Lempoyang Rhizome
Zingiber zerumbet (L.) Smith
Zingiberaceae
DEFINITION
Lempoyang rhizome consists of dried rhizomes of Z. zerumbet (L.) Smith (Zingiberaceae).
SYNONYM
Zingiber spurium J. Koenig, Zingiber littorale Val., Zingiber aromaticum Noronha, Amomum zerumbet Linn, Amomum sylvestre Lam [ 1 ].
VERNACULAR NAMES
Shampoo ginger, wild ginger, zerumbet ginger (English), lempoyang (Malay) [ 2 ], hong qiu jiang, nan jiang (Chinese) [ 3 , 4 ], araniyacaranai (Tamil) [ 5 ].
CHARACTER
| Colour | Dark dull yellow-brown |
| Odour | Fragrant, aromatic |
| Taste | Spicy and slightly bitter in taste |
IDENTIFICATION
Plant Morphology
Z. zerumbet is perennial, grows to about 2.1 m tall. Stems usually short, replaced by pseudostems formed by leaf sheaths. Tuberous or non-tuberous rhizomes, often with tuber-bearing roots. Leaves with long narrow arranged oppositely along the stem, having 10-12 blade-shaped leaves with 15-20 cm long, grow on thin, upright stem. Inflorescence resembled by separate stalks grows out of the ground with green cone-shaped bracts drought season and turns red over a couple of weeks. 3-10 cm long with overlapping scales, enclosing small yellowish-white flowers, fill with an aromatic, slimy liquid and turn a brighter red colour when matured, flower stalks usually remain hidden beneath the leaf stalks. Flowers small, creamy yellow appear on the reddish-green cone. Fruit a capsule, fleshy or dry, dehiscent or indehiscent, sometimes berrylike. Seeds few to many, arillate; aril often lobed or lacerate [ 6 , 7 ].
Microscopy
Powder is dull to dark yellowish brown; characterized by numerous fragments of thin, wrinkled walls of isolated parenchyma and parenchyma cells containing starch granules; fragments of thin-walled isolated fibres; epidermis rectangular and elongated followed by thin walled cork cells, irregularly elongated; fragments of tracheids are non-lignified and have reticulate, scalariform, pitted vessels, isolated spiral vessels, and spiral vessels accompanied by secretory cells; yellow to orange coloured of oleoresin in fragments or droplets with secretory cells scattered in parenchyma; numerous starch granules, simple, mostly globose, ovoid and irregularly rounded [ 8 ].



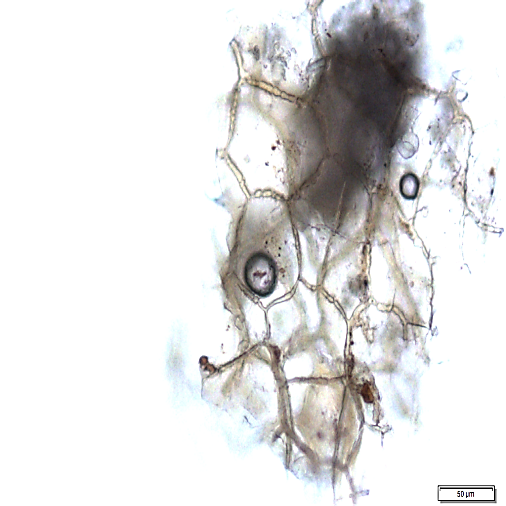

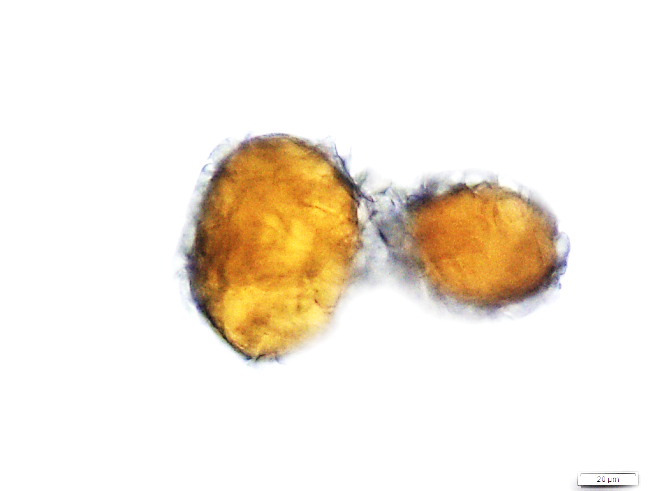
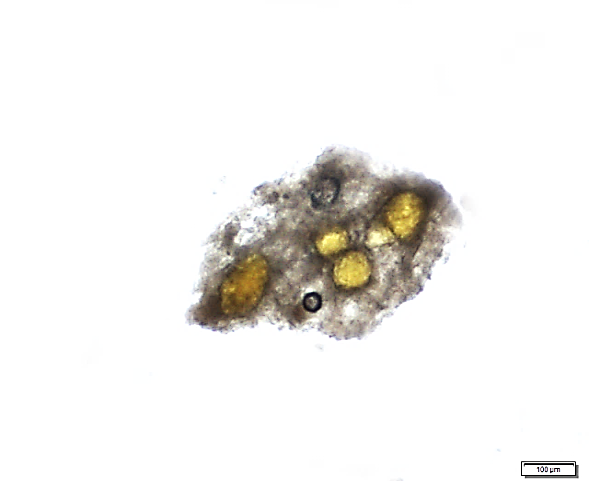

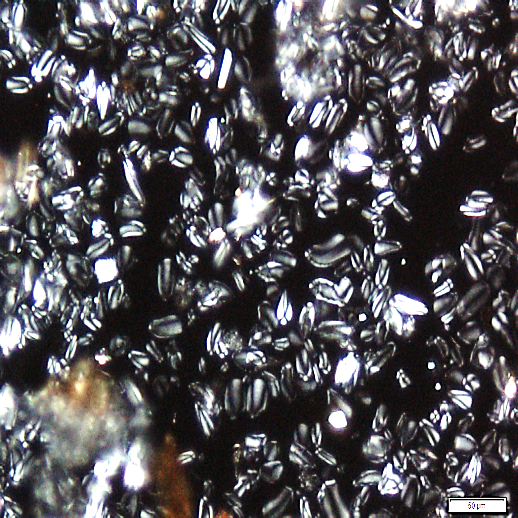

Figure 2 : Microscopic characters of Z. zerumbet rhizome powder. (a) Fragment of spirally thickened vessels; (b) fragment of parenchyma cells; (c) solitary crystals; (d) fragments of parenchyma; (e) groups of irregular starch granules; (f) secretory cells; (g) secretory cells; (h) fragment of fibres; (i) groups of starch (under polarized filter); (j) fragments of spirally thickened vessel with secretory cells attached (under polarized filter). [Scale bars: c = 10 µm; a, e, f, j = 20 µm; b, d, h, i = 50 µm; g = 100 µm]
Colour Tests
| HCl (conc.) | Brown |
| 5% NaOH | Light brown |
| 25% NH4OH | Light brown |
Thin Layer Chromatography (TLC)
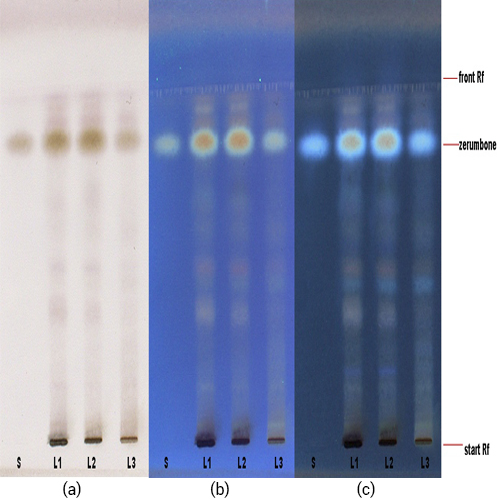
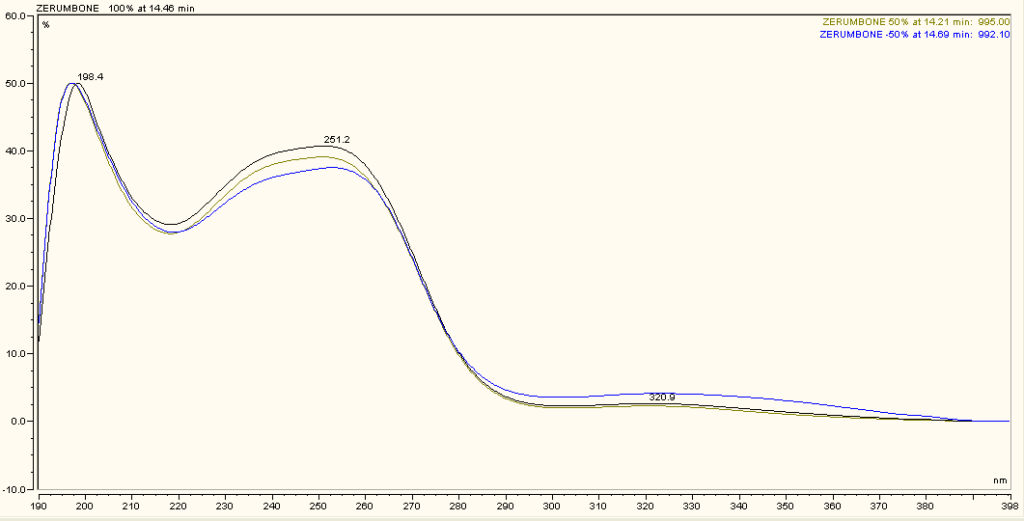
Figure 3 : TLC profiles of zerumbone (Rf: 0.84) (S) and ethanol extract of Z. zerumbet dried rhizome powder (L1, L2, L3) observed under (a) visible light (b) UV at 254 nm and (c) UV at 366 nm after spray with 10% methanolic sulphuric acid solution.
| Test Solutions | Weigh about 5 g of Z. zerumbet dried rhizome powder in a 50 mL screw cap conical flask and add 25 mL of ethanol (99.4%). Sonicate the mixture for 15 min at room temperature. Filter and collect the mixture in round bottom flask. Evaporate the filtrate using rotary evaporator at 60°C. Reconstitute the residue with 20 mL of methanol. Filter the solution and use the filtrate as test solution. |
| Standard solution | Dissolve 1.0 mg of zerumbone standard [CAS no.: 471-05-6] in 1 mL methanol to produce 1000 µg/mL solution. |
| Stationary Phase | HPTLC glass silica gel 60 F254, 10 x 10 cm (pre-heated in oven at 100°C for 20 minutes) |
| Mobile phase | Hexane : ethyl acetate (9.0:2.0) (v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
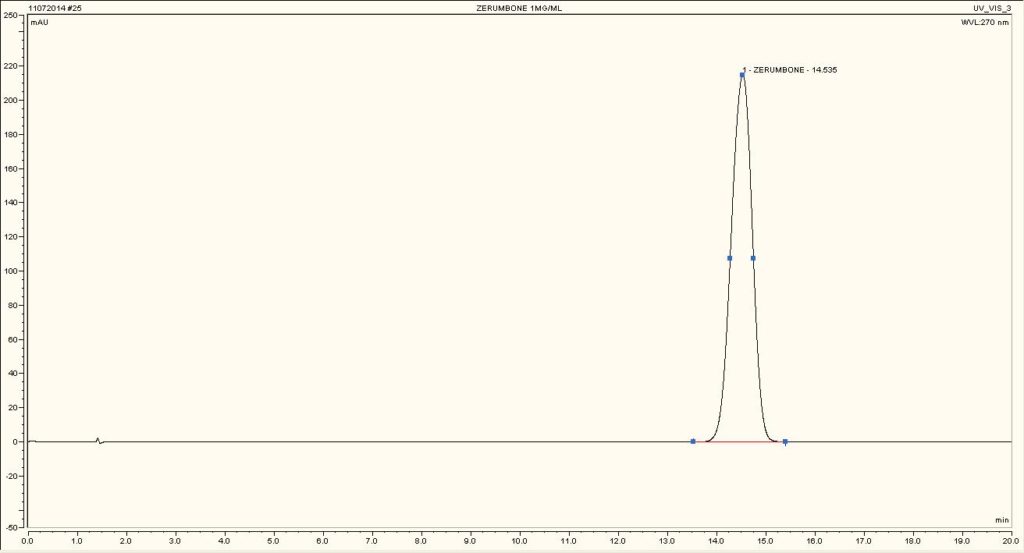
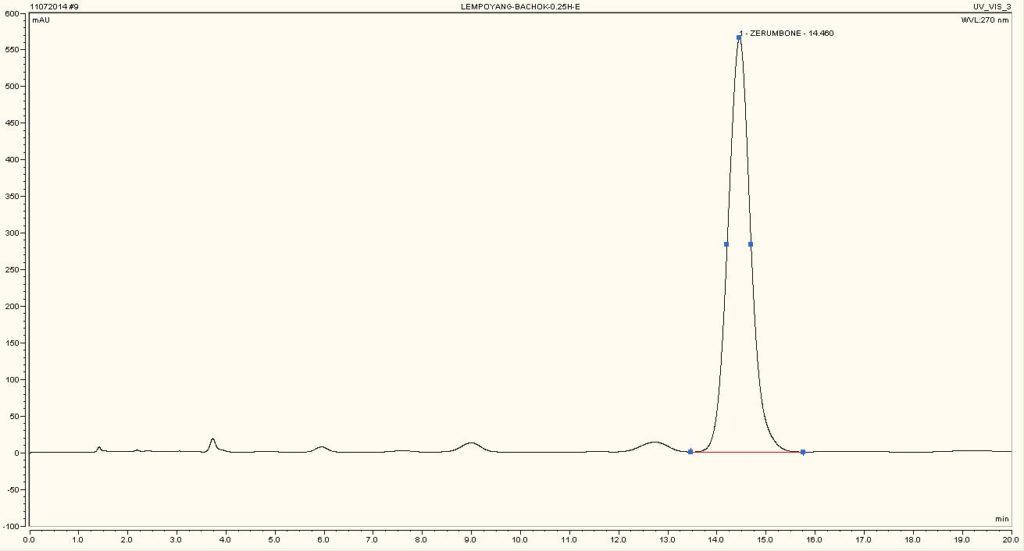
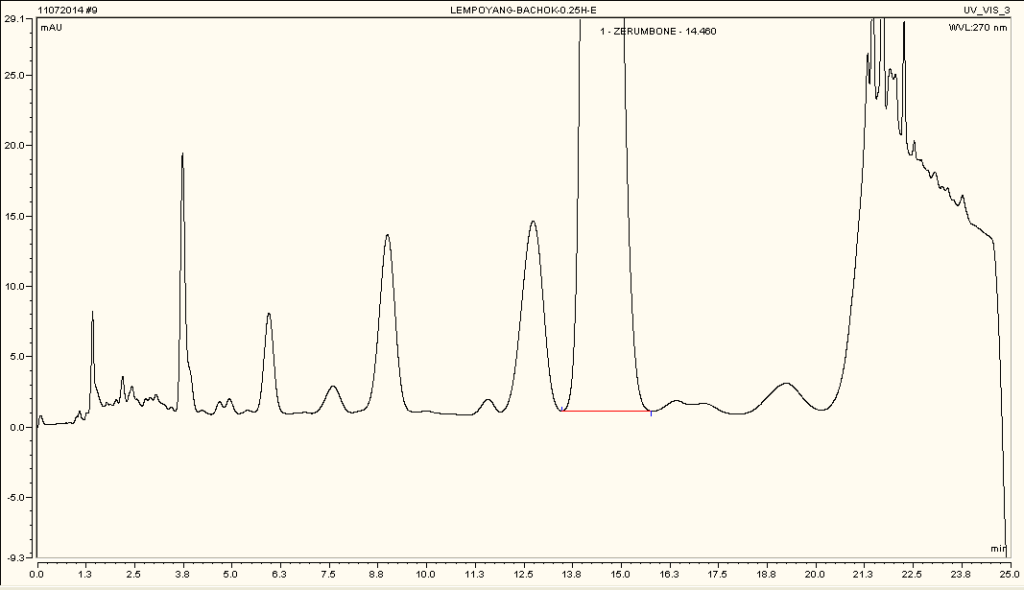
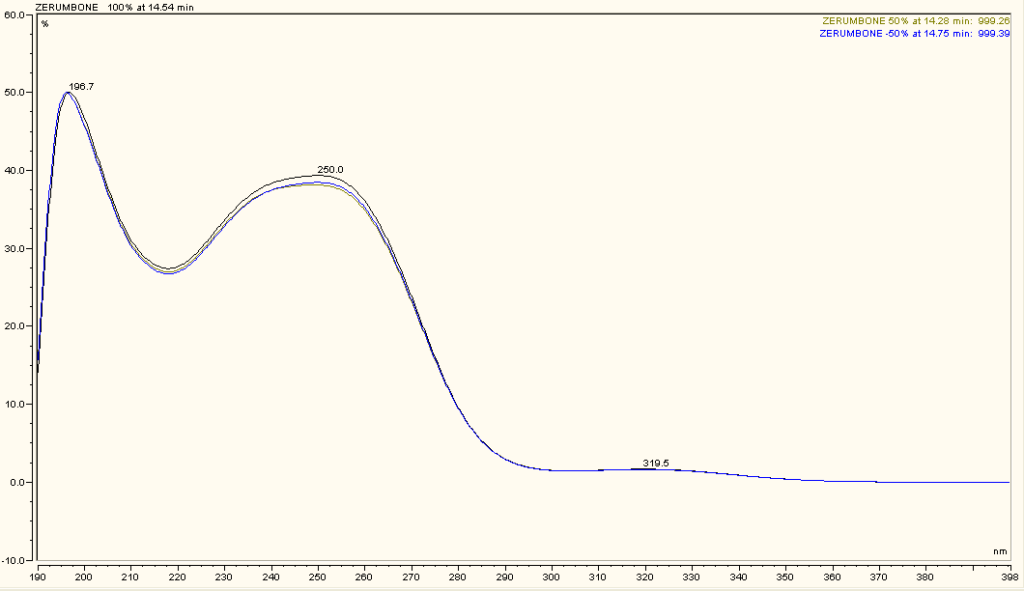
High Performance Liquid Chromatography (HPLC)
| Test solution | Weigh 5 g of dried powder of Z. zerumbet rhizome in a 100 mL conical flask and add 25 mL of absolute ethanol. Sonicate the mixture for 15 min at room temperature. Filter the mixture with Whatman 1 filter paper. The filtrate was by dried off with rotary evaporator at 60°C and 150 mbar. Dissolve the extract yield in methanol. Sonicate for 10 min and then centrifuge for 2 min. | |||||||||||||||||||||
| Standard solution | 1.3 mg zerumbone standard solid [CAS no.: 471-05-6] was weighed and vortexed to dissolved in 1 mL methanol | |||||||||||||||||||||
| Chromatographic system |
Detector: UV 270 nm Column: C18, 150 X 4.6 mm, 5 µm Column oven temperature: 40oC Flow rate: 1.2 mL/min Injection volume: 10 µL |
|||||||||||||||||||||
| Mobile Phase (Isocratic mode) |
|
|||||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the standard solutions (1 mg/mL). The requirements of the system suitability parameters are as follow:
|
|||||||||||||||||||||
| Acceptance criteria |
|
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 10% |
| Acid-insoluble ash | Not more than 2% |
| Loss on Drying |
| Not more than 13% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 30% |
| Cold method | Not less than 9% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 4% |
| Cold method | Not less than 3% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 25 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Methanol extract of Z. zerumbet dried rhizome has been reported to contain sesquiterpenes (e.g. zerumbone, 5-hydroxyzerumbone, zerumboneoxide) [ 9 , 10 ].
Essential oil of Z. zerumbet rhizomes has been reported to contain sesquiterpenes (e.g. zerumbone, α-humulene, p-caryophyllene, 6-cadinene, (Z)-nerolidol, (E)-nerolidol, caryophyllene oxide and humulene oxide II); monoterpene (e.g. camphene, tricyclene, α-thujene, β-pinene, myrcene, α-phellandrene, 3-carene, p-cymene,); terpene (e.g. α-pinene, limonene, 1,8 cineole-γ-terpinene, fenchone, terpinolene, terpinen-4-ol, isoborneol, borneol); terpene alcohol (e.g. linalool); terpenoid (e.g. camphor citronellal); monoterpene alcohol (e.g. α-terpineol); Ester (e.g. bornyl acetate) [ 11 ].
MEDICINAL USES
Uses described in folk medicine,not supported by experimental or clinical data
A decoction of Z. zerumbet rhizome was used for stomachache [ 12 ].
Biological and pharmacological activities supported by experimental data
Antiparasitic activity
Chloroform extract of Z. zerumbet rhizome showed anti-amoebic activity with inhibition concentration at 50% (IC50) of 196.9 ± 37.0 µg/mL compared to metronidazole (IC50 = 1.1 ± 0.10 µg/mL) against Entamoeba histolytica trophozoites [ 13 ].
Chloroform extract of Z. zerumbet rhizome (31.25–1000 µg/mL) showed antigiardial activity with IC50 of 69.02 ± 0.92 µg/mL compared to metronidazole (IC50 = 0.48 ± 0.02 µg/mL) against Giardia intestinalis trophozoites [ 14 ].
Antimicrobial activity
Crude ethanol extract of Z. zerumbet rhizome and its fractions of petroleum ether and chloroform (400 µg/disc) inhibited the growth of Candida albicans, Aspergillus niger and Sacharomyces cerevaceae with zone of inhibition ranging from 6-9 mm compared to kanamycin (31 mm) using disc diffusion method [ 3 ].
Crude ethanol extract of Z. zerumbet rhizome and its fractions, petroleum ether and chloroform (400 µg/disc) respectively inhibited the growth of Bacillus cereus, Bacillus megaterium, Bacillus subtilis, Staphylococcus aureus, Sarcina lutea, Escherichia coli, Pseudomonas aeruginosa, Salmonella paratyphi, Salmonella typhi, Shigella boydii, Shigella dysenteriae, Vibrio mimicus and Vibrio parahemolyticus with zone of inhibition ranging from 6-10 mm compared to Kanamycin (30-31 mm) using disc diffusion method. The minimum inhibitory concentration (MIC) for B. cereus, S. lutea, V. parahemolyticus, E. coli, S. typhi and P. aeruginosa were 128-256 µg/mL for all the studied extract and fractions
[ 3 ].
Zerumbone from aqueous extract of Z. zerumbet rhizome (0.13–13.00 mg/mL) inhibited the growth of Salmonella choleraesuis with inhibition zone diameter ranging from 7-11 mm compared to streptomycin (17 mm) using disc diffusion assay [ 15 ].
Anti-inflammatory activity
Methanol (80%) extract of Z. zerumbet rhizome (25-100 mg/kg) was administered subcutaneously to Sprague Dawley rats (180-200 g; 8-10 weeks old) 30 min before induction of paw edema using carrageenan. After 120 min, different concentrations of the extract showed significant (p < 0.05) reduction in paw edema volume (0.34-0.12 mL) compared to untreated control group (0.42-0.3 mL) [ 2 ].
The methanol extract of Z. zerumbet rhizome (25-100 mg/kg) administered subcutaneously to Sprague Dawley rats (180-200 g; 8-10 weeks old) 30 min before induction of inflammation using cotton-pellet significantly (p < 0.05) decreased the weight of exudate (0.3-0.5 g) and cells in granuloma tissues (0.06-0.08 g), in a dose dependent manner, compared to untreated control group (exudates: 0.68 g; cells: 0.06 g) [ 2 ].
Antinociceptive activity
Methanol (80%) extract of Z. zerumbet rhizome (100 mg/kg) administered to male Balb/c mice (25-30 g; 5-7 weeks old) 30 min before induction of abdominal constriction using acetic acid significantly (p<0.05) decreased the number of writhing (54.4 ± 8.1) compared to untreated control group (120.3 ± 10.2) [ 2 ].
The methanol extract of Z. zerumbet rhizome (100 mg/kg) administered to male Balb/c mice (25-30 g; 5-7 weeks old) 30 min before induction of pain using hot plate showed significant (p < 0.05) increase in the reaction time (7.1 ± 0.6 s) compared to untreated control group (4.1 ± 0.3 s) [ 2 ].
The methanol extract of Z. zerumbet rhizome (25-100 mg/kg) administered to Sprague Dawley rats (180-200 g; 8-10 weeks old) 30 min before induction of pain using formalin showed significant (p < 0.05) decreased in duration of paw licking (19.1-14.3 s) compared to untreated control group (26.1 s) [ 2 ].
The methanol extract of Z. zerumbet rhizome (5-10 µg/mL) administered to Sprague Dawley rats (180-200 g; 8-10 weeks old) 30 min before induction of rat ileum contraction using bradykinin showed significant (p < 0.05) dose-dependent decreased (3.0-1.2 g) compared to untreated control group (7.4-2.2 g) [ 2 ].
The methanol extract of Z. zerumbet rhizome (5-10 µg/mL) administered to Sprague Dawley rats (180-200 g; 8-10 weeks old) 30 min before induction of rat ileum contraction using prostaglandin showed significant (p < 0.05) dose-dependent decreased (4.0-1.2 g) compared to untreated control group (7.8-1.8 g) [ 2 ].
The methanol extract of Z. zerumbet rhizome (5-10 µg/mL) administered to Sprague Dawley rats (180-200 g; 8-10 weeks old) 30 min before induction of rat ileum contraction using histamine showed significant (p < 0.05) dose-dependent decreased (8.8-2.4 g) compared to untreated control group (8.8-3.0 g) [ 2 ].
Ethanol extract of Z. zerumbet rhizome (10.0-100.0 mg/kg) administered to male Balb/C mice (25-30 g) 30 min before induction of abdominal constriction using acetic acid significantly (p < 0.05) decreased the number of writhing (5.33-51.30), in a dose-dependent manner, at 10 min compared to morphine (0.2 mg/kg: 33.45; 0.8 mg/kg: 2.01) [ 16 ].
Antiproliferative activity
Zerumbone isolated from essential oil of Z. zerumbet rhizome significantly inhibited the proliferation of cervical cancer cell line (HeLa cell) in time-dependent manner (24, 48 and 72 hours). The IC50 of zerumbone towards cell viability was 20.30 ± 1.2 µM/mL compared to cisplatin (5.45 ± 0.44 µM/mL) [ 15 ].
Antitumour activity
Diethyl ether fraction of ethanol (95%) extract of Z. zerumbet rhizome showed antitumour activity on murine lymphoid neoplasm (P-388D1) cell with IC50 of 60.67 µg/mL at 12 hours and 12.41 µg/mL at 72 hours compared to positive control adriamycin (12 hours: 0.20 µg/mL and 72 hours: 0.05 µg/mL) using MTT assay [ 4 ].
Diethyl ether fraction of ethanol (95%) extract of Z. zerumbet rhizome (2.5–10.0 mg/kg) significantly (p < 0.05) prolonged the lifespan of P-388D1 tumor-bearing CDF1 5-week old male mice (25.8–26.2 days: 119.4–121.3% ILS, percentage increase in lifespan) compared to control (21.6 days: 100.0%) [ 4 ].
Antihypersensitive activity
Aqueous extract of Z. zerumbet rhizome (100 mg/kg) was administered orally to female specific-pathogen-free (SPF) ICR mice (4 weeks old) for a duration of 56 days. Aqueous extract of Z. zerumbet rhizome decreased the level of tumour necrosis factor-alpha and interleukin-4 (IL-4) in vitro. In vivo, mice treated with the aqueous extract of Z. zerumbet (100 mg/kg) for 56 days show higher ratios of interferon-gamma (IFN-γ) / IL-4 mRNA in their splenocytes compared to the control group (p<0.05). The results show that the aqueous extract has the capacity in diminishing hypersensitive reaction since increased level of IFN-γ/IL-4 indicates reduction of allergic asthmatic reaction [ 16 ].
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Genotoxicity
Zerumbone isolated from methanol extract of Z. zerumbet (1000 mg/kg) administered intraperitoneally to male Sprague-Dawley rats (170-200 g; 6-8 weeks old) for a duration of 24 hours. The extract significantly (p < 0.05) increased the number of micronuclei in polychromatic erythrocytes (MN-PCEs) 0.76 ± 0.16% compared to untreated control group (0.15 ± 0.04%) [ 17 ].
Zerumbone isolated from methanol extract Z. zerumbet (40 µM) was treated in Chinese hamster ovary (CHO) cells lines for duration of 48 hours. The extract significantly (p < 0.05) increase in the frequency of binucleated cells with micronucleus (57) compared to ethanol treated group (13). The extract also showed a dose-dependent reduction in cell proliferation reaching statistical significance (p < 0.05) at highest concentration tested, 80 µM compared to untreated control group (2.072) [ 17 ].
Zerumbone isolated from methanol extract Z. zerumbet (80 µM) was treated in human lymphocytes for duration of 24 hours. The extract significantly (p < 0.05) increase in the frequency of binucleated cells with micronucleus (7.5%) compared to ethanol treated group (3.0%). The extract also showed a dose-dependent reduction in cell proliferation reaching statistical significance (p < 0.05) at highest concentration tested, 80 µM compared to untreated control group (1.804)[ 17 ].
Clinical studies
Information and data have not been established.
Others (Adverse reaction, contraindication, side effect, warning, precaution)
Information and data have not been established.
DOSAGE
Information and data have not been established.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The plant list. [Internet] Zingiber zerumbet. Version 1.1; [cited on 9th December 2014]. Available from: http://www.theplantlist.org/tpl1.1/search?q=zingiber+zerumbet.
- Zakaria ZA, Mohamad AS, Chear CT, Wong YY, Israf DA, Sulaiman MR. Antiinflammatory and antinociceptive activities of Zingiber zerumbet methanol extract in experimental model systems. Medical Principles and Practice. 2010;19:287–294.
- Golam K, Farjana N, Mohammad AR, Tanzima Y. Antimicrobial activities of the rhizome extract of Zingiber zerumbet Linn. Asian Pacific Journal of Tropical Biomedicine. 2011;1(5):409-412.
- Huang GC, Chien TY, Chen LG, Wang CC.Antitumor effects of zerumbone from Zingiberzerumbetin P-388D1 cells in vitro and in vivo. Planta Medica. 2005;71(3):219-224.
- Flowers of India. [Internet] Shampoo ginger; [cited on 4th December 2014]. Available from: http://www.flowersofindia.net/catalog/slides/Shampoo%20Ginger.html
- Floridata. [Internet] Zingiber zerumbet (1996-2012); [cited on 3rd December 2014].Available from:http://www.floridata.com/ref/z/zing_zer.cfm
- National tropical botanical garden. [Internet] Zingiber zerumbet(2014);[cited on 3rd December 2014]. Available from: http://ntbg.org/plants/plant_details.php?plantid=11663
- Prakash RO, Kumar RK, Rabinarayan A, Kumar MS. Pharmacological and phytochemical studies of Zingiber zerumbet (L.) SM. Rhizome. International Journal of Research in Ayurveda & Pharmacy. 2011;2(3):698-703
- Jang DS, Min HY, Kim MS, Han AR, Windono T, Jeohn GH, Kang SS, Lee SK, Seo EK. Humulene Derivatives from Zingiber zerumbet with the inhibitory effects on lipopolysaccharide-induced nitric oxide production. Chemical & Pharmaceutical Bulletin. 2005;53(7):829-831.
- Abdul ABH, Al-Zubairi AS, Tailan ND, Wahab SIA, Zain ZNM, Ruslay S, Syam MM. Anticancer activity of natural compound (zerumbone) extracted from Zingiber zerumbet in human hela cervical cancer cells. International Journal of Pharmacology. 2008;4(3):160-168.
- Jimmy CMJ, Robert V, Chalchat JC. Chemical composition of essential oils from rhizomes, leaves and flowers of Zingiber zerumbet Smith from Reunion Island. Journal of Essential Oil Research. 2013; 15(3):202-205.
- Burkill IH. A dictionary of the economic products of the Malay peninsula. Vol. 2. London; Published on behalf of the governments of the Straits settlements and Federated Malay states by the Crown agents for the colonies. 1935;p.2303-2304.
- Sawangjaroen N, Phongpaichit S, Subhadhirasakul S, Visutthi M, Srisuwan N, Thammapalerd N. The anti-amoebic activity of some medicinal plants used by AIDS patients in southern Thailand. Parasitology Research. 2006;98(6):588-592.
- Sawangjaroen N, Subhadhirasakul S, Phongpaichit S, Siripanth C, Jamjaroen K, Sawangjaroen K. The in vitro anti-giardial activity of extracts from plants that are used for self-medication by AIDS patients in southern Thailand. Parasitology Research. 2005;95(1):17-21.
- Ahmad BA, Siddig IA, Adel SA, Manal ME, Syam MM. Anticancer and antimicrobial activities of zerumbone from the rhizomes of Zingiber zerumbet. International Journal of Pharmacology. 2008;4(4):301-304.
- Chaung HC, Ho CT, Huang TC. Anti-hypersensitive and anti-inflammatory activities of water extract of Zingiber zerumbet (L.) Smith. Food and Agricultural Immunology. 2008;19(2):117-129.
- Al-Zubairi, Adel S, Abdul AB, Mohammed Y, Siddig IA, Manal ME, Syam M. In vivo and in vitro genotoxic effects of zerumbone. Caryologia. 2010;63(1):11-17.