Chronological Development
| Year/ Century | Type of Medicinal System & Ethnic group |
| 4th Century BCE and the 5th Century CE | Surgical treatment decreased but on the other hand, medicinal use of plants and herbs [1] had developed. |
| 7th Century | The arrival of Arab Muslim traders |
| 1100AD | Invasion of Muslims brought in Unani medicine [2] |
| Late 15th Century to 16th Century | Foreign traders from Portugal, Dutch, and British East India Company came to India with doctors from their native lands and hence indirectly brought in their medical system respectively. |
| 1795-1689 | A German physician and geologist came to India around 1810 A.D to treat patient using homeopathy. In 1839, Dr. John Martin Honigberger came to treat Maharaja Ranjit using the same method [3]. |
Current Practice
In India, the Ayurveda healthcare system is well established. State hospitals and dispensaries of traditional medicine have always been available as the traditional system of medicines (Ayurveda, Siddha, Unani, homeopathy, yoga and naturopathy) are well integrated into the national healthcare system. These systems have been providing services to most Indians, especially in the rural areas. On the other hand, traditional medicine is not always accepted by the modern medical practitioners and sometimes they found difficulties to integrate traditional medicine into allopathic medicine. Scientific evidence is much needed for rationale of using this method
Prevalance
- In India, traditional medicine is widely used and accepted especially in rural areas where most of them live. It is estimated that 70% of Indian population live in rural areas as compared to the urban areas (4). They use herbs, ayurveda, siddha, unani and etc for medicinal purpose.
- By far, Ayurveda is the most common traditional medicine used in India as compared to other types of treatment.
Administration
Official Body
In 1995, The Government of India created a Department of Indian Systems of Medicine and Homeopathy (ISM&H) to plan and regulate the Indian traditional system of medicines. This department was later renamed as the Department of Ayurveda, Yoga, Unani, Siddha and Homeopathy (AYUSH) in the year of 2003.
In 2014, this Department of AYUSH became the Ministy of AYUSH, which was created under the Union Ministry of Government of India and headed by a minister of state.
Main Therapies
Department of AYUSH covers 7 India therapies.
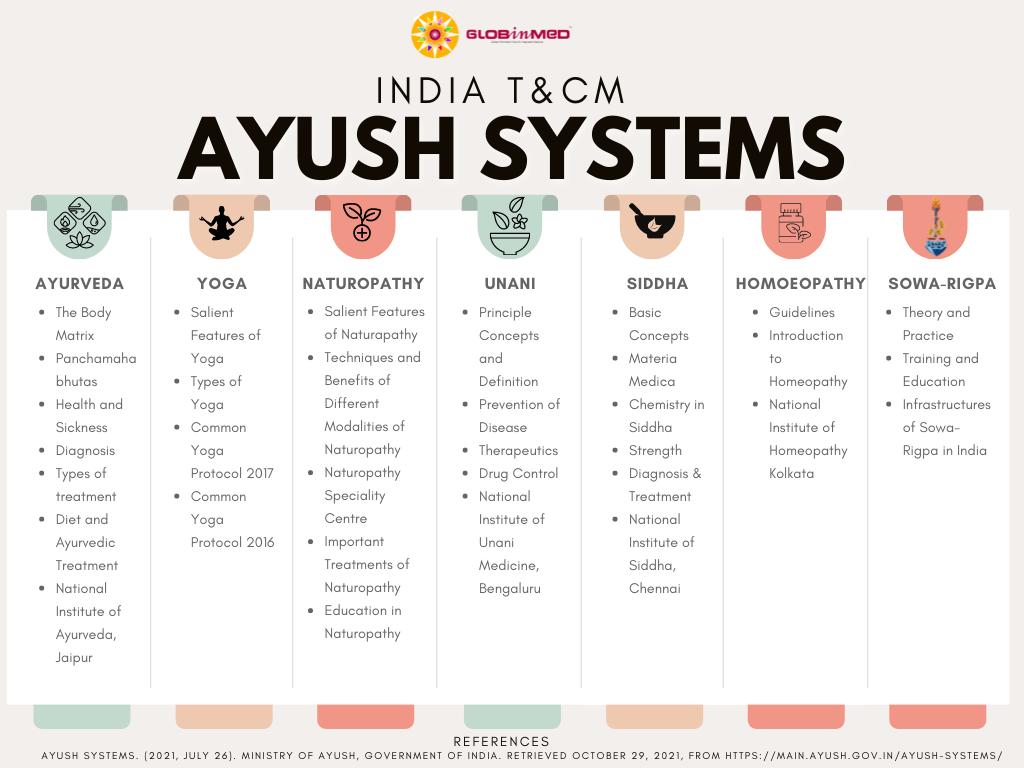
Policy & Regulations
Policy
- National Policy in Traditional & Complementary Medicine (T&CM): National Policy on Indian Systems of Medicine and Homeopathy, 2002
- India’s National Health Policy, 1983.
- The policy proposed an initiative aimed at developing traditional Indian medicine. This policy emphasizes integration of traditional medicine into mainstream healthcare system and every plan must be done gradually phase by phase in due time.
- A national plan for integrating T&CM into national health delivery began in 2014. Government and public research funding is allocated towards T&CM. (7)
Act
- The Indian medicine Central Council Act, 1970
- The Homeopathy Central Council Act, 1973
- Traditional medicine is also governed by the Drugs and Cosmetics Act, 1940
- The State Government is responsible of implementing the licensing of manufacturers and also provisions of the Drugs Act.
- There are other policies and regulations pertaining to this country in its endeavor to regulate traditional and complementary/alternative medicine (TM/CAM). For further information, please visit www.indianmedicine.nic.in.
Facilities
- There are about 25,000 dispensaries, 3000 hospitals with a bed capacity of 65,000 in the government sector and on the whole can be stated to be on a par with the allopathic institutions (5).
- However there are only 30,000 to 40,000 doctors working in the government sector where majority of them work privately.
- Traditional medicine is widely used in rural areas.
Insurance coverage
Not many having medical insurance in India, for those who have, it does cover traditional (6).
Research Institute
- The government of India established a Central Council for Research on Indian Medicine and Homeopathy in 1969 with the aim to develop scientific research on Indian systems of medicine namely Ayurveda, Unani, Siddha, Yoga, Homeopathy and Naturopathy.
- Later, in 1978, this was split into 4 separate Councils
There are 4 apex research councils doing research in India (7):
- Central Council for Research in Ayurveda and Siddha Medicines (CCRAS)
- Central Council for Research in Unani (CCRUM)
- Central Council for for Research in Homeopathy (CCRH)
- Central Council for Research in Yoga and Naturopathy (CCRYN)
Training & Education
In India, all six traditional systems of medicine with official recognition (Ayurveda, Yoga, Naturopathy, Unani Medicine, Siddha and Homeopathy) have institutionalized education systems. Updated in 2013, India has 508 colleges with an annual admission capacity of 25, 586 undergraduate students. Among these, 117 colleges are also admitting 2493 postgraduate students. Colleges can only be established with the permission of central government and the prior approval of their infrastructure, syllabi and course curricula. Annual and surprise inspections ensure that educational and infrastructural standards are met. Central Government has the power to recognize or rescind any qualification of a college. (9)
- The Department of AYUSH (Ayurveda, Siddha, Unani and Homeopathy) is regulating education and research in these systems.
- Traditional Medicine (TM) education system is available in the country, providing degree, short to midterm courses, postgraduate and PhD.
- There are Pharmacopoeia Committees and laboratories for Ayurveda, Siddha and Unani medicines.
- The Central Council of Indian Medicine and Central Council of Homeopathy functions to set the standard for traditional practitioners in terms of training.
- Seven national institutes(8) :
- National Institute of Homeopathy – Bachelor’s and Master’s Degree (MD) in homeopathy.
- National Institute of Naturopathy
- National Academy of Ayurveda
- National Institute of Postgraduate Teaching and Research in Ayurveda – offers Master’s Degree (MD) and PhD.
- National Institute of Ayurveda – PhD and Master’s Degree (MD) in Ayurveda
- National Institute of Yoga – offers one year diploma in yoga.
- National Institute of Unani Medicine – postgraduate research.
Practice
There are national and state level regulations that apply to providers of ayurvedic , homeopathic and Unani medicine.
The Indian Government issues the that T&CM practitioners required license to practise. Licences and certificates are both issued after graduation and subsequent completion of compulsory rotating internship. In India, Bachelor’s degree, Master’s degree, PhDs and clinical doctorate degrees in T&CM are available at the university level.
Regulations for homeopathy practitioners were updated in 2014 and the list of registered TM practitioners was updated in 2016. A consumer education programme for self-help care using T&CM has been in place since 1997. T&CM services are reimbursed by both public and private health insurance in the end of 2016. Numbers of T&CM practitioners registered (as at 1st January 2016) under each practice are as follows: Ayurveda, 419 217; Unani, 48 196; Siddha, 8528; naturopathy, 2220; and homeopathy, 293 307. The total number of T&CM practitioners is 771 468 (6.4 per 10 000 population (7).
Regulatory Status of Herbal Medicine
Herbal medicines are regulated under Ayurveda, Siddha and Unani drugs provision in the Drugs and Cosmetics Act.
They are categorized as prescription medicines and non-prescription medicines, and are sold with medical claims, health claims and nutrient content claims. Regulations for herbal medicines were updated in 2006 and 2017, and the list of registered herbal medicines was updated in 2016. The herbal medicines included in the India’s National Essential Medicine List (NEML) were updated in 2013.
The Ayurveda pharmacopoeia of India, the Unani pharmacopoeia of India and the Siddha pharmacopoeia of India are used as a guideline in the regulations and are legally binding. There are also monographs on single herbs and formularies. The Indian herbal pharmacopoeia is also used but is not legally binding.
Good Manufacturing Practice (GMP) exist for Ayurveda, Unani and Siddha drugs, which include herbal medicines. There are exclusive regulations for GMP, separate from those for conventional pharmaceuticals, that apply to the manufacturing of herbal medicines to ensure their quality. Adherence to manufacturing information in pharmacopoeias and monographs is required. Compliance mechanisms include periodic inspections by authorities at the manufacturing plants or laboratories, the requirement for manufacturers to submit samples of their medicines to a government approved laboratory for testing and to assign a person to the role of ensuring compliance. Licenses given to manufacturing units are renewed every 3 years, to ensure compliance with GMP. Traditional use without demonstrated harmful effects is considered sufficient for safety assessment of herbal medicines.
Herbal medicines are also included under Schedule E of the Drugs and Cosmetics Rules. There is a separate essential drug list for Ayurveda and Unani medicines. Inclusion of a herbal medicine is based on its traditional use and long-term historical use, as well as disease-wise classification. Herbal medicines which are categorized as prescription medicines are sold in pharmacies while herbal medicines which are categorized as non-prescription medicines, self-medication or OTC medicines are sold in pharmacies and other outlets, by licensed practitioners. (7)
Essential medicines are defined as those medicines that satisfy the priority health care needs of the population, India do have herbal medicines included in their National Essential Medicine List (NEML). List of countries who use and refer Ayurveda pharmacopoeia of India and Indian Traditional Medicine are Nepal, Brunei Darussalam, Malaysia, Singapore, South Africa, Canada, Oman, Pakistan, Syrian Arab Republic, Nepal, and Sri Lanka (7).
References
- Rao MS, The history of medicine in India and Burma.Pg 55
- Elizabeth Williamson,2006, System of Traditional Medicine From South and South East Asia : The Pharmaceutical Journal, volume 276:539
- AYUSH in India 2005, Planning and Evaluation Cell, Department of Ayurveda,Yoga and Naturopathy,Unani, Siddha and Homeopathy (AYUSH), Ministry of health and Family Welfare, Government of India,pg 13.
- Legal Status of Traditional Medicine and Complementary/Alternative Medicine: A worldwide review, WHO 2001
- Review of Traditional medicine in the south east asia region, Report of the Regional Working Group Meeting New Delhi, India, 16-17 August 2004,WHO, Pg 8
- Legal Status of Traditional Medicine and Complementary/Alternative Medicine:A Worldwide Review,WHO 2001
- World Health Organization. (2019). WHO global report on traditional and complementary medicine 2019. World Health Organization.
- Legal Status of Traditional Medicine and Complementary/Alternative Medicine: A worldwide review,WHO 2001
- World Health Organization. (2013). WHO traditional medicine strategy: 2014-2023. World Health Organization

