Chronological development
| Year | Chronology |
| Early settlers | Singaporeans practiced their traditional folk medicine [1]. |
| 19th century | The first western trained doctor arrived and treated the East India Company officials exclusively [1]. |
| 1844 | A leposarium was built at Pearl’s Hill [1]. |
| 1905 | College of Medicine was founded [1]. |
| 1909 | Tan Tock Seng Hospital was built followed by the Municipal Hospital [1]. |
| After the first world war |
The period that most of the existing hospital in singapore were built [1]:
|
| 1921 | Training of public health personnel to deal with the control infectious disease and the inspection of food premises, market and other public places were undertaken [1] |
| 1994 |
The Minister of Health (MOH) appointed of committee on Traditional Medicine (TCM) and recommend measure to safeguards patient interest and safety and to enhance the standards of training of TCM practicioners.[2] |
| 1995 |
The comittee submitted a reported on TCM to the Minister of Health and recommended a phased approach to regulation of TCM based on the following principles.[3] A TCM was setup in the MOH ( which was subsequently renamed TCM department) to coordinate the implementation of the committee recomendations.[4] |
| 1996 |
As part of self regulation of TCM practisoners, the following committes were formed to represent the local TCM community in Singapore.[3]
Chinese Propreitary Medicines (CPM) Unit was setup.[5] |
| 1998 | CPM product regulation was gazette under medicines acts [6] |
| 1999 | With effect from 1st September 1999, CPM product regulation was implemented to promote safety and quality of CPM products available in the Singapore markets. |
| 2000 | TCM practitioners act was passed 14th Nov 2000 by parliament [7],[8] |
| 2001 |
The Traditional Chinese Medicine in MOH was renamed the Traditional & Complementary Medicine Branch. Formation of Traditional Chinese Medicine Practitioner Board (TCMPB) [6]. TCMPB is responsible to register both acupuncturists and TCM physician: to accredited TCM Instituition and TCM courses for the purposes of registration; and to determine and regulate the conduct and ethics of registered practitioners.[9] Registration of TCM practitioners in phases.First to be registered : Accupuncturist [6],[8],[10] |
| 2002 |
Registration of TCM physician started.[6],[8] |
| 2004 |
From 1st January 2004 practioners who wish to practicise TCM in Singapore are eligible for registeration once they
|
| 2005 | The transmissible spongiform Encephalopathy (TSE) guideline for Minimizing the Risk of Contamination in Chinese proprietary Medicine, Traditional Medicine and Health Supplement was issued[5]. |
| 2006 |
The Ethical code and Ethical guidelines for TCM practitioners was published by TCM practitioners board [6] The Singapore College of TCM started a graduate diploma in acupuncture course, conducted in English to train registered medical and dental practitioners in acupuncture. Graduates of the course who passed the Singapore Acupuncture Registration Examination (SARE) can be registered as acupuncturists by the TCM practitioners boards. Voluntary listing of Chinese medicinal materials (CMM) dispensers ie:(TCM Pharmacist) |
Current Practice
At present in Singapore, allopathic medicine is the core medicinal system being utilized [5][6][11]. Nonetheless, it is common practice among the various ethnic groups to consult traditional practitioners for general ailments [11]. Traditional medicine comprising the following historical healing methods still enjoy considerable popularity [12]:
- Traditional Chinese Medicine (TCM) using acupuncture and Chinese medicinal materials (CMM or zhongyao) mainly from China
- Traditional Malay Medicine using Malay medicinal materials (or jamu) mainly from Indonesia and Malaysia; and
- Traditional Indian Medicine using Indian medicinal materials (or ayurveda) mainly from India
TCM products are Chinese Medicinal Materials (CMM) that include Chinese herbs and medicinal materials obtained from animal and mineral sources used in the practice of TCM. They are grouped as follows [2][12]:
- Raw CMM: CMM in natural states or undergone simple processing (e.g. cutting, drying etc).
- Chinese Proprietary Medicines (CPM): CMM preparations in final dosage form (e.g. tablets, pills, liquid preparations etc).
Prevalence
The survey in 2004, conducted by National University of Singapore, supported that Traditional Chinese Medicine (TCM) as the most frequently utilized Traditional Medicine (TM) by Singaporean followed by Traditional Malay Medicine (jamu) and Traditional Indian medicine [13].
| No. | Modality | Percentage (%) |
| 1 | Traditional Chinese medicine | 88 |
| 2 | Traditional Malay medicine | 8 |
| 3 | Traditional Indian Medicine | 3 |
| 4 | Other forms of CAM | 1 |
| Total | 100 |
Table 1: Utilization of T/CM by the Singaporean population based on categories
Table constructed based on information from: Lim, M. K. et al. Complementary and alternative medicine use in multiracial Singapore. Journal Complementary Therapies in Medicine, 2005.
It also exhibited that gender and ethnicity were important in determining factors that affect the utilization of TM/CAM. It showed that among the ethnics, 84% Chinese were using the TM/CAM, with Malay and Indian at 69% respectively [13].
Administration
Official Body
Contact Address:
Traditional & Complementary Medicine Branch
Ministry of Health
College of Medicine Building
16 College Road
Singapore 169854
Contact No. : (65) 6325 9220
Fax No : (65) 6235 9499
Email : [email protected]
Website : www.moh.gov.sg
Main therapies
In Singapore, Traditional Chinese Medicine is the most popular form of Traditional and Complementary Medicine [6][13]. Traditional Malay Medicine and Traditional Indian Medicine are also available as are ‘Western’ Complementary and Alternative Medicine (CAM) modalities such as aromatherapy and chiropractic [12][13][14].
Policy & Regulations
Regulation
- The TCM Practitioners Board oversees the regulation of TCM practice [11].
- The Health Sciences Authority (HSA) is the authorized body in charge of health product regulation in accordance to the Health Products Act. The Complementary Medicines Branch of HSA oversees the regulation of Chinese Proprietary Medicines (CPM), other Traditional Medicines and Health Supplements [5].
Policy
- The Singapore’s National Policy on Traditional/Complementary Medicine was issued in 1995 [3].
- The policy proposed:
- A phased line attempt to TCM regulation
- Promote standard of TCM training
- Control of Chinese medicinal materials
Act
- The Singapore Parliament passed The Traditional Chinese Medicine Practitioners Act on 14 November 2000 [6][7][11].
- The Act requires TCM practitioners who undertake the prescribed practice of TCM to be registered with the TCM Practitioners Board [6].
- Legislations regulating TCM Practice:
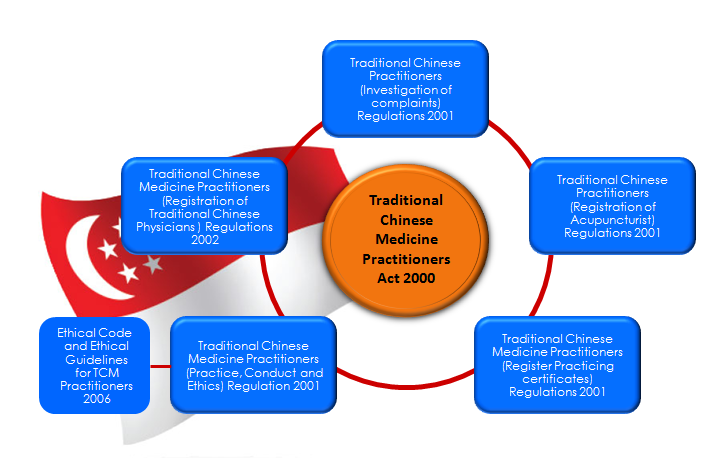
Diagram 1: Regulation of Traditional Chinese Medicine Practitioners [3].
Legislations regulating CPM products [3]:
- The Health Products Act
- The Medicines Act
- The Poisons Act
- The Sale of Drugs Act
- The Medicines (Advertisement and Sale) Act
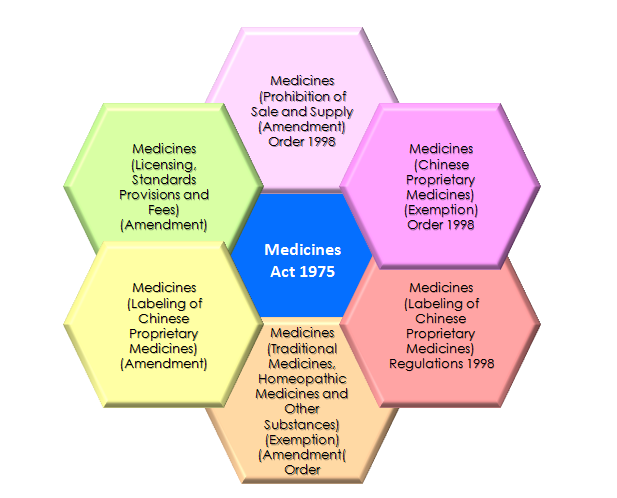
Diagram 2: Legislations of Chinese Proprietary Medicines [5].
Chinese Proprietary Medicines (CPM) regulation requires the local dealers to obtain the appropriate licences before they can import, wholesale, manufacture or assemble CPM. All CPM products must meet stipulated safety and quality criteria before they can be approved by the HSA [5].
Unlike CPM, it is currently not necessary for traditional medicines and homeopathic medicines to obtain approvals from HSA. The accountability of safety and quality of these products lies with the dealers and sellers. They must comply with legal requirements, such as the absence of legally prohibited substances, limits of toxic heavy metals and prohibition of legally stipulated strong medical claims e.g. cancer, diabetes.
An advertising permit is also required for advertisement and promotional activities for CPM and traditional medicines.
In addition to the pre-marketing activities, HSA carries out post-marketing surveillance activities to ensure timely detection and the removal of any unsafe products from the market. These surveillance activities include:
- Adverse reaction reporting;
- Testing of products sampled from the retail market using a risk-based approach;
- Intelligence gathering including investigation of feedback from members of the public and the media
- Information exchange with other national authorities.
(Note: There are other policies and regulations pertaining to this country in its endeavour to regulate TM/CAM. For further information, please review LINK TO POLICY)
Facilities
- In the past, the use of acupuncture was allowed on clinical research basis in several hospitals [7]:
- Ang Mo Kio Community Hospital
- National University Hospital
- Singapore General Hospital
- Tan Tock Seng Hospital
- Today, the Singapore government allows acupuncture as well as other TCM services (e.g. Chinese herbal medicine and Chinese therapeutic massage ‘Tui Na’) to be offered in hospitals and nursing homes. However, such services are only allowed on an outpatient basis and provided by a TCM clinic co-located within the premises of the healthcare institutions as a separate entity (except for acupuncture services which could be provided by the healthcare institutions as part of their medical services to their patients, and inpatients would be allowed to seek acupuncture treatment with a doctor’s referral) [15].
- In addition, registered medical and dental practitioners (who are also registered acupuncturists/TCM practitioners) are allowed to provide acupuncture services as part of their package of services to their patients in their medical clinics. [15].
- Apart from the above, hospitals and medical centres that have a co-located TCM clinic within their premises include:
- Singapore General Hospital
- Changi General Hospital
- Alexandra Hospital
- Raffles Hospital
- Camden Medical Centre
- National University Hospital
Insurance coverage
- Currently, there is no information on insurance coverage for any T&CM modalities in Singapore.
Research Institute/s
- In 1995, an Acupuncture Research Clinic was set up by the Singapore Ministry of Health at the Ang Mo Kio Community Hospital [3].
- Other institutions that have carried out TCM research are:
- National University of Singapore
- Nanyang Technological University
- Singapore General Hospital
- National University Singapore
- Tan Tock Seng Hospital
- KK Women’s and Children’s Hospital
- The Health Sciences Authority
- The National Cancer Centre
- Research projects conducted include use of TCM and acupuncture in pain control, stroke rehabilitation and smoking cessation.
Training & Education
In 2002, Singapore Ministry of Health worked with the local Chinese Medicinal Material (CMM) community to conduct a formal training course in CMM herbal dispensing jointly with China’s Beijing University of Chinese Medicine. Graduates of the course have been listed voluntarily with the TCM Practitioners Board in preparation for future registration of this group of practitioners.
Presently, there are three local institutions accredited by the TCM Practitioners Board to provide TCM training courses:
- Singapore College of Traditional Chinese Medicine
- Institute of Chinese medical Studies
- Nanyang Technological University
Links
- Singapore Ministry of Health : http://www.moh.gov.sg/mohcorp/default.aspx
- Traditional Chinese Medicines Practitioners Board : http://www.tcmpb.gov.sg/tcm/
- Health Sciences Authority : http://www.hsa.gov.sg
Special Issue/s
For this moment, Singapore government requires acupuncturists and TCM physicians to be registered with the TCM Practitioners Board. Statutory registration is not necessary for the practitioners of other traditional and complementary medicine [15].
References
- Singapore Ministry of Health Singapore 1975. Singapore: Ministry of Health; 1975.
- Singapore Ministry of Health. Traditional Chinese Medicine. The Report by the Committee on Traditional Chinese Medicine.[homepage on the intrenet]. [cited Oct 1995 ). Available from http://www.gov.sg/moh
- Country Report for ASEAN Ad-hoc Working Group Meeting on Traditional & Complementary Medicine (AWGTCM): 2004 April 17 Penang, Malaysia.
- World Health Organization. National policy on traditional medicine and regulation of herbal medicine. Report of a WHO Global Survey. Geneva: WHO; 2005. Pg. 137
- Health Sciences Authority. Overview. [homepage on the internet]. [cited 2014 August 8]. Available from: http://www.hsa.gov.sg/.
- Heng C. H. The Regulation of traditional Chinese medicine in Singapore. Presented at: 2006 Government Forum on Traditional Medicine (2006GFTRM); 2006 Oct 26-27; Beijing.
- Lee, T. L. (2006). Complementary and alternative medicine and traditional Chinese medicine: time for critical engagement. Ann Acad Med Singapore. [serial online]. 2006 Nov [cited 2008 July 2];35(11):749-52. Available from www.annals.edu.sg/PDF/35VolNo11Nov2006/V35N11p749.pdf.
- Ministry of Singapore. [cited 2007 August 1]. Available from: http://www.moh.gov.sg/mohcorp
- Traditional Chinese Medicine Practitioner Board. [homepage on the internet]. [cited 2007 July 31]. Available from http://www.tcmpb.gov.sg
- Singapore Chinese Physicians’ Association. Singapore. [homepage in the internet].[cited 2008 Jun 13]. Available from http://www.singaporetcm.com
- World Health Organization (2001). Legal status of traditional medicine and complementary/alternative medicine: a worldwide review. Geneva: World Health Organization; 2001.
- Presentation Dr. Cheah C., in Integrating Traditional and Complementary Medicine into the National Healthcare-Singapore’s Experience.
- Lim, M. K., Sdarangani, P., Chan, H.L., Heng, J.Y. (2005). Complementary and alternative medicine use in multiracial Singapore. Complementary therapies in medicine;13:16-24
- Ministrial Advisory Committee on Complementary and Alternative Health, Ministry of Health (2003). Complementary and Alternative Medicine. Current Policies and Policy Issues in New Zealand and Selected Countries. A discussion document 2003. New Zealand: Ministry of Health; 2003.
- Ministry of Health Singapore. [homepage on the internet].[cited 2007 August 1]. Available from: http://www.moh.gov.sg/mohcorp



