008;18:4544-4546.
- Siddiqui BS, Gulzar T, Begum S, Afshan F, Sultana R. A new natural product and insecticidal amides from seeds of Piper nigrum Linn. Natural Product Research. 2008;22(13):1107-1111.
- Siddiqui BS, Gulzar T, Begum S, Afshan F. Piptigrine, a new insecticidal amide from Piper nigrum Linn. Natural Product Research. 2004;18(5):473-477.
- Siddiqui BS, Gulzar T, Begum S, Afshan F, Sattar FA. Two new insecticidal amide dimers from fruits of Piper nigrum Linn. Helvetica Chemica Acta 2004;87:660-666.
- Daulatabad CD, Mulla GM, Mirajkar AM. Venolic and cyclopropenoic fatty acid in Piper nigrum seed oil. European Journal of Lipid Science and Technology. 1995;97:453-454
- Ao P. Essential oil analysis and trace element study of the roots of Piper nigrum L. [Article in Chinese] Zhongguo Zhong Yao Za Zhi. Jan1998;23(1):42-43,63.
- Pereira J. The Elements of Materia Medica and Therapeutics: Longman, Brown, Green, and Longmans; 1842, p. 1099-1102.
- Burkill IH, Birtwistle W, Foxworthy FW, Scrivenor JB, Watson JG. A Dictionary of the Economic Products of the Malay Peninsula: Governments of Malaysia and Singapore by the Ministry of Agriculture and Co-operatives; 1966.
- Thornton RJ, Bewick T. A New Family Herbal: Or, Popular Account of the Natures and Properties of the Various Plants Used in Medicine, Diet and the Arts. The Plants Drawn from Nature: Richard Phillips; 1810, p. 30-33.
- Dioscorides P, Osbaldeston TA, Wood RPA. De Materia Medica: Being an Herbal with Many Other Medicinal Materials Written in Greek in the First Century of the Common Era: Ibidis; 2000, p. 316-318.
- Pérez C, Anesini C. Antibacterial activity of alimentary plants against Staphylococcus aureus growth. Am J Chin Med. 1994;22(2):169-174.
- Dorman HJ, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88(2):308-316.
- Gulcin I. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int J Food Sci Nutr. 2005;56(7):491-499.
- Nahak G, Sahu RK. Phytochemical evaluation and antioxidant activity of Piper cubeba and Piper nigrum. J Appl Pharm Sci 2011;1(8):153-157.
- Singh R, Singh N, Saini BS, Rao HS. In vitro antioxidant activity of pet ether extract of black pepper. Indian J Pharmacol. 2008;40(4):147-151.
- Politeo O, Jukić M, Miloš M. Chemical composition and antioxidant activity of essential oils of twelve spice plants. Croatica Chemica Acta. 2006;79(4):545-552.
- Gülcin I. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int J Food Sci Nutr. 2005;56(7):491-499.
- Vijayakumar RS, Surya D, Nalini N. Antioxidant efficacy of black pepper (Piper nigrum L.) and piperine in rats with high fat diet induced oxidative stress. Redox Rep. 2004;9(2):105-110
- Natarajan KS, Narasimhan M, Shanmugasundaram KR, Shanmugasundaram ER. Antioxidant activity of a salt-spice-herbal mixture against free radical induction. J Ethnopharmacol. 2006;105(1-2):76-83
- Mujumdar AM, Dhuley JN, Deshmukh VK, Raman PH, Naik SR. Anti-inflammatory activity of piperine. Japanese J Med Sci Biol. 1990;43:95-100.
- Dhuley JN, Raman PH, Mujumdar AM, Naik SR. Inhibition of lipid peroxidation by piperine during experimental inflammation in rats. Indian J Exp Biol. 1993;31:443-445.
- Chun H, Shin DH, Hong BS, Cho WD, Cho HY, Yang HC. Biochemical properties of polysaccharides from black pepper. Biol Pharm Bull. 2002;25(9):1203-1208.
- Majdalawieh AF, Carr RI. In vitro investigation of the potential immunomodulatory and anti-cancer activities of black pepper (Piper nigrum) and cardamom (Elettaria cardamomum). J Med Food. 2010;13(2):371-381.
- Khajuria A, Johrn RK, Zutshi U, Bedi KL. Piperine modulation of carcinogen induced oxidative stress in intestinal mucosa. Phytomedicine. 1998;6(5):351-355.
- Nalini N, Manju V, Menon VP. Effect of spices on lipid metabolism in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. J Medl Food. 2006;9(2):237-242.
- Wrba H, el-Mofty MM, Schwaireb MH, Dutter A. Carcinogenicity testing of some constituents of black pepper (Piper nigrum). Exp Toxicol Pathol. 1992;44(2):61-65.
- Pradeep CR, Kuttan G. Effect of piperine on the inhibition of lung metastasis induced B16F-10 melanoma cells in mice. Clin Exp Metastasis. 2002;19(8):703-708.
- El Hamss R, Idaomar M, Alonso-Moraga A, Serrano AM. Antimutagenic properties of bell and black peppers. Food Chem Toxicol. 2003;41:41-47.
- Lin Z, Hoult JR, Bennett DC, Raman A. Stimulation of mouse melanocyte proliferation by Piper nigrum fruit extract and its main alkaloid, piperine. Planta Medica. 1999;65(7):600-603.
- Lin Z, Liao Y, Venkatasamy R, Hider RC, Soumyanath A. Amides from Piper nigrum L. with dissimilar effects on melanocyte proliferation in-vitro. J Pharm Pharmacol. 2007;59:529-536.
- Matsuda H, Kawaguchi Y, Yamazaki M, et al. Melanogenesis stimulation in murine B16 melanoma cells by Piper nigrum leaf extract and its lignan constituents. Biol Pharm Bull. 2004;27(10):1611-1616.
- Platel K, Srinivasan K. Influence of digestive spices and their active principles on pancreatic digestive enzymes in albino rats. Nahrung. 2000;44:42-46.
- Mehmood MH, Gilani H. Pharmacological basis for the medicinal use of black pepper and piperine in gastrointestinal disorders. J Med Food. 2010;13(5):1086-1096.
- Naseri MKG, Yahyavi H. Spasmolytic activity of Piper nigrum fruit aqueous extract on rat non-pregnant uterus. Iranian J Pharmacol Ther. 2007;6(1):35-40.
- Naseri MK, Yahyavi H. Antispasmodic effect of Piper nigrum fruit hot water extract on rat ileum. Pak J Biol Sci. 2008;11(11):1492-1496.
- D’Hooge R, Pei YQ, Raes A, Lebrun P, van Bogaert PP, de Deyn PP. Anticonvulsant activity of piperine on seizures induced by excitatory amino acid receptor agonists. Arzneimittelforschung. 1996;46(6):557-560.
- Kaleem M, Sheema SH, Bano B. Protective effects of Piper nigrum and Vinca rosea in alloxan induced diabetic rats. Indian J Physiol Pharmacol. 2005;49(1):65-71.
- Lee SW, Rho MC, Park HR, et al. Inhibition of diacylglycerol acyltransferase by alkamides isolated from the fruits of Piper longum and Piper nigrum. J Agric Food Chem. 2006;54(26):9759-9763.
- Taqvi SI, Shah AJ, Gilani AH. Blood pressure lowering and vasomodulator effects of piperine. J Cardiovasc Pharmacol. 2008;52(5):452-458.
- Singh A, Rao AR. Evaluation of the modulatory influence of black pepper (Piper nigrum L.) on the hepatic detoxication system. Cancer Lett. 1993;72(1-2):5-9.
- Koul IB, Kapil A. Evaluation of the liver protective potential of piperine, an active principle of black and long peppers. Planta Medica. 1993;59(5):413-417.
- Ganesh Bhat B, Chandrasekhara N. Effect of black pepper and piperine on bile secretion and composition in rats. Die Nahrung.1987;31:913-916.
- Hirata N, Tokunaga M, Naruto S, Linuma M, Matsuda H. Testosterone 5 alpha-reductase inhibitory active constituents of Piper nigrum leaf. Biol Pharm Bull. 2007;30(12):2402-2405.
- Malini T, Manimaran RR, Arunakaran J, Aruldhas MM, Govindarajulu P. Effects of piperine on testis of albino rats. J Ethnopharmacol. 1999;64:219-225.
- Hiwale AR, Dhuley JN, Naik SR. Effect of co-administration of piperine on pharmacokinetics of beta-lactam antibiotics in rats. Indian J Exp Biol. 2002;40(3):277-281.
- Mujumdar AM, Dhuley JN, Deshmukh VK, Raman PH, Thorat SL, Naik SR. Effect of piperine on pentobarbitone induced hypnosis in rats. Indian J Exp Biol. 1990;28(5):486-487.
- Fan LS, Muhamad R, Omar D, Rahmani M. Insecticidal properties of Piper nigrum fruit extracts and essential oils against Spodoptera litura. Int J Agric Biol. 2011;13:517-522.
- Khani M, Awang RM, Omar D, Rahmani M. Toxicity, antifeedant, egg hatchability and adult emergence effect of Piper nigrum L. and Jatropha curcas L. extracts against rice moth, Corcyra cephalonica (Stainton). J Med Plants Res. 2013;7(18):1255-1262.
- Romão JA, Boccardo L, de Paula VF, Chagas RJ, Moreira BO. Toxicity of extracts from Piper nigrum, piperine and piperamides to the millipede Orthoporus fuscipes under laboratory conditions. Revista Brasileira de Toxicologia. 2008;21(1):33-38.
- Kumar S, Warikoo R, Wahab N. Larvicidal potential of ethanolic extracts of dried fruits of three species of peppercorns against different instars of an Indian strain of dengue fever mosquito, Aedes aegypti L. (Diptera: Culicidae). Parasitol Res. 2010;107(4):901-907.
- Kumar S, Warikoo R, Wahab N. Relative larvicidal efficacy of three species of peppercorns against dengue fever mosquito, Aedes aegypti L. J Entomol Res Soc. 2011;13(2):27-36.
- Park IK. Insecticidal activity of isobutylamides derived from Piper nigrum against adult of two mosquito species Culex pipiens pallensand Aedes aegypti. Nat Prod Res. 2012;26(22):2129-2131.
- Simas NK, Lima Eda C, Kuster RM, Lage CL, de Oliveira Filho AM. Potential use of Piper nigrum ethanol extract against pyrethroid-resistant Aedes aegypti larvae. Revista da Sociedade Brasileira de Medicina Tropical. 2007;40(4):405-407.
- Shwaireb MH, Wrba H, el-Mofty MM, Dutter A. Careinogenesis induced by black pepper (Piper nigrum) and modulated by vitamin A. Exp Pathol. 1990;40(4):233-238.
- El-Mofty MM, Soliman AA, Abdel-Gawad AF, Sakr SA, Shwaireb MH. Carcinogenicity testing of black pepper (Piper nigrum) using the Egyptian toad (Bufo regularis) as a quick biological test animal. Oncology. 1988;45(3):247-252.
- Piyachaturawat P, Glinsukon T, Toskulkao C. Acute and subacute toxicity of piperine in mice, rats and hamsters. Toxicol Lett. 1983;16(3-4):351-359.
- Shenoy NP, Choughuley AS. Characterization of potentially mutagenic products from the nitrosation of piperine. Cancer Lett. 1992;64:235-239.
- Mishra RK, Singh S. Antispermatogenic and antifertility effects of fruits of Piper nigrum L. in mice. Indian J Exp Biol. 2009;47:706-714.
- Badmaev V, Majeed M, Norkus EP. Piperine, an alkaloid derived from black pepper increases serum response of beta-carotene during 14-days of oral beta-carotene supplementation. Nutr Res. 1999;19:381-388.
- Rose JE, Behm FM. Inhalation of vapor from black pepper extract reduces smoking withdrawal symptoms. Drug Alcohol Depend. 1994;34(3):225-229.
- Badmaev V, Majeed M, Prakash L. Piperine derived from black pepper increases the plasma levels of coenzyme Q10 following oral supplementation. J Nutr Biochem. 2000;11:109-113.
- Shoba G, Joy D, Joseph TM, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353-356.
- Soumyanath A, Venkatasamy R, Joshi M, et al. UV irradiation affects melanocyte stimulatory activity and protein binding of piperine. Photochem Photobiol. 2006;82(6):1541-8.
- Ghadirian P, Ekoe JM, Thouez JP. Food habits and esophageal cancer: An overview. Cancer Detect Prev. 1992;16(3):163-8.
- Lis-Balchin M. Aromatherapy science: A guide for healthcare professionals. London: Pharmaceutical Press; 2006. p. 273.
- Marotta RB, Floch MH. Diet and nutrition in ulcer disease. Med Clin North Am. 1991;75(4):967-79.
- Department of Ayush, Ministry of Health and Family Welfare, Government of India. The Ayurvedic pharmacopoeia of India. Part I, VolUME III. New Delhi: Ministry of Health and Family Welfare. 2001. p. 117.
- Chauhan R, Dwivedi J, Siddiqui AA. Chemical standardization and quantification of piperin from methanolic extract of Piper nigrum by HPLC method on the basis of isolated markers. Int J Chem Sci. 2008;6(4):1726-1733.


