Product Registration With NPCB (National Pharmaceutical Control Bureau)
For Malaysia, product registration is conducted through National Pharmaceutical Control Bureau.
Documents required for Health supplement product with Disease Risk Reduction claims (high claim).
|
NO. |
FIELD |
NO. |
FIELD |
|
|
GENERAL REQUIREMENTS*apply for general/nutritional claims, functional claims and disease risk reduction claims. |
||||
|
1 |
Product name |
22 |
Finished product quality specifications |
|
|
2 |
Brand name and product name |
23 |
Stability data |
|
|
3 |
Product description |
24 |
Label for immediate container |
|
|
4 |
Dosage form |
25 |
Label for outer carton |
|
|
5 |
Product indication/usage |
26 |
Proposed package insert/Product information leaflet |
|
|
6 |
Dose/Use instruction |
27 |
Company name and address of product owner |
|
|
7 |
Contraindication, if applicable |
28 |
Company name and address of manufacturer(s) |
|
|
8 |
Warning/Precautions, if applicable |
29 |
Company name and address of repacker (if applicable) |
|
|
9 |
Drug interaction, if applicable |
30 |
Common name and address of other manufacturer (if applicable) |
|
|
10 |
Side effects/ Adverse reactions, if applicable |
31 |
Store address(s) |
|
|
11 |
Signs and symptoms of overdose and treatment, if applicable |
32 |
Importer (s) |
|
|
12 |
Storage condition |
33 |
Letter of authorization from product owner to product registration holder (if applicable) |
|
|
13 |
Shelf life |
34 |
Letter of Appointment of Contract Manufacturer/Repacker from Product Owner (if applicable) |
|
|
14 |
Therapeutic code |
35 |
Letter of Acceptance from Contract Manufacturer/Repacker (if applicable) |
|
|
15 |
Batch Manufacturing Formula |
36 |
Certificate of Pharmaceutical Product (CPP) |
|
|
16 |
List of active ingredient(s) |
37 |
Certificate of Free Sale (CFS) |
|
|
17 |
List of excipient(s) |
38 |
Certificate of Good Manufacturing Practice (GMP) |
|
|
18 |
Attachment of Batch Manufacturing Formula – Product owner’s/manufacturer original letterhead, product details, date and signature & designation of authorized personnel |
39 |
Attachment of protocol analysis |
|
|
19 |
Manufacturing process |
40 |
Attachment of Certificate of finished product (COA of finished product) |
|
|
20 |
Attachment of manufacturing process Document or manufacturing flow diagram |
41 |
Attachment of Specifications and Certificate of Analysis (COA) of Active Ingredient |
|
|
21 |
In-process quality control |
|
|
|
|
ADDITIONAL REQUIREMENTS* apply for Disease Risk Reduction claims. |
||||
|
|
PRODUCT |
|
SUBSTANCE |
|
|
NO. |
FIELD |
NO. |
FIELD |
|
|
1 |
Description & composition |
1 |
General information |
|
|
2 |
Pharmaceutical development |
2 |
Manufacture |
|
|
3 |
Manufacturer |
3 |
Characterization |
|
|
4 |
Control of excipients |
4 |
Control of health supplement substance |
|
|
5 |
Control of finished products |
5 |
Reference standards/materials |
|
|
6 |
Reference standards/materials |
9 |
Container closure system |
|
|
7 |
Container closure system |
7 |
Stability |
|
|
8 |
Stability |
|
|
|
|
9 |
Product Interchangeability/Equivalent evidence |
|
|
|
Complete stability study conducted at 30 ± 2 ºC / RH 75 ± 5%. IPQC, FPQC, protocol analysis and COA of finished product are required to be submitted 2 years after product registration with SAMPLE of the products. Failure on submission will cause the product be suspended until complete documents are submitted, the registration of the product will be terminated if the complete documents still cannot be produced upon renewal of product registration.
| Non Clinical Data (for new active ingredients , new combination of active ingredients, new dose) | Clinical Data |
| Overview of non-clinical testing strategy | Clinical overview |
| Pharmacology | Production development rationale |
| Pharmacokinetics | Overview of Bio-pharmaceutics |
| Toxicology | Overview of Clinical pharmacology |
| Integrated overview and conclusion | Overview of Efficiency |
| Other toxicities study , if available | Overview of Safety |
| References | References |
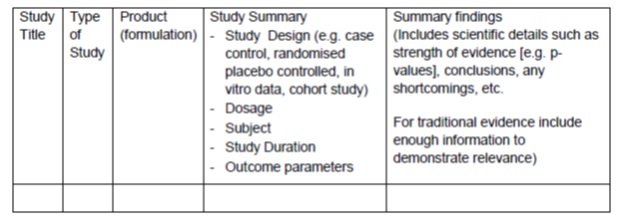
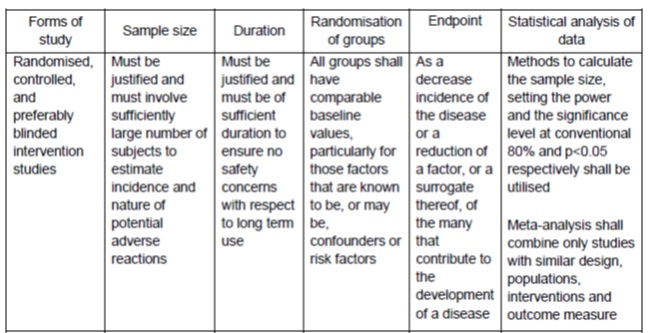
Drug Registration Guidance Document (DRGD), First Edition. January 2013
Information obtained from Drug Registration Guidance Document (DRGD), First Editiondownload from http://npra.moh.gov.my/index.php/guidelines-central on 27th May 2014.


