Pecah Beling Leaves
Strobilanthes crispus (L.) Bremek
Acanthaceae
DEFINITION
Pecah beling leaves consist of the dried leaves of Strobilanthes crispus (L.) Bremek (Acanthaceae).
SYNONYM
Cericocalyx crispus (L.) Brmek [ 1 ].
VERNACULAR NAMES
Yellow strobilanthus (English); pecah kaca, pecah beling, karang jin, bayam karang (Malay) [ 2 ]; hei mian jiang jun (Chinese) [ 3 ].
CHARACTER
| Colour | Dark green |
| Odour | Aroma |
| Taste | Slightly bitter taste |
IDENTIFICATION
Plant Morphology
S. crispus is a woody spreading shrub which reaches to 1-1.5 m height. Stem has a diameter of between 0.2-0.7 mm with the external bark being purplish in color when young and brown when matured; branches usually 4-angled, often sulcate, basally becoming woody and hollow with age. Leaves are elliptical in shape, 5-8 cm long and 2-5 cm wide, oblong-lanceolate, rather obtuse and shallowly crenate crispate and have rough surface, covered with short hairs, upper surface of the leaves is darker green in colour and less rough as compared to underside. Inflorescence is terminal with lanceolate bract. Flowers are short, dense, panicle spikes, yellow in colour, funnel-shaped. Seeds usually ovate or orbicular in outline and lenticular by being flattened, usually pubescent with appressed mucilaginous trichomes [ 2 ].
Microscopy
Powdered material consists of spongy mesophyll cells; epidermis cells of adaxial leaves are straight to wavy anticlinal wall and attached with stomata; epidermis cells of petiole are found in fragmented form with spiral vessels; trichome simple or multicellular; cystolith cells; fiber and adaxial epidermis cells.








Figure 2 : Microscopic characters of S. crispus leaves powder. (a) Spongy mesophyll cells (magnification 40x); (b) epidermis cells attached with stomata (magnification 40x); (c) spiral vessels (magnification 60x); (d) simple multicellular trichome (magnification 40x); (e) spiral vessels (magnification 60x); (f) cystolith cells (magnification 80x); (g) fiber (magnification 40x); (h) adaxial epidermis cells. [Scale bars: a, b, c, d, e, f, g = 20 µm]
Colour Tests
Observed colour of solution after treatment with various reagents:
| H2SO4 (conc.) | Brown |
| NaOH (5%) | Green |
| KOH (5%) | Green |
Thin Layer Chromatography (TLC)

Figure 3 : TLC profiles of caffeic acid (S), ethanol extract of S. crispus dried leaves powder (L) observed under (a) UV at 254 nm before derivatization, (b) UV at 366 nm before derivatization and (c) UV at 366 nm after derivatization with 5% ethanolic sulphuric acid.
| Test Solutions | Weigh about 1.0 g of S. crispus dried leaves powder in a round flask and add 10 mL of ethanol. Reflux at 80oC until boil (about 2 min). Turn off the heater but continue reflux for the next 30 min. Filter the solution and use the filtrate as test solution. |
| Standard solution | Dissolve caffeic acid [CAS no: 331-39-5] in ethanol to produce a standard concentration 1.0 mg/mL solution |
| Stationary Phase | HPTLC Glass silica gel 60 F254, 10 x 10 cm. |
| Mobile phase | Toluene : ethyl acetate : formic acid; (5 : 4 : 1) (v/v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
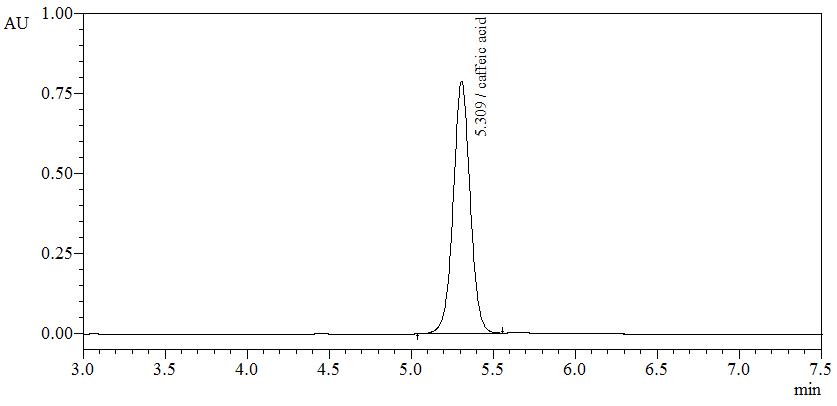
High Performance Liquid Chromatography (HPLC)
| Test solution | Weigh about 1.0 g of S. crispus dried leaves powder in a round flask and add 10 mL of ethanol. Reflux at 80oC until boil (about 2 min). Turn off the heater but continue reflux for the next 30 min. Filter through a 0.45 µm syringe filter and inject the filtrate into the HPLC column. | ||||||||||||||||||
| Standard solution | Dissolve caffeic acid [CAS no: 331-39-5] in ethanol to produce a standard concentration 1.0 mg/mL solution. | ||||||||||||||||||
| Chromatographic system |
Detector: UV 320 nmColumn: C18 column (5.0 µm, 4.6 mm I.D x 250 mm)(Zorbax Eclipse unless necessary)Column oven temperature: 35°CFlow rate: 1.0 mL/minInjection volume: 10 µL |
||||||||||||||||||
| Mobile Phase (gradient mode) |
|
||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of caffeic acid (1.0 mg/mL). The requirements of the system suitability parameters are as follow:
|
||||||||||||||||||
| Acceptance criteria |
|
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 8% |
| Acid-insoluble ash | Not more than 2% |
| Loss on Drying |
| Not more than 14% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 23% |
| Cold method | Not less than 18% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 7% |
| Cold method | Not less than 4% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Aqueous and ethanol extract of S.crispus dried leaves was found to contain phenolic acids (caffeic acid, ferulic acid, gallic acid, chlorogenic acid, trans-cinnamic acid) and flavonoids (quercetin, rutin, catechin, apigenin, naringenin,kaempferol) [ 4 ].
Ethanol extract of S.crispus dried leaves was found to contain phenolic acids (p-hydroxybenzoic acid, p-coumaric acid, caffeic acid, vanillic acid, gentinic acid, ferulic acid and syryngic acid), caffeine, tannin alkaloid [ 5 ].
Ethanol extract of S.crispus dried leaves was found to contain flavonoid compounds ((+)-catechin, (−)-epicatechin, rutin, myricetin, luteolin, apigenin, naringenin and kaempferol)
[ 6 ].
Dichloromethane extract of S.crispus dried leaves was found to contain fatty acid esters (β-amyrin, taraxerone, taraxerol, taraxerol) [ 7 ].
Hexane extract of S.crispus dried leaves was found to contain non-volatile constituents (1-heptacosanol, tetracosanoic acid, stigmasterol) [ 7 ].
MEDICINAL USES
Uses described in folk medicine, not supported by experimental or clinical data
Traditionally used as a laxative and to treat constipation [ 8 ]. Poultice of its leaves was used for ague in children, coughs and as a poultice on the chest [ 9 ].
Biological and pharmacological activities supported by experimental data
Antioxidant activity
Ethanol extract of S. crispus leaves (0.2%) showed antioxidant activity with Fe2+ reducing ability (180%) compared to vitamin E (78%) using ferric reducing antioxidant potential (FRAP) assay [ 5 ].
Aqueous (100%) extract of S. crispus leaves (1 mg/mL) showed antioxidant activity with Fe2+ reducing ability (1182 mmol/g) compared to Gallic acid (1216.67 mmol/g) using FRAP assay [ 10 ].
Ethanol extract of S. crispus leaves (1 mg/mL) showed antioxidant activity with Fe2+ reducing ability (108 mmol/g) compared to gallic acid (1216.67 mmol/g) using FRAP assay
[ 10 ].
Antiulcerogenic activity
Aqueous extract of S. crispus leaves (250, 500 and 1000 mg/kg, body weight) administered orally to adult male Sprague Dawley rats (180-200 g) that were pretreated with 500 or 1000 mg/kg leaf extract had significantly (p < 0.05) reduced formation of gastric ulcers induced by ethanol compared to rats that were pre-treated with omeprazole (20 mg/kg)
[ 11 ].
Antidiabetic activity
Leaves juice (4%) of S. crispus (1, 1.5 and 2 mL/kg) orally administered to alloxan-induced diabetic male and female Sprague Dawley rats (150-200 g) for a duration of 30 days significantly (p < 0.01) reduced the serum glucose level compared to the initial level from 42.65 to 5.16 mmol/L in male and 41.37 to 6.93 mmol/L in female rats at day 30 [ 12 ].
Hot water extract of S. crispus leaves (2%, fermented and unfermented tea) orally administered to streptozocin-induced diabetic male Sprague Dawley rats (200-250 g) for a duration of 21 days significantly (p < 0.01) reduced the serum glucose from the initial level of 16.30 to 7.80 mmol/L (fermented) and from 16.30 to 7.70 mmol/L (unfermented) at day 21 [ 13 ].
Cytotoxicity activity
Methanol extract of S. crispus leaves (25 mg/mL) showed cytotoxic activity on ductal breast epithelial tumor cell line (T-47D) with inhibition concentration at 50% of growth (IC50) of 121.53 µg/mL and breast cancer cell line (MCF-7) (160.16 µg/mL) using MTT assay [ 14 ].
Subfractions of dichloromethane extract of S. crispus leaves (0-100 µg/mL) showed cytotoxic activity on breast cancer cell lines (MCF-7) with median effective concentration (EC50) of 8.5 µg/mL, breast cancer cell lines (MDA-MB-231) (10.0 µg/mL), prostate cancer cell line (DU-145) (7.4 µg/mL) and prostate cancer cell line (PC-3) (7.2 µg/mL) using cytotoxicity detection kit assay [ 15 ].
Ethanol extract of S. crispus leaves (30 μg/mL) showed cytotoxic activity on breast cancer cell lines (MCF-7) with IC50 of 30 ± 3.1 µg/mL compared to doxorubicin (2.7 µg/mL)
[ 16 ].
Dichloromethane extract of S. crispus leaves (10 μg/mL) showed cytotoxic activity on breast cancer cell lines MCF-7 (72%) and MDA-MB-231 (79%) after 48 hr of treatment compared to tamoxifen (MCF-7 = 73%; MDA-MB-231 = 95%) [ 17 ].
Methanol extracts of S. crispus showed cytotoxic activity on colon cancer (Caco-2) with IC50 of 22.3 μg/mL, human breast cancer (MDA-MB-231) (27.2 μg/mL) and liver cancer (HepG-2) (29.3 μg/mL) compared to catechin with (IC50) value more than 100 μg/mL [ 18 ].
Anti-angiogenic activity
Aqueous extract of S. crispus leaves (100 μg/mL) showed moderate anti-angiogenic activity (16.67%) compared to suramin (69.44%) [ 14 ].
Hypolipidemic activity
Hot water extract of S. crispus leaves (2%, fermented and unfermented tea) administered orally to streptozocin-induced diabetic male Sprague Dawley rats (200-250 g) for a duration of 21 days significantly (p < 0.01) reduced the level of plasma cholesterol (fermented 0.95 and unfermented 1.03 mmol/L), triglycerides (fermented 0.46 and unfermented 0.34 mmol/L), compared to diabetic control (cholesterol = 1.71 mmol/L and triglycerides: 0.68 mmol/L). The extract also significantly (p < 0.05) increase high density lipoprotein (HDL) (fermented: 0.74 mmol/L and unfermented: 0.64 mmol/L) while significantly (p < 0.05) decrease the low density lipoprotein (LDL) (fermented: 0.74 and unfermented: 0.49 mmol/L) compared to diabetic control (HDL = 0.57 mmol/L and LDL = 1.00 mmol/L) [ 13 ].
Wound healing activity
Ethanol (95% v/v) extract of S. crispus leaves (100 and 200 mg/mL/day) was applied topically twice a day on the dorsal neck excision wound of adult male Sprague Dawley rats (220-250 g, 8 weeks old). After 15 days, the histology showed less scarring tissue at wound closure with fewer inflammatory cells observed compared to placebo group. The percentage healing in placebo control group wounds was significantly lower than those of S. crispus treated groups and reference standard control wounds [ 19 ].
Clinical studies
Information and data have not been established.
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute toxicity
Ethanol (95%) extract of S. crispus leaves (150, 300 and 600 mg/kg) administered orally to female Sprague Dawley rats (160 ± 10 g) for 0, 3, 7 and 14 days showed neither toxic signs nor toxicity on liver, kidney, heart, lung and spleen of rats after 24 hr and median lethal oral dose (LD50) was greater than 600 mg/kg [ 20 ].
Juice of S. crispus leaves (700, 2100, 3500 and 4900 mg/kg) administered orally to normal female and male Sprague Dawley rats for 14 days did not show toxicity for 14 days and LD50 was greater than 4900 mg/kg [ 21 ].
Ethanol (95%) extract of S. crispus leaves (1, 2 and 5 g/kg) was administered orally to female SD rats (220-250 g; 8 weeks old) for 14 days. All the animals remained alive and did not manifest any significant visible of toxicity at these doses [ 19 ].
Oral single dose acute toxicity study on female Sprague Dawley rats ( aged between 8 and 12 weeks old) using aqueous extract of S. crispus leaves showed no toxic effect on the parameters observed, including behaviors, body weight , food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. Approximate lethal dose (LD50) is more than 2,000 mg/kg body weight [ 22 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- Bhore S, Tiong O. Bacterial endophytes of therapeutically important Strobilanthes crispa (L.) Bremek and Vernonia amygdalina Del. Journal of Pharmaceutical and Biomedical Sciences. 2012;14:1-3.
- Nurraihana H, Norfarizan-Hanoon N. Phytochemistry, pharmacology and toxicology properties of Strobilanthes crispus. International Food Research Journal. 2013;20(5):2045-2056.
- Friends of the Penang Botanic Gardens Society. [Internet]Strobilanthes crispus. [cited on 18 Sept 2014]. Available from: http://www.botanikapenang.org.my/index.php?option=com_content&view=article&id=11.
- Ghasemzadeh A, Jaafar HZE, Rahmat A. Phytochemical constituents and biological activities of different extracts of Strobilanthes crispus (L.) Bremek leaves grown in different locations of Malaysia. BMC Complementary and Alternative Medicine (2015) 15:422.
- Ismail M, Manickam E, Danial AM, Rahmat A, Yahaya A. Chemical composition and antioxidant activity of Strobilanthes crispus leaf extract. The Journal of Nutritional Biochemistry. 2000;11(11):536-542.
- Liza M, Abdul Rahman R, Mandana B, Jinap S, Rahmat A, Zaidul I, et al. Supercritical carbon dioxide extraction of bioactive flavonoid from Strobilanthes crispus (Pecah kaca). Food and Bioproducts Processing. 2010;88(2):319-326.
- Koay YC, Wong KC, Osman H, Eldeen I, Asmawi MZ. Chemical constituents and biological activities of Strobilanthes crispus L. Records of Natural Products. 2013;7(1):59-64.
- Perry LM, Metzger J. Medicinal plants of east and southeast Asia: attributed properties and uses. MIT press. 1980.
- Burkill IH, Haniff M. Malay Village Medicine: Prescriptions Collected by: University Press. 1930.
- Qader SW, Abdulla MA, Chua LS, Najim N, Zain MM, Hamdan S. Antioxidant, total phenolic content and cytotoxicity evaluation of selected Malaysian plants. Molecules. 2011;16(4):3433-3443.
- Mahmood A, Fard AA, Harita H, Amin ZA, Salmah I. Evaluation of gastroprotective effects of Strobianthes crispus leaf extract on ethanol-induced gastric mucosal injury in rats. Scientific Research and Essays. 2011;6(11):2306-2314.
- Norfarizan-Hanoon N, Asmah R, Rokiah M, Fauziah O, Faridah H. Antihyperglycemic, hypolipidemic and antioxidant enzymes effect of Strobilanthes crispus juice in normal and streptozotocin-induced diabetic male and female rats. International Journal of Pharmacology. 2009;5(3):200-207.
- Fadzelly AM, Asmah R, Fauziah O. Effects of Strobilanthes crispus tea aqueous extracts on glucose and lipid profile in normal and streptozotocin-induced hyperglycemic rats. Plant Foods for Human Nutrition. 2006;61(1):6-11.
- Muslim N, Ng K, Itam A, Nassar Z, Ismail Z, Majid A. Evaluation of cytotoxic, anti-angiogenic and antioxidant properties of standardized extracts of Strobilanthes crispus leaves. International Journal of Pharmacology. 2010;6(5):591-599.
- Yaacob NS, Hamzah N, Kamal NNNM, Abidin SAZ, Lai CS, Navaratnam V, et al. Anticancer activity of a sub-fraction of dichloromethane extract of Strobilanthes crispus on human breast and prostate cancer cells in vitro. BioMedCentral (BMC) Complementary and Alternative Medicine. 2010;10(1):42.
- Chong HZ, Rahmat A, Yeap SK, Akim AM, Alitheen NB, Othman F, et al. In vitro cytotoxicity of Strobilanthes crispus ethanol extract on hormone dependent human breast adenocarcinoma MCF-7 cell. BioMedCentral (BMC) Complementary and Alternative Medicine. 2012;12(1):35.
- Yaacob NS, Kamal NN, Norazmi MN. Synergistic anticancer effects of a bioactive subfraction of Strobilanthes crispus and tamoxifen on MCF-7 and MDA-MB-231 human breast cancer cell lines. BioMedCentral (BMC) Complementary and Alternative Medicine. 2014;14(1):252.
- Rahmat A, Edrini S, Akim AM, Ismail P, Hin TYY, Bakar MFA. Anticarcinogenic properties of Strobilanthes crispus extracts and its compounds in vitro. International Journal of Cancer Research. 2006;2(1):47-49.
- Al-Henhena N, Mahmood A, Al-Magrami A, Nor Syuhada A, Zahra A, Summaya M, et al. Histological study of wound healing potential by ethanol leaf extract of Strobilanthes crispus in rats. Journal of Medicinal Plants Research. 2011;5(16):3666-3669.
- Lim KT, Lim V, Chin JH. Sub-acute oral toxicity study of ethanolic leaves extracts of Strobilanthes crispus in rats. Asian Pacific Journal of Tropical Biomedicine. 2012;2(12):948-952.
- NA N-H, MY R. Absence of toxicity of Strobilanthes crispa juice in acute oral toxicity study in sprague dawley rats. Sains Malaysiana. 2012;41(4):403-409.
- Syed Muhammad Asyraf ST, Nor Zahirah A, Nor Kasmini S, Wan Abdul Hakim WL, Wan Mohammad Adham Afiq WZ, Teh BP, Hussin M. Acute oral toxicity study of selected Malaysian medicinal herbs on Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2015. Report no.: HMRC 11-045/01/SC/L/P.